Defining the Inflammatory Plasma Proteome in Pediatric Hodgkin Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
| Protein | Alternative Protein Name(s) | Significance in Study Cohort | Previously Reported in HL | Previously Reported Significance |
|---|---|---|---|---|
| C-C motif chemokine 17 (CCL17) | CC chemokine TARC, Small-inducible cytokine A17, Thymus and activation-regulated chemokine (TARC) | Elevated in HL vs. Controls | Adult HL | Pretreatment levels were associated with clinical risk factors, therapy response [6]. |
| Adult HL | Disease response marker [2]. | |||
| Adult HL | Increased levels correlated with Ann Arbor stage in NS HL; majority showed decreased levels after treatment [18]. | |||
| Adult HL | Accurately reflects disease activity, correlates with treatment response [5]. | |||
| Adult HL | Baseline levels correlate with tumor burden, serial levels correlate with therapy response [19]. | |||
| Adult HL | Change in TARC is not prognostic [20]. | |||
| C-C motif chemokine 22 (CCL22) | Macrophage-derived chemokine (MDC), MDC (1–69), CC chemokine STCP-1, Small-inducible cytokine A22, Stimulated T-cell chemotactic protein 1 | None | Adult HL | Increased levels correlated with Ann Arbor stage in nodular sclerosing HL. Majority showed decreased levels after treatment [18]. |
| Fibroblast growth factor 2 (FGF-2) | Basic fibroblast growth factor (bFGF), Heparin-binding growth factor 2 (HBGF-2) | Elevated in HL vs. Controls | Adult HL | Cases with CD30+ cells carrying an FGF2+/SDC1+ immunophenotype had shortened survival [21]. |
| Interleukin-1 receptor antagonist protein (IL-1RA) | None | None | Adult HL | High levels were independent poor prognosis factors (EFS, OS) [22]. |
| Interleukin-2 (IL-2) | None | None | Pediatric HL | Very high levels were significantly associated with poor outcomes in HL [23]. |
| Interleukin-6 (IL-6) | None | Elevated in HL vs. Controls | Adult HL | High levels were independent poor prognosis factors (OS, EFS) [22]. |
| Adult HL | Associated with increased relapse, poor survival, sCD30 and TARC levels [4]. | |||
| Adult HL | Useful for disease monitoring [24]. | |||
| Interleukin-10 (IL-10) | None | Elevated in HL vs. Controls, associated with risk | Adult HL | Useful for disease monitoring [24]. |
| Adult HL | Associated with early treatment failure [25]. | |||
| Pediatric HL | Increased levels were associated with HL symptoms; pre-treatment levels were higher in non-responders (multiple diagnoses) [26]. | |||
| Interleukin-12 (IL-12) | None | None | Pediatric HL | Pre-treatment IL-12 was lower in non-responders (multiple diagnoses) [26]. |
| Interleukin-13 (IL-13) | None | None | Adult HL | Useful for disease monitoring [24]. |
