Computed Tomography Radiomics for Residual Positron Emission Tomography-Computed Tomography Uptake in Lymph Nodes after Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
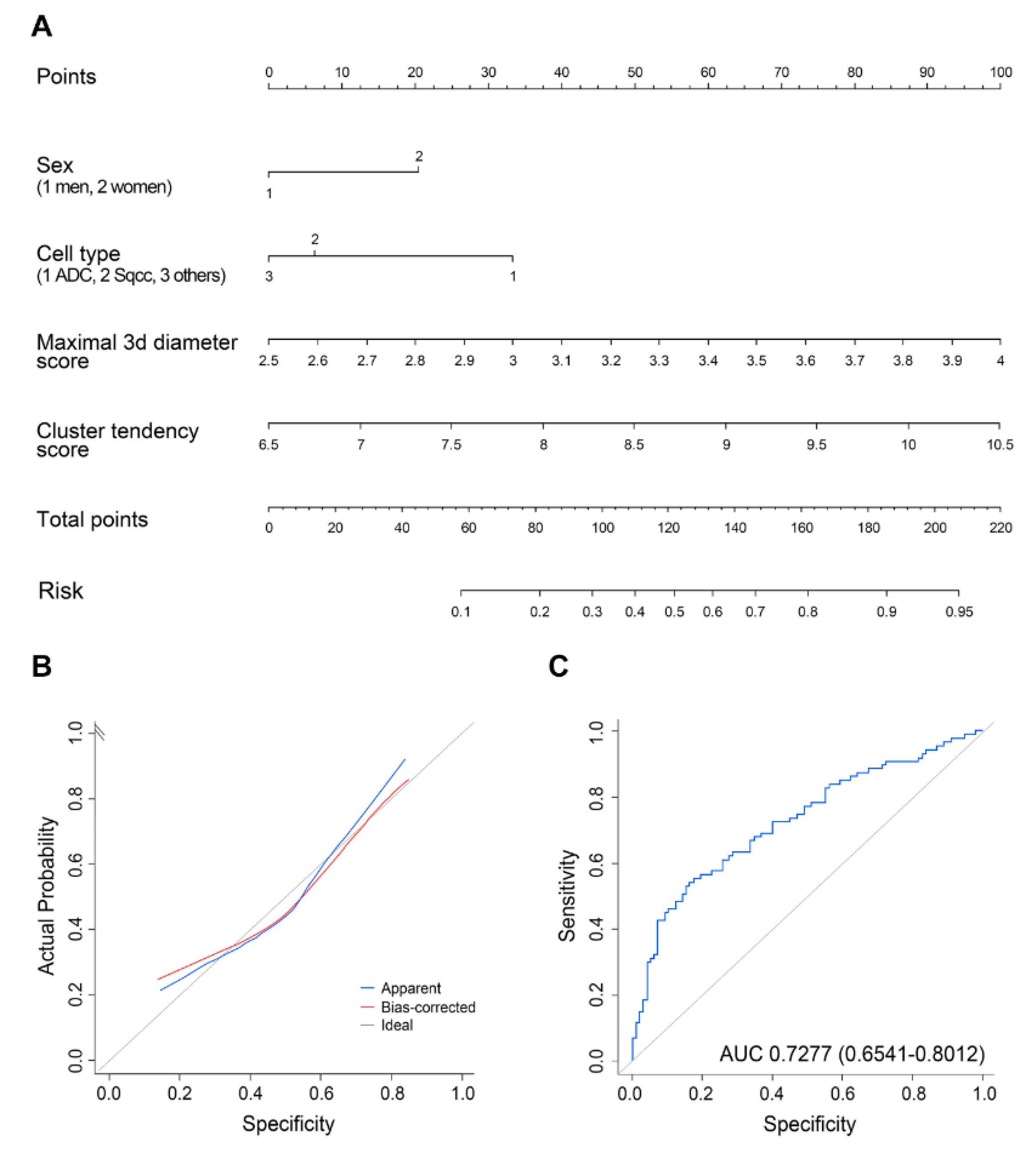
2.2. Analyses of Clinical/Radiomics Variables