Timing of Development of Symptomatic Brain Metastases from Non-Small Cell Lung Cancer: Impact on Symptoms, Treatment, and Survival in the Era of Molecular Treatments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Demographic and Baseline Clinical Data
2.2. Treatment of BM
2.3. Driver Mutation Status
2.4. Clinical Status
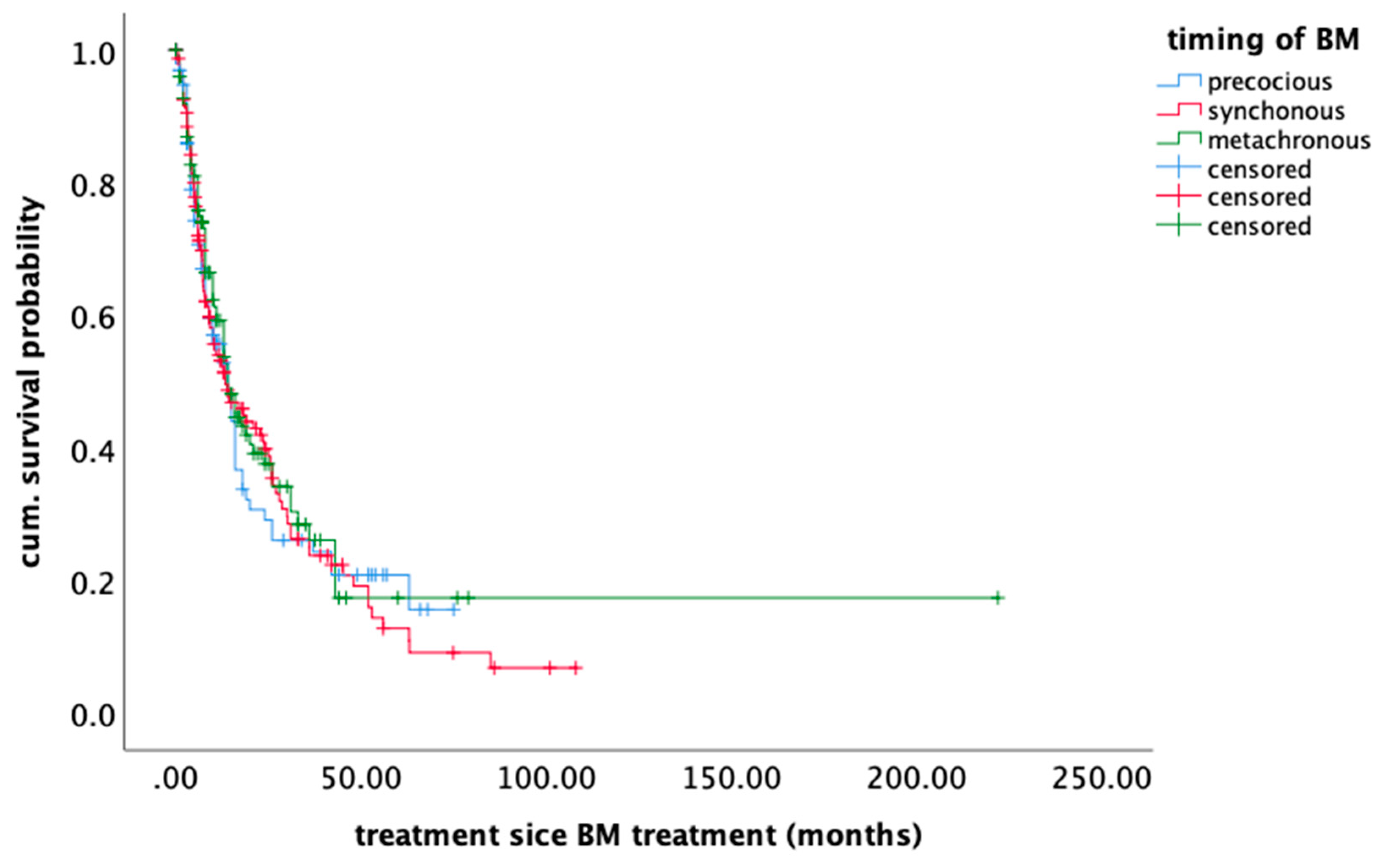
2.5. Survival Outcome
3. Discussion
4. Patients and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALK | anaplastic lymphoma kinase |
| BM | brain metastasis |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| CT | computer tomography |
| EGFR | epidermal growth factor recetor |
| ErbB2 | Erb-B2 Receptor Tyrosine Kinase 2 |
| FGFR | fibroblast growth factor receptor |
| HR | Hazard ratio |
| KPS | Karnofsky performance scale |
| KRAS | Kirsten rat sarcoma viral oncogene |
| LINAC | linear accelerator |
| MET | Mesenchymal–epithelial transition |
| (c)MRI | (cranial)magnetic resonance imaging |
| N/A | not applicable |
| NSCLC | non-small cell lung cancers |
| OS | overall survival |
| PIK3CA | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha |
| ROS | (reactive oxygen species |
| RPA | recursive partitioning analysis |
| RTOG | Radiation Therapy Oncology Group |
| SRS | stereotactic radiosurgery |
| WBRT | whole brain radiation therapy |
References
- Nolan, C.; Deangelis, L.M. Overview of metastatic disease of the central nervous system. Handb. Clin. Neurol. 2018, 149, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Gonçalves, A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012, 32, 4655–4662. [Google Scholar] [PubMed]
- Shibahara, I.; Kanamori, M.; Watanabe, T.; Utsunomiya, A.; Suzuki, H.; Saito, R.; Sonoda, Y.; Jokura, H.; Uenohara, H.; Tominaga, T. Clinical Features of Precocious, Synchronous, and Metachronous Brain Metastases and the Role of Tumor Resection. World Neurosurg. 2018, 113, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.D.; Haylock, B.; Shenoy, A.; Husband, D.; Javadpour, M. Management of cerebral metastasis: Evidence-based approach for surgery, stereotactic radiosurgery and radiotherapy. Eur. J. Cancer 2011, 47, 649–655. [Google Scholar] [CrossRef]
- Ho, V.K.Y.; Gijtenbeek, J.M.M.; Brandsma, D.; Beerepoot, L.V.; Sonke, G.S.; van der Heiden-van der Loo, M. Survival of breast cancer patients with synchronous or metachronous central nervous system metastases. Eur. J. Cancer 2015, 51, 2508–2516. [Google Scholar] [CrossRef]
