Immune Cell Infiltrate and Prognosis in Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Tissue Microarray
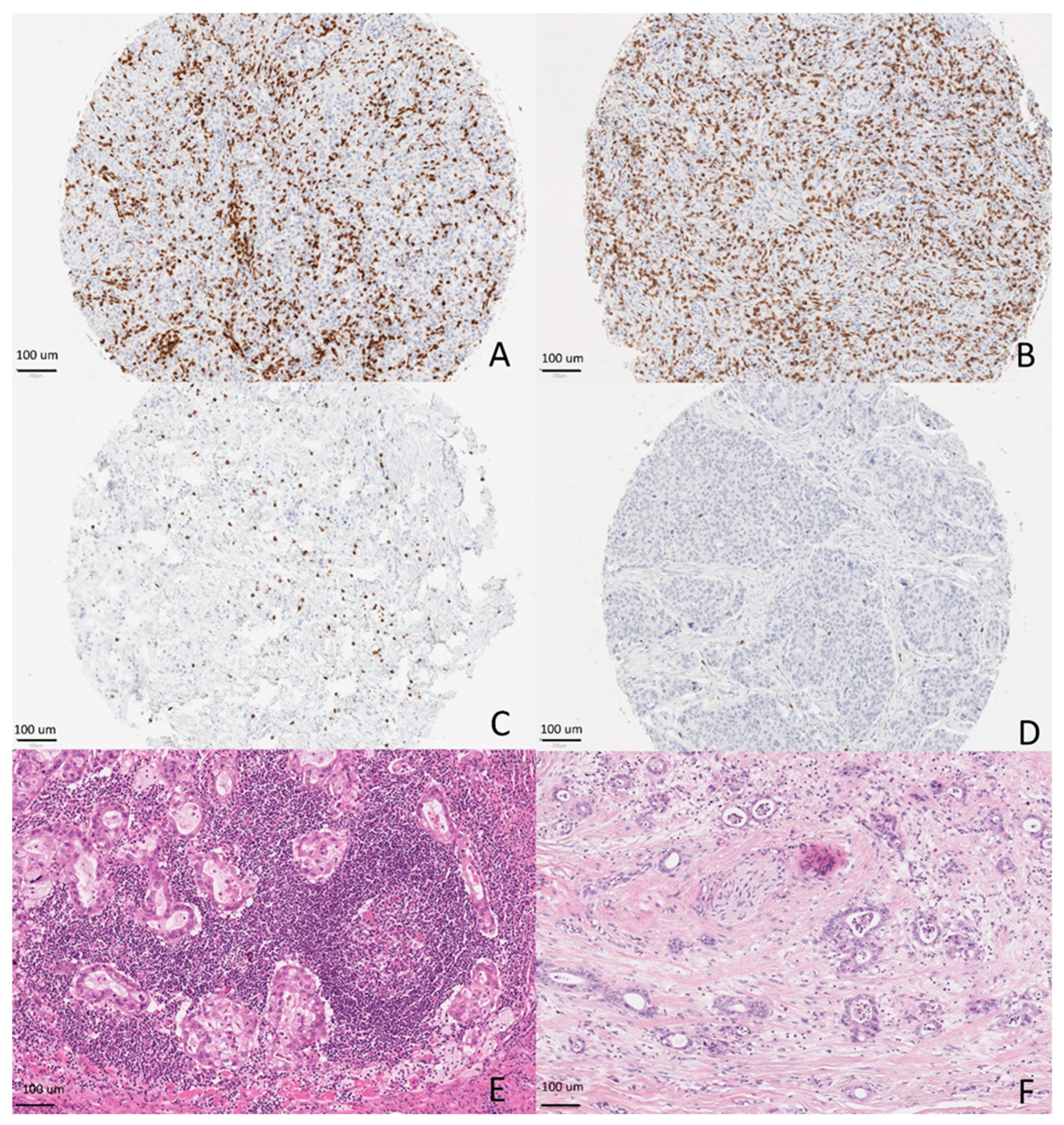
2.4. Immunohistochemical Stainings
2.5. Immune Cell Score
2.6. KM Grade
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Patients
3.2. Assessment of ICS
3.3. ICS and Survival
3.4. Assessment of Klintrup–Mäkinen Grade
3.5. Klintrup–Mäkinen Grade and Survival
3.6. Comparison of ICS and Klintrup–Mäkinen Grade
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Sano, T.; Coit, D.G.; Kim, H.H.; Roviello, F.; Kassab, P.; Wittekind, C.; Yamamoto, Y.; Ohashi, Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 2017, 20, 217–225. [Google Scholar] [CrossRef]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Rugge, M.; Carneiro, F.; Fukayama, M.; Yasu, W.; Grabsch, H. Gastric adenocarcinoma. In WHO Classification of Tumors: Digestive System Tumours, 5th ed.; Arends, M., Fukayama, M., Klimstra, D., Lam, A., Nagtegaal, I., Odze, R., Paradis, V., Wittekind, C., Eds.; IARC Press: Lyon, France, 2019. [Google Scholar]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202. [Google Scholar] [CrossRef] [Green Version]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, Q.; Hu, Y.; Li, T.; Yu, J.; Zhao, L.; Ye, G.; Deng, H.; Mou, T.; Cai, S.; et al. ImmunoScore Signature: A Prognostic and Predictive Tool in Gastric Cancer. Ann. Surg. 2018, 267, 504–513. [Google Scholar] [CrossRef]
- Klintrup, K.; Makinen, J.M.; Kauppila, S.; Vare, P.O.; Melkko, J.; Tuominen, H.; Tuppurainen, K.; Makela, J.; Karttunen, T.J.; Makinen, M.J. Inflammation and prognosis in colorectal cancer. Eur. J. Cancer (Oxf. Engl. 1990) 2005, 41, 2645–2654. [Google Scholar] [CrossRef]
- Park, J.H.; McMillan, D.C.; Powell, A.G.; Richards, C.H.; Horgan, P.G.; Edwards, J.; Roxburgh, C.S. Evaluation of a tumor microenvironment-based prognostic score in primary operable colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 882–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.H.; Ji, C.D.; Zhu, J.; Xiao, H.L.; Zhao, H.B.; Cui, Y.H.; Bian, X.W. The prognostic value and pathobiological significance of Glasgow microenvironment score in gastric cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Chae, Y.S.; Kim, C.H.; Lee, Y.; Lee, J.H.; Kim, J.Y. Tumor microenvironmental factors have prognostic significances in advanced gastric cancer. Apmis 2018, 126, 814–821. [Google Scholar] [CrossRef]