| Macrophage colony-stimulating factor 1 (M-CSF) | None | None | Adult HL | Increased levels correlate with bulky mediastinal disease, systemic symptoms [27]. |
| Tumor Necrosis Factor Receptor (TNF receptor) | None | None | Adult HL | Associated with lower EFS/OS [22]. |
| Vascular endothelial growth factor (VEGF) | None | Elevated in HL vs. Controls | Pediatric HL | Independent risk factor for treatment failure [10]. |
| Pediatric HL | Changes in levels correlate with treatment response [9]. |
2. Results
2.1. Cohort Characteristics
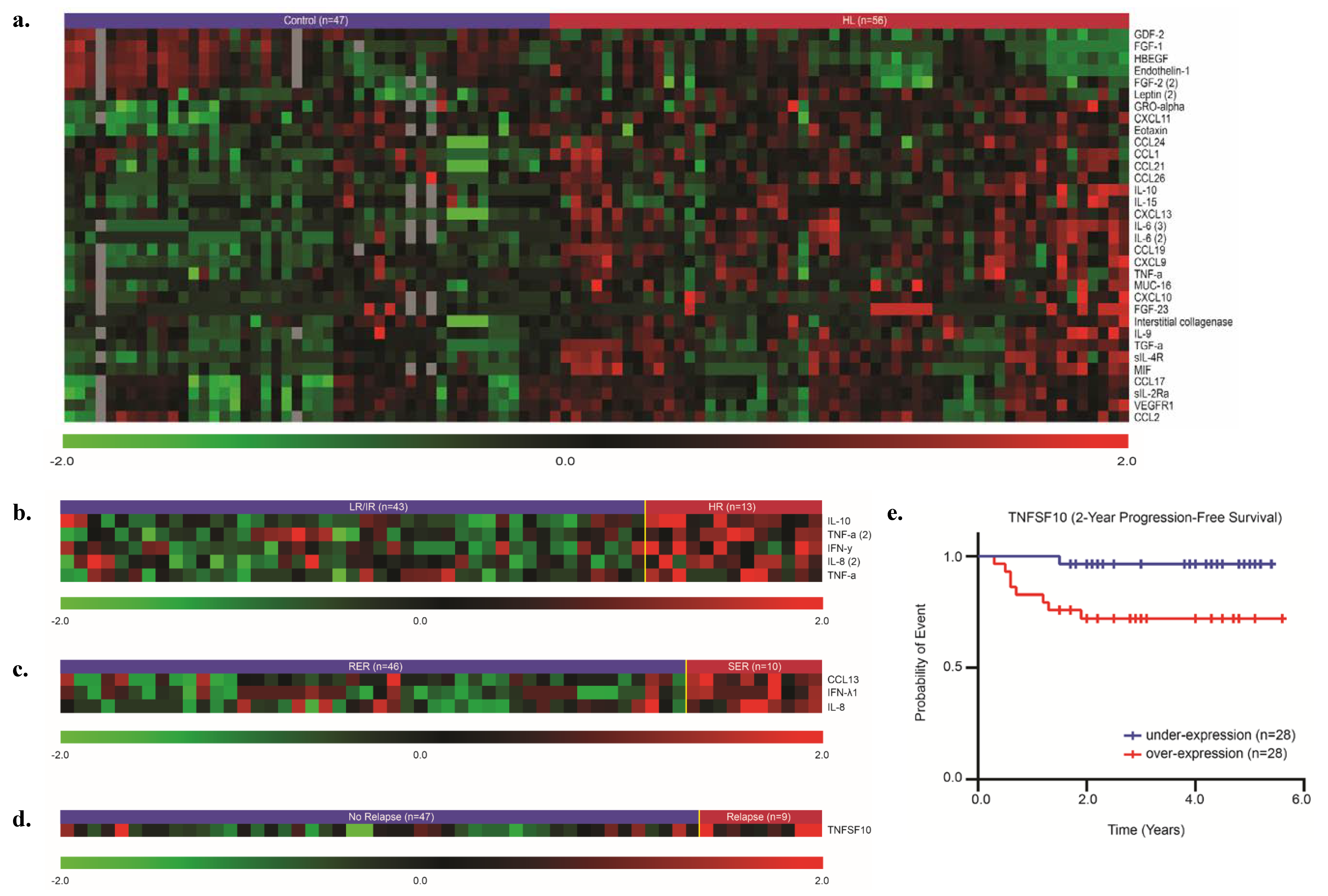
2.2. The Pre-Therapy Plasma Inflammatory Proteome in Subjects with HL Is Distinct from Controls
2.3. An Inflammatory Signature Distinguishes Subjects with HR HL from Those with LR/IR HL
2.4. CCL13, Interferon Lambda-1 (IFN-λ1), and IL-8 Are Elevated in HL Subjects Who Are Slow Early Responders
2.5. Tumor Necrosis Factor Ligand Superfamily Member 10 (TNFSF10) Is Elevated in Subjects with Relapsed HL and Is Predictive of EFS
2.6. Cytokines or Chemokines Are Not Significantly Associated with Specific Demographic or Clinical Features
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Assessment of Plasma Cytokine/Chemokine Profile
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Poppema, S. Immunobiology and pathophysiology of Hodgkin lymphomas. Hematol. Am. Soc. Hematol. Educ. Program 2005, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.; Vari, F.; Keane, C.; Crooks, P.; Nourse, J.P.; Seymour, L.A.; Gottlieb, D.; Ritchie, D.; Gill, D.; Gandhi, M.K. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin. Cancer Res. 2013, 19, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Kamper, P.; Ludvigsen, M.; Bendix, K.; Hamilton-Dutoit, S.; Rabinovich, G.A.; Moller, M.B.; Nyengaard, J.R.; Honoré, B.; d’Amore, F. Proteomic analysis identifies galectin-1 as a predictive biomarker for relapsed/refractory disease in classical Hodgkin lymphoma. Blood 2011, 117, 6638–6649. [Google Scholar] [CrossRef] [Green Version]
- Marri, P.R.; Hodge, L.S.; Maurer, M.J.; Ziesmer, S.C.; Slager, S.L.; Habermann, T.M.; Link, B.K.; Cerhan, J.R.; Novak, A.J.; Ansell, S.M. Prognostic significance of pretreatment serum cytokines in classical Hodgkin lymphoma. Clin. Cancer Res. 2013, 19, 6812–6819. [Google Scholar] [CrossRef] [Green Version]
- Plattel, W.J.; Alsada, Z.N.; van Imhoff, G.W.; Diepstra, A.; van den Berg, A.; Visser, L. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: Comparison of sGalectin-1, sCD163 and sCD30 with TARC. Br. J. Haematol. 2016, 175, 868–875. [Google Scholar] [CrossRef]
- Sauer, M.; Plutschow, A.; Jachimowicz, R.D.; Kleefisch, D.; Reiners, K.S.; Ponader, S.; Engert, A.; von Strandmann, E.P. Baseline serum TARC levels predict therapy outcome in patients with Hodgkin lymphoma. Am. J. Hematol. 2013, 88, 113–115. [Google Scholar] [CrossRef]