- Wroński, M.; Arbit, E.; Burt, M.; Galicich, J.H. Survival after surgical treatment of brain metastases from lung cancer: A follow-up study of 231 patients treated between 1976 and 1991. J. Neurosurg. 1995, 83, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, X.; Liang, X.; Huang, R.; Chu, Z.; Jiang, J.; Zhan, Q. A study of patients with brain metastases as the initial manifestation of their systemic cancer in a Chinese population. J. Neurooncol. 2011, 103, 649–655. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, X.; Liang, X.; Huang, R.; Chu, Z.; Jiang, J.; Zhan, Q. Brain metastases as the first symptom of lung cancer: A clinical study from an Asian medical center. J. Cancer Res. Clin. Oncol. 2013, 139, 403–408. [Google Scholar] [CrossRef]
- Füreder, L.M.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Hainfellner, J.A.; Bartsch, R.; Zielinski, C.C.; Preusser, M.; Berghoff, A.S. Brain metastases as first manifestation of advanced cancer: Exploratory analysis of 459 patients at a tertiary care center. Clin. Exp. Metastasis 2018, 35, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.J.; Rock, J.P.; Johnson, C.C.; Weiss, L.; Jacobsen, G.; Rosenblum, M.L. Survival of patients with synchronous brain metastases: An epidemiological study in southeastern Michigan. J. Neurosurg. 2000, 93, 927–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getman, V.; Devyatko, E.; Dunkler, D.; Eckersberger, F.; End, A.; Klepetko, W.; Marta, G.; Mueller, M.R. Prognosis of patients with non-small cell lung cancer with isolated brain metastases undergoing combined surgical treatment. Eur. J. Cardiothorac. Surg. 2004, 25, 1107–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussi, A.; Pistolesi, M.; Lucchi, M.; Janni, A.; Chella, A.; Parenti, G.; Rossi, G.; Angeletti, C.A. Resection of single brain metastasis in non-small-cell lung cancer: Prognostic factors. J. Thorac. Cardiovasc. Surg. 1996, 112, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Granone, P.; Margaritora, S.; D’Andrilli, A.; Cesario, A.; Kawamukai, K.; Meacci, E. Non-small cell lung cancer with single brain metastasis: The role of surgical treatment. Eur. J. Cardiothorac. Surg. 2001, 20, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Billing, P.S.; Miller, D.L.; Allen, M.S.; Deschamps, C.; Trastek, V.F.; Pairolero, P.C. Surgical treatment of primary lung cancer with synchronous brain metastases. J. Thorac. Cardiovasc. Surg. 2001, 122, 548–553. [Google Scholar] [CrossRef] [Green Version]