- Kemi, N.; Eskuri, M.; Herva, A.; Leppanen, J.; Huhta, H.; Helminen, O.; Saarnio, J.; Karttunen, T.J.; Kauppila, J.H. Tumour-stroma ratio and prognosis in gastric adenocarcinoma. Br. J. Cancer 2018, 119, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Kemi, N.; Eskuri, M.; Ikalainen, J.; Karttunen, T.J.; Kauppila, J.H. Tumor Budding and Prognosis in Gastric Adenocarcinoma. Am. J. Surg. Pathol. 2019, 43, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Vayrynen, J.P.; Vornanen, J.O.; Sajanti, S.; Bohm, J.P.; Tuomisto, A.; Makinen, M.J. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch. Int. J. Pathol. 2012, 460, 455–465. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Gancberg, D.; Di Leo, A.; Rouas, G.; Jarvinen, T.; Verhest, A.; Isola, J.; Piccart, M.J.; Larsimont, D. Reliability of the tissue microarray based FISH for evaluation of the HER-2 oncogene in breast carcinoma. J. Clin. Pathol. 2002, 55, 315–317. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.C.; Robinson, R.A.; Trask, D.K. Validation of tissue microarrays using p53 immunohistochemical studies of squamous cell carcinoma of the larynx. Mod. Pathol. 2003, 16, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Rosen, D.G.; Huang, X.; Deavers, M.T.; Malpica, A.; Silva, E.G.; Liu, J. Validation of tissue microarray technology in ovarian carcinoma. Mod. Pathol. 2004, 17, 790–797. [Google Scholar] [CrossRef]
- Kim, K.J.; Yang, H.K.; Kim, W.H.; Kang, G.H. Combined prognostic effect of PD-L1 expression and immunoscore in microsatellite-unstable advanced gastric cancers. Oncotarget 2017, 8, 58887–58902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, K.; Guo, Q.; Cheng, J.; Shen, L.; Cao, Y.; Wu, J.; Shi, J.; Cao, H.; Liu, B.; et al. Tumor-infiltrating immune cells and prognosis in gastric cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 62312–62329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Zhang, D.; Liu, H.; Chen, T.; Xie, J.; Peng, L.; Zheng, X.; Xu, B.; Li, Q.; Jiang, J. The High Level of Tertiary Lymphoid Structure Is Correlated with Superior Survival in Patients with Advanced Gastric Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]