- Venkataraman, G.; Mirza, M.K.; Eichenauer, D.A.; Diehl, V. Current status of prognostication in classical Hodgkin lymphoma. Br. J. Haematol. 2014, 165, 287–299. [Google Scholar] [CrossRef]
- Abdelrazik, N.; Fouda, M.; Zaghloul, M.H.; Abbas, D. Serum level of intercellular adhesion molecule-1 in children with malignant lymphoma. Med. Princ. Pract. 2008, 17, 233–238. [Google Scholar] [CrossRef]
- Ben Arush, M.W.; Ben Barak, A.; Maurice, S.; Livne, E. Serum VEGF as a significant marker of treatment response in hodgkin lymphoma. Pediatr. Hematol. Oncol. 2007, 24, 111–115. [Google Scholar] [CrossRef]
- Mizia-Malarz, A.; Sobol, G.; Janowska, J.; Wos, H.; Zahorska-Markiewicz, B. Prognostic value of proangiogenic cytokines in children with lymphomas. Pediatr. Blood Cancer 2009, 53, 1195–1199. [Google Scholar] [CrossRef]
- Tacyildiz, N.; Yavuz, G.; Gozdasoglu, S.; Unal, E.; Ertem, U.; Duru, F.; Ikinciogullari, A.; Babacan, E.; Ensari, A.; Okcuoglu-Cavdar, A. Serum levels and differential expression of intercellular adhesion molecule-1 in childhood leukemia and malignant lymphoma: Prognostic importance and relationship with survival. Pediatr. Hematol. Oncol. 1999, 16, 149–158. [Google Scholar]
- Nagpal, P.; Akl, M.R.; Ayoub, N.M.; Tomiyama, T.; Cousins, T.; Tai, B.; Carroll, N.; Nyrenda, T.; Bhattacharyya, P.; Harris, M.B.; et al. Pediatric Hodgkin lymphoma: Biomarkers, drugs, and clinical trials for translational science and medicine. Oncotarget 2016, 7, 67551–67573. [Google Scholar] [CrossRef] [Green Version]
- Castellino, S.M.; Geiger, A.M.; Mertens, A.C.; Leisenring, W.M.; Tooze, J.A.; Goodman, P.; Stovall, M.; Robison, L.L.; Hudson, M.M. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood 2011, 117, 1806–1816. [Google Scholar] [CrossRef] [Green Version]
- Friedman, D.L.; Chen, L.; Wolden, S.; Buxton, A.; McCarten, K.; FitzGerald, T.J.; Kessel, S.; De Alarcon, P.A.; Chen, A.R.; Kobrinsky, N.; et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: A report from the Children‘s Oncology Group Study AHOD0031. J. Clin. Oncol. 2014, 32, 3651–3658. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Daw, S.; Wynn, R.; Wallace, H. Management of relapsed and refractory classical Hodgkin lymphoma in children and adolescents. Br. J. Haematol. 2011, 152, 249–260. [Google Scholar] [CrossRef]
- Diefenbach, C.S.; Connors, J.M.; Friedberg, J.W.; Leonard, J.P.; Kahl, B.S.; Little, R.F.; Baizer, L.; Evens, A.M.; Hoppe, R.T.; Kelly, K.M.; et al. Hodgkin Lymphoma: Current Status and Clinical Trial Recommendations. J. Natl. Cancer Inst. 2017, 109, 249. [Google Scholar] [CrossRef] [Green Version]