- Antuña, A.R.; Vega, M.A.; Sanchez, C.R.; Fernandez, V.M. Brain Metastases of Non-Small Cell Lung Cancer: Prognostic Factors in Patients with Surgical Resection. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2018, 79, 101–107. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecci, P.E.; Champion, C.D.; Hoj, J.; McKernan, C.M.; Goodwin, C.R.; Kirkpatrick, J.P.; Anders, C.K.; Pendergast, A.M.; Sampson, J.H. The Evolving Modern Management of Brain Metastasis. Clin. Cancer Res. 2019, 25, 6570–6580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brastianos, P.; Davies, M.A.; Margolin, K.; Yu, H.A. Modern Management of Central Nervous System Metastases in the Era of Targeted Therapy and Immune Oncology. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e59–e69. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ou, Q.; Li, D.; Qin, T.; Bao, H.; Hou, X.; Wang, K.; Wang, F.; Deng, Q.; Liang, J.; et al. Genes associated with increased brain metastasis risk in non-small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non-small cell lung cancer (Guangdong Association Study of Thoracic Oncology 1036). Cancer 2019, 125, 3535–3544. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A New Prognostic Index and Comparison to Three Other Indices for Patients with Brain Metastases: An Analysis of 1960 Patients in the RTOG Database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Churilla, T.M.; Chowdhury, I.H.; Handorf, E.; Collette, L.; Collette, S.; Dong, Y.; Alexander, B.M.; Kocher, M.; Soffietti, R.; Claus, E.B.; et al. Comparison of Local Control of Brain Metastases With Stereotactic Radiosurgery vs Surgical Resection: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 5, 243–247. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Scott, C.; Murray, K.; Curran, W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1001–1006. [Google Scholar] [CrossRef]
- Peifer, M.; Fernández-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef]
- Castiglione, R.; Alidousty, C.; Holz, B.; Wagener, S.; Baar, T.; Heydt, C.; Binot, E.; Zupp, S.; Kron, A.; Wolf, J.; et al. Comparison of the genomic background of MET-altered carcinomas of the lung: Biological differences and analogies. Mod. Pathol. 2019, 32, 627–638. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Precocious (n = 99) | Synchronous (n = 152) | Metachronous (n = 126) | All (n = 377) | p-Value |
|---|---|---|---|---|---|
| Age [median; range] | 62 (38–87) | 62 (32–85) | 60 (25–83) | 61 (25–87) | 0.76 |
| Male gender [n;%] | 56 (56.6) | 88 (57.9) | 63 (50.0) | 207 (54.9) | 0.26 |
| Controlled primary disease [n;(%)] | 0 (0) | 37 (24.3) | 57 (45.2) | 94 (24.9) | <0.0001 |
| Histology [n;%] | 0.10 | ||||
| Adeno | 78 (78.8) | 127 (83.5) | 104 (82.5) | 309 (82.0) | |
| Squamous cell | 14 (14.1) | 13 (8.6) | 19 (15.1) | 46 (12.2) | |
| Neuro-endocrine | 0 (0) | 3 (2.0) | 2 (1.6) | 5 (1.3) | |
| NOS | 7 (7.1) | 9 (5.9) | 1 (0.8) | 17 (4.5) | |
| Previous treatment for primary disease | <0.0001 | ||||