| Clinicopathological Variable | Low Immune Cell Score (n = 119) | Intermediate Immune Cell Score (n = 384) | High Immune Cell Score (n = 155) | p-Value |
|---|---|---|---|---|
| Year of surgery | 0.11 | |||
| ≥2000 | 55 (46.2%) | 218 (56.8.2%) | 88 (56.8%) | |
| <2000 | 64 (53.8%) | 166 (43.2%) | 67 (43.2%) | |
| Mean age at diagnosis | 68.7 | 66.5 | 67.7 | 0.14 |
| Sex | 0.67 | |||
| Man | 69 (58.0%) | 226 (58.9%) | 97 (62.6%) | |
| Woman | 50 (42.0%) | 158 (41.1%) | 58 (37.4%) | |
| Neoadjuvant chemotherapy | 0.93 | |||
| Yes | 4 (3.4%) | 16 (4.2%) | 7 (4.5%) | |
| No | 115 (96.6%) | 368 (95.8%) | 148 (95.5%) | |
| Tumor stage | 0.048 | |||
| 1 or 2 | 64 (53.8%) | 238 (62.0%) | 106 (68.4%) | |
| 3 or 4 | 55 (46.2%) | 146 (38.0%) | 49 (31.6%) | |
| Lauren class | 0.001 | |||
| Intestinal | 59 (49.6%) | 170 (44.3%) | 100 (64.5%) | |
| Diffuse | 56 (47.1%) | 200 (52.1%) | 52 (33.5%) | |
| Mixed | 4 (3.4%) | 14 (3.6%) | 3 (1.9%) | |
| Histological grade in intestinal type | 0.004 | |||
| I or II | 42 (71.2%) | 116 (68.2%) | 50 (50.0%) | |
| III | 17 (28.8%) | 54 (31.8%) | 50 (50.0%) | |
| Radicality of resection | 0.002 | |||
| R0 | 82 (68.9%) | 300 (78.1%) | 134 (86.5%) | |
| R1 or R2 | 37 (31.1%) | 84 (21.9%) | 21 (13.5%) |
| Survival Model | Number of Patients | Low Immune Cell Score HR (95% CI) | Intermediate Immune Cell Score HR (95% CI) | High Immune Cell Score HR (95% CI) |
|---|---|---|---|---|
| 5-year survival | ||||
| All patients (Crude) | 658 | 1.00 (Reference) | 0.74 (0.58–0.94) | 0.59 (0.44–0.79) |
| All patients (Model 1) a | 658 | 1.00 (Reference) | 0.86 (0.67–1.09) | 0.70 (0.52–0.94) |
| All patients (Model 2) b | 658 | 1.00 (Reference) | 0.90 (0.70–1.15) | 0.87 (0.63–1.20) |
| Subgroup analysis | ||||
| Intestinal-type (Crude) | 329 | 1.00 (Reference) | 0.81 (0.57–1.14) | 0.53 (0.36–0.79) |
| Intestinal-type (Model 1) c | 329 | 1.00 (Reference) | 0.80 (0.56–1.14) | 0.54 (0.36–0.81) |
| Intestinal-type (Model 2) d | 329 | 1.00 (Reference) | 0.85 (0.60–1.22) | 0.68 (0.43–1.07) |
| Diffuse-type (Crude) | 308 | 1.00 (Reference) | 0.74 (0.53–1.04) | 0.74 (0.47–1.17) |
| Diffuse-type (Model 1) e | 308 | 1.00 (Reference) | 0.95 (0.67–1.35) | 0.92 (0.58–1.46) |
| Diffuse-type (Model 2) f | 308 | 1.00 (Reference) | 0.97 (0.68–1.39) | 1.05 (0.66–1.69) |