- Niens, M.; Visser, L.; Nolte, I.M.; van der Steege, G.; Diepstra, A.; Cordano, P.; Jarrett, R.F.; Te Meerman, G.J.; Poppema, S.; van den Berg, A. Serum chemokine levels in Hodgkin lymphoma patients: Highly increased levels of CCL17 and CCL22. Br. J. Haematol. 2008, 140, 527–536. [Google Scholar] [CrossRef]
- Plattel, W.J.; van den Berg, A.; Visser, L.; van der Graaf, A.M.; Pruim, J.; Vos, H.; Hepkema, B.; Diepstra, A.; van Imhoff, G.W. Plasma thymus and activation-regulated chemokine as an early response marker in classical Hodgkin‘s lymphoma. Haematologica 2012, 97, 410–415. [Google Scholar] [CrossRef]
- Cuccaro, A.; Annunziata, S.; Cupelli, E.; Martini, M.; Calcagni, M.L.; Rufini, V.; Giachelia, M.; Bartolomei, F.; Galli, E.; D‘Alo, F.; et al. CD68+ cell count, early evaluation with PET and plasma TARC levels predict response in Hodgkin lymphoma. Cancer Med. 2016, 5, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Gharbaran, R.; Goy, A.; Tanaka, T.; Park, J.; Kim, C.; Hasan, N.; Vemulapalli, S.; Sarojini, S.; Tuluc, M.; Nalley, K.; et al. Fibroblast growth factor-2 (FGF2) and syndecan-1 (SDC1) are potential biomarkers for putative circulating CD15+/CD30+ cells in poor outcome Hodgkin lymphoma patients. J. Hematol. Oncol. 2013, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Rautert, R.; Schinkothe, T.; Franklin, J.; Weihrauch, M.; Boll, B.; Pogge, E.; Bredenfeld, H.; Engert, A.; Diehl, V.; Re, D. Elevated pretreatment interleukin-10 serum level is an International Prognostic Score (IPS)-independent risk factor for early treatment failure in advanced stage Hodgkin lymphoma. Leuk. Lymphoma 2008, 49, 2091–2098. [Google Scholar] [CrossRef]
- Pui, C.H.; Hudson, M.; Luo, X.; Wilimas, J.; Evans, W.; Crist, W.M. Serum interleukin-2 receptor levels in Hodgkin disease and other solid tumors of childhood. Leukemia 1993, 7, 1242–1244. [Google Scholar]
- Gaiolla, R.D.; Domingues, M.A.; Niero-Melo, L.; de Oliveira, D.E. Serum levels of interleukins 6, 10, and 13 before and after treatment of classic Hodgkin lymphoma. Arch. Pathol. Lab. Med. 2011, 135, 483–489. [Google Scholar]
- Connors, J.M. Risk assessment in the management of newly diagnosed classical Hodgkin lymphoma. Blood 2015, 125, 1693–1702. [Google Scholar] [CrossRef] [Green Version]
- Bien, E.; Balcerska, A.; Adamkiewicz-Drozynska, E.; Rapala, M.; Krawczyk, M.; Stepinski, J. Pre-treatment serum levels of interleukin-10, interleukin-12 and their ratio predict response to therapy and probability of event-free and overall survival in childhood soft tissue sarcomas, Hodgkin‘s lymphomas and acute lymphoblastic leukemias. Clin. Biochem. 2009, 42, 1144–1157. [Google Scholar] [CrossRef]