| none | 99 (100) | 16 (10.5) | 4 (3.2) | 119 | |
| Surgery | 0 (0) | 27 (17.8) | 68 (54.0) | 95 | |
| Neo-adjuvant Chemotherapy | 0 (0) | 14 (9.2) | 20 (15.9) | 34 | |
| Neo-adjuvant radiotherapy | 0 (0) | 0 (0) | 12 (9.5) | 12 | |
| Chemotherapy | 0 (0) | 63 (41.4) | 89 (70.6) | 152 | |
| Radiotherapy | 0 (0) | 21 (13.8) | 47 (37.3) | 68 | |
| Molecular treatment | 0 (0) | 6 (3.9) | 26 (20.6) | 32 | |
| Unknown | 0 (0) | 5 (3.3) | 5 (4.0) | 10 | |
| BM count [n;%] | 0.11 | ||||
| 1 BM | 60 (60.6) | 80 (52.6) | 59 (46.8) | 199 (52.8) | |
| 2–3 BM | 29 (29.3) | 51 (33.6) | 38 (30.2) | 118 (31.3) | |
| ≥4 BM | 10 (10.1) | 30 (19.8) | 29 (23.0) | 69 (18.3) | |
| Mutational status [n;%] | 0.002 | ||||
| N/A | 29 (29.3) | 79 (52.0) | 34 (27.0) | 142 (37.7) | |
| Wild type | 36 (36.4) | 18 (11.8) | 40 (31.7) | 94 (24.9) | |
| EGFR | 4 (4.0) | 28 (18.4) | 19 (15.1) | 51 (13.6) | |
| KRAS | 7 (17.2) | 20 (13.2) | 21(16.7) | 58 (15.4) | |
| MET | 6 (6.1) | 3 (2.0) | 4 (3.2) | 13 (3.4) | |
| BRAF | 1 (1.0) | 0 (0) | 1 (0.8) | 2 (0.5) | |
| ALK | 1 (1.0) | 1 (0.7) | 1 (0.8) | 3 (0.8) | |
| ROS | 0 (0) | 0 (0) | 3 (2.4) | 3 (0.8) | |
| FGFR | 2 (2.0) | 1 (0.7) | 1 (0.8) | 4 (1.1) | |
| PIK3CA | 0 (0) | 2 (1.3) | 0 (0) | 2 (0.5) | |
| ErbB2 | 3 (3.0) | 0 (0) | 2 (1.6) | 5 (1.3) | |
| Tumor location [n;(%)] | 0.17 | ||||
| Supratentorial | 46 (46.5) | 86 (56.6) | 64 (50.8) | 196 (52.0) | |
| Infratentorial | 22 (22.2) | 25 (16.4) | 11 (8.7) | 58 (15.4) | |
| Supra- and infratentorial | 31 (31.3) | 41 (27.0) | 51 (40.5) | 123 (32.6) | |
| Symptomatic BM [n;(%)] | <0.0001 | ||||
| Neurological deficits [n] | 97 (98.0) | 90 (59.2) | 71 (56.3) | 258 (68.4) | |
| Seizures | 15 | 21 | 12 | 48 | |
| Aphasia | 14 | 11 | 8 | 33 | |
| Hemiparesis | 26 | 28 | 21 | 75 | |
| Visual field defects | 1 | 13 | 5 | 19 | |
| Cerebellar signs | 39 | 10 | 20 | 69 | |
| Signs of elevated intracranial pressure | 30 | 26 | 18 | 74 | |
| KPS at presentation [median; range] | 80 (40–100) | 90 (40–100) | 90 (50–100) | 80 (40–100) | 0.03 |
| RPA class prior to BM treatment | <0.0001 | ||||
| Class I | 0 | 0 | 34 | 34 | |
| Class II | 79 | 133 | 72 | 284 | |
| Class III | 20 | 19 | 20 | 59 | |
| RPA class after BM treatment | <0.0001 | ||||
| Class I | 0 | 1 | 34 | 35 | |
| Class II | 97 | 139 | 78 | 314 | |
| Class III | 2 | 12 | 14 | 28 | |
| Treatment modality | <0.0001 | ||||
| Surgery + postoperative radiotherapy | 84 (84.8) | 91 (59.9) | 70 (55.6) | 245 (65.0) | |
| Stereotactic radiosurgery | 15 (15.2) | 61 (40.1) | 56 (44.4) | 132 (35.0) | |
| Systemic medical treatment after BM | 0.09 | ||||
| Treatment modality (n = 238) | 68 (68.7) | 102 (67.1) | 71 (56.3) | 241 (63.9) | |