| Overall survival | ||||
| All patients (Crude) | 658 | 1.00 (Reference) | 0.71 (0.57–0.89) | 0.65 (0.50–0.84) |
| All patients (Model 1) a | 658 | 1.00 (Reference) | 0.80 (0.64–1.00) | 0.71 (0.55–0.93) |
| All patients (Model 2) b | 658 | 1.00 (Reference) | 0.84 (0.67–1.05) | 0.85 (0.64–1.14) |
| Subgroup analysis | ||||
| Intestinal-type (Crude) | 329 | 1.00 (Reference) | 0.78 (0.57–1.07) | 0.64 (0.45–0.91) |
| Intestinal-type (Model 1) c | 329 | 1.00 (Reference) | 0.81 (0.59–1.12) | 0.64 (0.45–0.92) |
| Intestinal-type (Model 2) d | 329 | 1.00 (Reference) | 0.86 (0.62–1.19) | 0.78 (0.52–1.16) |
| Diffuse-type (Crude) | 308 | 1.00 (Reference) | 0.69 (0.50–0.95) | 0.70 (0.46–1.06) |
| Diffuse-type (Model 1) e | 308 | 1.00 (Reference) | 0.85 (0.61–1.18) | 0.82 (0.53–1.25) |
| Diffuse-type (Model 2) f | 308 | 1.00 (Reference) | 0.87 (0.63–1.21) | 0.93 (0.60–1.44) |
| Clinicopathological Variable | Low KM Grade (n = 599) | High KM Grade (n = 142) | p-Value |
|---|---|---|---|
| Year of surgery | 0.26 | ||
| ≥2000 | 331 (55.3%) | 71 (50.0%) | |
| <2000 | 268 (44.7%) | 71 (50.0%) | |
| Mean age at diagnosis | 66.4 | 69.3 | 0.009 |
| Sex | |||
| Man | 359 (59.9%) | 80 (56.3%) | 0.45 |
| Woman | 240 (40.1%) | 62 (43.7%) | |
| Neoadjuvant chemotherapy | 0.28 | ||
| Yes | 32 (5.3%) | 4 (2.8%) | |
| No | 567 (96.6%) | 138 (97.2%) | |
| Tumour stage | <0.001 | ||
| 1 or 2 | 349 (58.3%) | 116 (81.7%) | |
| 3 or 4 | 250 (41.7%) | 26 (18.3%) | |
| Lauren class | <0.001 | ||
| Intestinal | 271 (45.2%) | 91 (64.1%) | |
| Diffuse | 309 (51.6%) | 46 (32.4%) | |
| Mixed | 19 (3.2%) | 5 (3.5%) | |
| Histological grade in intestinal type | 0.08 | ||
| I or II | 180 (66.4%) | 51 (56.0%) | |
| III | 91 (33.6%) | 40 (44.0%) | |
| Radicality of resection | <0.001 | ||
| R0 | 450 (75.1%) | 129 (90.8%) | |
| R1 or R2 | 149 (24.9%) | 13 (9.2%) |
| Survival Model | Number of Patients | Low KM Grade HR (95% CI) | High KM Grade HR (95% CI) |
|---|---|---|---|
| 5-year survival | |||
| All patients (Crude) | 741 | 1.00 (Reference) | 0.51 (0.39–0.66) |
| All patients (Model 1) a | 741 | 1.00 (Reference) | 0.59 (0.45–0.77) |
| All patients (Model 2) b | 658 | 1.00 (Reference) | 0.60 (0.44–0.82) |
| Subgroup analysis | |||