- Kowalska, M.; Tajer, J.; Chechlinska, M.; Fuksiewicz, M.; Kotowicz, B.; Kaminska, J.; Walewski, J. Serum macrophage colony-stimulating factor (M-CSF) in patients with Hodgkin lymphoma. Med. Oncol. 2012, 29, 2143–2147. [Google Scholar] [CrossRef]
- Appel, B.E.; Chen, L.; Buxton, A.B.; Hutchison, R.E.; Hodgson, D.C.; Ehrlich, P.F.; Constine, L.S.; Schwartz, C.L. Minimal Treatment of Low-Risk, Pediatric Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the Children‘s Oncology Group. J. Clin. Oncol. 2016, 34, 2372–2379. [Google Scholar] [CrossRef]
- Castellino, S.M.; Parsons, S.K.; Pei, Q.; McCarten, K.; Kessel, S.; Punnet, A.; Horton, T.M.; Henderson, T.O.; Hodgson, D.; Dave, H.; et al. A randomized Phase III trial of Brentuximab vedotin (Bv) for de novo High-Risk Classical Hodgkin Lymphoma (cHL) in children and adolescents-Study Design and Incorporation of secondary endoints in Children’s Oncology Gorup (COG) AHOD1331. Klin. Padiatr. 2020, 232, 82–83. [Google Scholar]
- Kelly, K.M.; Cole, P.D.; Pei, Q.; Bush, R.; Roberts, K.B.; Hodgson, D.C.; McCarten, K.M.; Cho, S.Y.; Schwartz, C. Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): A report from the Children’s Oncology Group. Br. J. Haematol. 2019, 187, 39–48. [Google Scholar] [CrossRef]
- Skinnider, B.F.; Mak, T.W. The role of cytokines in classical Hodgkin lymphoma. Blood 2002, 99, 4283–4297. [Google Scholar] [CrossRef] [Green Version]
- Axdorph, U.; Sjoberg, J.; Grimfors, G.; Landgren, O.; Porwit-MacDonald, A.; Bjorkholm, M. Biological markers may add to prediction of outcome achieved by the International Prognostic Score in Hodgkin’s disease. Ann. Oncol. 2000, 11, 1405–1411. [Google Scholar] [CrossRef]
- Casasnovas, R.O.; Mounier, N.; Brice, P.; Divine, M.; Morschhauser, F.; Gabarre, J.; Blay, J.Y.; Voillat, L.; Lederlin, P.; Stamatoullas, A.; et al. Plasma cytokine and soluble receptor signature predicts outcome of patients with classical Hodgkin’s lymphoma: A study from the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 2007, 25, 1732–1740. [Google Scholar] [CrossRef]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef]
- Foss, H.D.; Herbst, H.; Gottstein, S.; Demel, G.; Araujo, I.; Stein, H. Interleukin-8 in Hodgkin’s disease. Preferential expression by reactive cells and association with neutrophil density. Am. J. Pathol. 1996, 148, 1229–1236. [Google Scholar]
- Agur, A.; Amir, G.; Paltiel, O.; Klein, M.; Dann, E.J.; Goldschmidt, H.; Goldschmidt, N. CD68 staining correlates with the size of residual mass but not with survival in classical Hodgkin lymphoma. Leuk. Lymphoma 2015, 56, 1315–1319. [Google Scholar] [CrossRef]