| Chemotherapy | 42 | 72 | 57 | 171 | |
| Molecular therapy | 39 | 39 | 24 | 102 | |
| Dead by time of analysis | 62 (62.6) | 103 (67.8) | 67 (53.2) | 232 (61.5) | |
| Cause of death | <0.0001 | ||||
| Unknown | 8 (12.9) | 67 (65.0) | 23 (34.3) | 101 (43.5) | |
| Neurological | 4 (6.5) | 7 (6.8) | 10 (14.9) | 21(9.1) | |
| Systemic disease progression | 46 (74.1) | 26 (25.2) | 34 (50.7) | 106 (45.7) | |
| others | 4 (6.5) | 3 (2.9) | 6 (9.0) | 13 (5.6) |
| Parameter | Univariate (Log Rank) [p-Value] | Multivariate (Cox Regression) [HR 95%CI; p-Value] |
|---|---|---|
| Age ≤ 65 | 0.88 | |
| Controlled systemic status | 0.15 | |
| Timing | 0.76 | |
| Precocious vs. synchronous | 0.71 | |
| Precocious vs. metachronous | 0.82 | |
| Synchronous vs. metachronous | 0.33 | |
| KPS ≥ 70 post-BM-treatment | <0.0001 | 0.38, 0.28–0.58, p < 0.0001 |
| BM count | ||
| Single vs. oligo vs. multi | 0.99 | |
| Systemic treatment after BM | <0.0001 | 0.48, 0.39–0.63, p < 0.0001 |
| Radiosurgery only | 0.002 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jünger, S.T.; Schödel, P.; Ruess, D.; Ruge, M.; Brand, J.-S.; Wittersheim, M.; Eich, M.-L.; Schmidt, N.-O.; Goldbrunner, R.; Grau, S.; et al. Timing of Development of Symptomatic Brain Metastases from Non-Small Cell Lung Cancer: Impact on Symptoms, Treatment, and Survival in the Era of Molecular Treatments. Cancers 2020, 12, 3618. https://doi.org/10.3390/cancers12123618
Jünger ST, Schödel P, Ruess D, Ruge M, Brand J-S, Wittersheim M, Eich M-L, Schmidt N-O, Goldbrunner R, Grau S, et al. Timing of Development of Symptomatic Brain Metastases from Non-Small Cell Lung Cancer: Impact on Symptoms, Treatment, and Survival in the Era of Molecular Treatments. Cancers. 2020; 12(12):3618. https://doi.org/10.3390/cancers12123618
Chicago/Turabian StyleJünger, Stephanie T., Petra Schödel, Daniel Ruess, Maximilian Ruge, Julia-Sarita Brand, Maike Wittersheim, Marie-Lisa Eich, Nils-Ole Schmidt, Roland Goldbrunner, Stefan Grau, and et al. 2020. "Timing of Development of Symptomatic Brain Metastases from Non-Small Cell Lung Cancer: Impact on Symptoms, Treatment, and Survival in the Era of Molecular Treatments" Cancers 12, no. 12: 3618. https://doi.org/10.3390/cancers12123618
APA StyleJünger, S. T., Schödel, P., Ruess, D., Ruge, M., Brand, J.-S., Wittersheim, M., Eich, M.-L., Schmidt, N.-O., Goldbrunner, R., Grau, S., & Proescholdt, M. (2020). Timing of Development of Symptomatic Brain Metastases from Non-Small Cell Lung Cancer: Impact on Symptoms, Treatment, and Survival in the Era of Molecular Treatments. Cancers, 12(12), 3618. https://doi.org/10.3390/cancers12123618