| Intestinal type (Crude) | 362 | 1.00 (Reference) | 0.59 (0.43–0.81) |
| Intestinal type (Model 1) c | 362 | 1.00 (Reference) | 0.61 (0.44–0.85) |
| Intestinal type (Model 2) d | 329 | 1.00 (Reference) | 0.66 (0.45–0.97) |
| Diffuse type (Crude) | 355 | 1.00 (Reference) | 0.40 (0.25–0.67) |
| Diffuse type (Model 1) e | 355 | 1.00 (Reference) | 0.52 (0.31–0.86) |
| Diffuse type (Model 2) f | 308 | 1.00 (Reference) | 0.57 (0.33–1.00) |
| Overall survival | |||
| All patients (Crude) | 741 | 1.00 (Reference) | 0.60 (0.49–0.75) |
| All patients (Model 1) a | 741 | 1.00 (Reference) | 0.66 (0.53–0.82) |
| All patients (Model 2) b | 658 | 1.00 (Reference) | 0.69 (0.53–0.89) |
| Subgroup analysis | |||
| Intestinal type (Crude) | 362 | 1.00 (Reference) | 0.70 (0.54–0.91) |
| Intestinal type (Model 1) c | 362 | 1.00 (Reference) | 0.69 (0.53–0.91) |
| Intestinal type (Model 2) d | 329 | 1.00 (Reference) | 0.72 (0.52–1.00) |
| Diffuse type (Crude) | 355 | 1.00 (Reference) | 0.47 (0.32–0.70) |
| Diffuse type (Model 1) e | 355 | 1.00 (Reference) | 0.56 (0.38–0.85) |
| Diffuse type (Model 2) f | 308 | 1.00 (Reference) | 0.61 (0.39–0.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemi, N.; Hiltunen, N.; P. Väyrynen, J.; Pohjanen, V.-M.; Helminen, O.; Junttila, A.; Mrena, J.; Böhm, J.; Huhta, H.; Leppänen, J.; et al. Immune Cell Infiltrate and Prognosis in Gastric Cancer. Cancers 2020, 12, 3604. https://doi.org/10.3390/cancers12123604
Kemi N, Hiltunen N, P. Väyrynen J, Pohjanen V-M, Helminen O, Junttila A, Mrena J, Böhm J, Huhta H, Leppänen J, et al. Immune Cell Infiltrate and Prognosis in Gastric Cancer. Cancers. 2020; 12(12):3604. https://doi.org/10.3390/cancers12123604
Chicago/Turabian StyleKemi, Niko, Niko Hiltunen, Juha P. Väyrynen, Vesa-Matti Pohjanen, Olli Helminen, Anna Junttila, Johanna Mrena, Jan Böhm, Heikki Huhta, Joni Leppänen, and et al. 2020. "Immune Cell Infiltrate and Prognosis in Gastric Cancer" Cancers 12, no. 12: 3604. https://doi.org/10.3390/cancers12123604
APA StyleKemi, N., Hiltunen, N., P. Väyrynen, J., Pohjanen, V.-M., Helminen, O., Junttila, A., Mrena, J., Böhm, J., Huhta, H., Leppänen, J., J. Karttunen, T., & H. Kauppila, J. (2020). Immune Cell Infiltrate and Prognosis in Gastric Cancer. Cancers, 12(12), 3604. https://doi.org/10.3390/cancers12123604