- Greaves, P.; Clear, A.; Coutinho, R.; Wilson, A.; Matthews, J.; Owen, A.; Shanyinde, M.; Lister, T.A.; Calaminici, M.; Gribben, J.G. Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J. Clin. Oncol. 2013, 31, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Kayal, S.; Mathur, S.; Karak, A.K.; Kumar, L.; Sharma, A.; Bakhshi, S.; Raina, V. CD68 tumor-associated macrophage marker is not prognostic of clinical outcome in classical Hodgkin lymphoma. Leuk. Lymphoma 2014, 55, 1031–1037. [Google Scholar] [CrossRef]
- Steidl, C.; Farinha, P.; Gascoyne, R.D. Macrophages predict treatment outcome in Hodgkin’s lymphoma. Haematologica 2011, 96, 186–189. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Gerdes, J.; Kretschmer, C.; Zahn, G.; Ernst, M.; Jones, D.B.; Flad, H.D. Immunoenzymatic assessment of interferon-gamma in Hodgkin and Sternberg-Reed cells. Cytokine 1990, 2, 307–310. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.P.; Levidou, G.; Milionis, V.; Hartmann, S.; Lakiotaki, E.; Sepsa, A.; Thymara, I.; Ntailiani, P.; Spanou, K.; Angelopoulou, M.K.; et al. Thioredoxin-1, chemokine (C-X-C motif) ligand-9 and interferon-γ expression in the neoplastic cells and macrophages of Hodgkin lymphoma: Clinicopathologic correlations and potential prognostic implications. Leuk. Lymphoma 2017, 58, 2227–2239. [Google Scholar] [CrossRef]
- Karachaliou, N.; Gonzalez-Cao, M.; Crespo, G.; Drozdowskyj, A.; Aldeguer, E.; Gimenez-Capitan, A.; Teixido, C.; Molina-Vila, M.A.; Viteri, S.; de Los Llanos Gil, M.; et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther. Adv. Med. Oncol. 2018, 10, 1758834017749748. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Feng, X.; Guo, D.; Tan, W.; Zhang, M. Interleukin-29 modulates proinflammatory cytokine production in synovial inflammation of rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R228. [Google Scholar] [CrossRef] [Green Version]
- Blanpain, C.; Migeotte, I.; Lee, B.; Vakili, J.; Doranz, B.J.; Govaerts, C.; Vassart, G.; Doms, R.W.; Parmentier, M. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood 1999, 94, 1899–1905. [Google Scholar] [CrossRef]
- Maggio, E.; van den Berg, A.; Diepstra, A.; Kluiver, J.; Visser, L.; Poppema, S. Chemokines, cytokines and their receptors in Hodgkin’s lymphoma cell lines and tissues. Ann. Oncol. 2002, 13 (Suppl. S1), 52–56. [Google Scholar] [CrossRef]
- Zhang, L.; Blackwell, K.; Workman, L.M.; Chen, S.; Pope, M.R.; Janz, S.; Habelhah, H. RIP1 Cleavage in the Kinase Domain Regulates TRAIL-Induced NF-kappaB Activation and Lymphoma Survival. Mol. Cell. Biol. 2015, 35, 3324–3338. [Google Scholar] [CrossRef] [Green Version]
- Hinz, M.; Loser, P.; Mathas, S.; Krappmann, D.; Dorken, B.; Scheidereit, C. Constitutive NF-kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells. Blood 2001, 97, 2798–2807. [Google Scholar] [CrossRef]
- Kahn, J.M.; Kelly, K.M.; Pei, Q.; Bush, R.; Friedman, D.L.; Keller, F.G.; Bhatia, S.; Henderson, T.O.; Schwartz, C.L.; Castellino, S.M. Survival by Race and Ethnicity in Pediatric and Adolescent Patients With Hodgkin Lymphoma: A Children’s Oncology Group Study. J. Clin. Oncol. 2019, 37, 3009–3017. [Google Scholar] [CrossRef]
| Variation | Value |
|---|---|
| Sex, n (%) | |
| Male | 32 (57) |
| Female | 24 (43) |
| Age, mean (range) | 13 years (3–18 years) |
| Ethnicity, n (%) | |
| non-Hispanic white | 21 (38) |
| non-Hispanic black | 9 (16) |
| Hispanic | 24 (43) |
| non-Hispanic Asian | 2 (3) |
| HL Subtype, n (%) | |
| Nodular Sclerosing | 35 (63) |
| Mixed Cellularity | 12 (21) |
| Lymphocyte Rich | 1 (2) |
| Classical HL NOS | 5 (9) |
| Nodular Lymphocyte Predominant | 3 (5) |
| EBV positive, n (%) | |
| Yes | 18 (32) |
| No | 37 (66) |
| Unknown | 1 (2) |
| Stage, n (%) | |
| I | 4 (7) |
| II | 26 (46) |
| III | 10 (18) |
| IV | 16 (29) |
| Risk Category, n (%) | |
| LR/IR | 43 (77) |
| HR | 13 (23) |
| HL Therapy Protocol, n (%) | |
| TXCH-HD-12A | 41 (73) |
| AHOD03P1 | 2 (4) |
| AHOD0831 | 12 (21) |
| AHOD1331 | 1 (2) |
| Therapy Response, n (%) | |
| RER | 46 (82) |
| SER | 10 (18) |
| Event-Free Survival, n (%) | |
| Relapse | 9 (16) |
| No Relapse | 47 (84) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrusa, J.E.; Scull, B.P.; Abhyankar, H.A.; Lin, H.; Ozuah, N.W.; Chakraborty, R.; Eckstein, O.S.; Gulati, N.; Fattah, E.A.; El-Mallawany, N.K.; et al. Defining the Inflammatory Plasma Proteome in Pediatric Hodgkin Lymphoma. Cancers 2020, 12, 3603. https://doi.org/10.3390/cancers12123603
Agrusa JE, Scull BP, Abhyankar HA, Lin H, Ozuah NW, Chakraborty R, Eckstein OS, Gulati N, Fattah EA, El-Mallawany NK, et al. Defining the Inflammatory Plasma Proteome in Pediatric Hodgkin Lymphoma. Cancers. 2020; 12(12):3603. https://doi.org/10.3390/cancers12123603
Chicago/Turabian StyleAgrusa, Jennifer E., Brooks P. Scull, Harshal A. Abhyankar, Howard Lin, Nmazuo W. Ozuah, Rikhia Chakraborty, Olive S. Eckstein, Nitya Gulati, Elmoataz Abdel Fattah, Nader K. El-Mallawany, and et al. 2020. "Defining the Inflammatory Plasma Proteome in Pediatric Hodgkin Lymphoma" Cancers 12, no. 12: 3603. https://doi.org/10.3390/cancers12123603
APA StyleAgrusa, J. E., Scull, B. P., Abhyankar, H. A., Lin, H., Ozuah, N. W., Chakraborty, R., Eckstein, O. S., Gulati, N., Fattah, E. A., El-Mallawany, N. K., Rouce, R. H., Dreyer, Z. E., Brackett, J., Margolin, J. F., Lubega, J., Horton, T. M., Bollard, C. M., Gramatges, M. M., Kamdar, K. Y., ... Allen, C. E. (2020). Defining the Inflammatory Plasma Proteome in Pediatric Hodgkin Lymphoma. Cancers, 12(12), 3603. https://doi.org/10.3390/cancers12123603