for Predicting Malignant LNs
3. Discussion
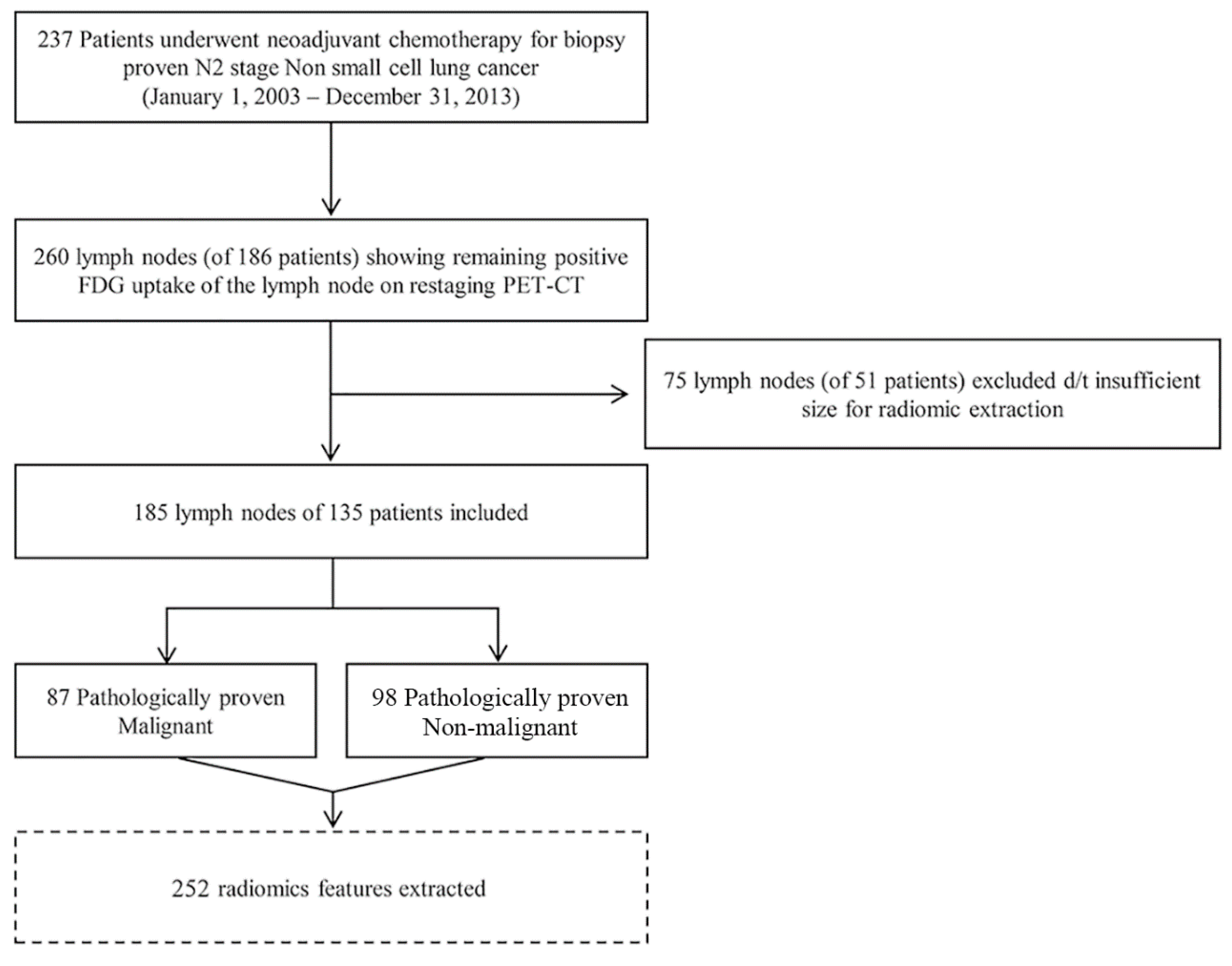
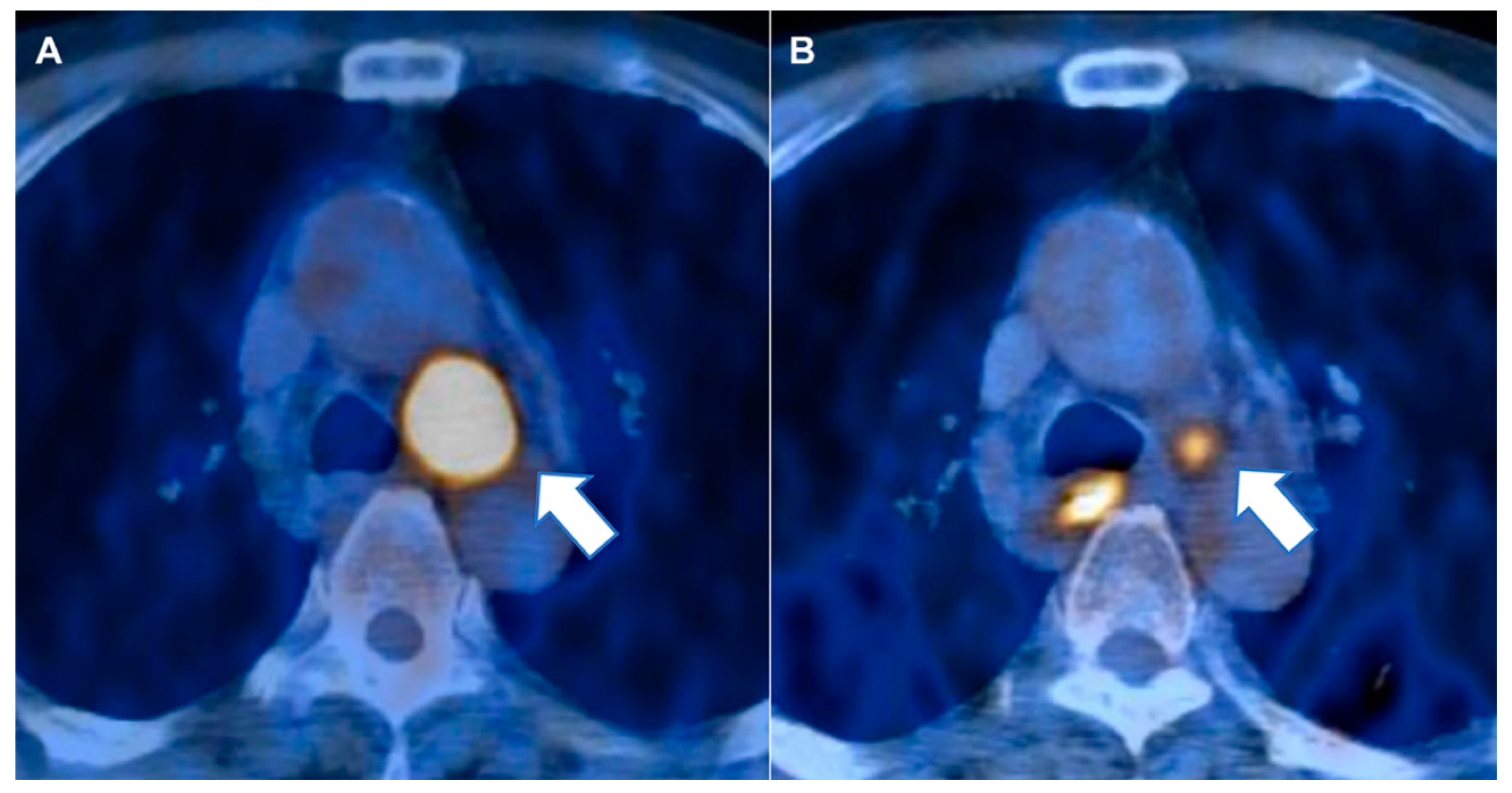
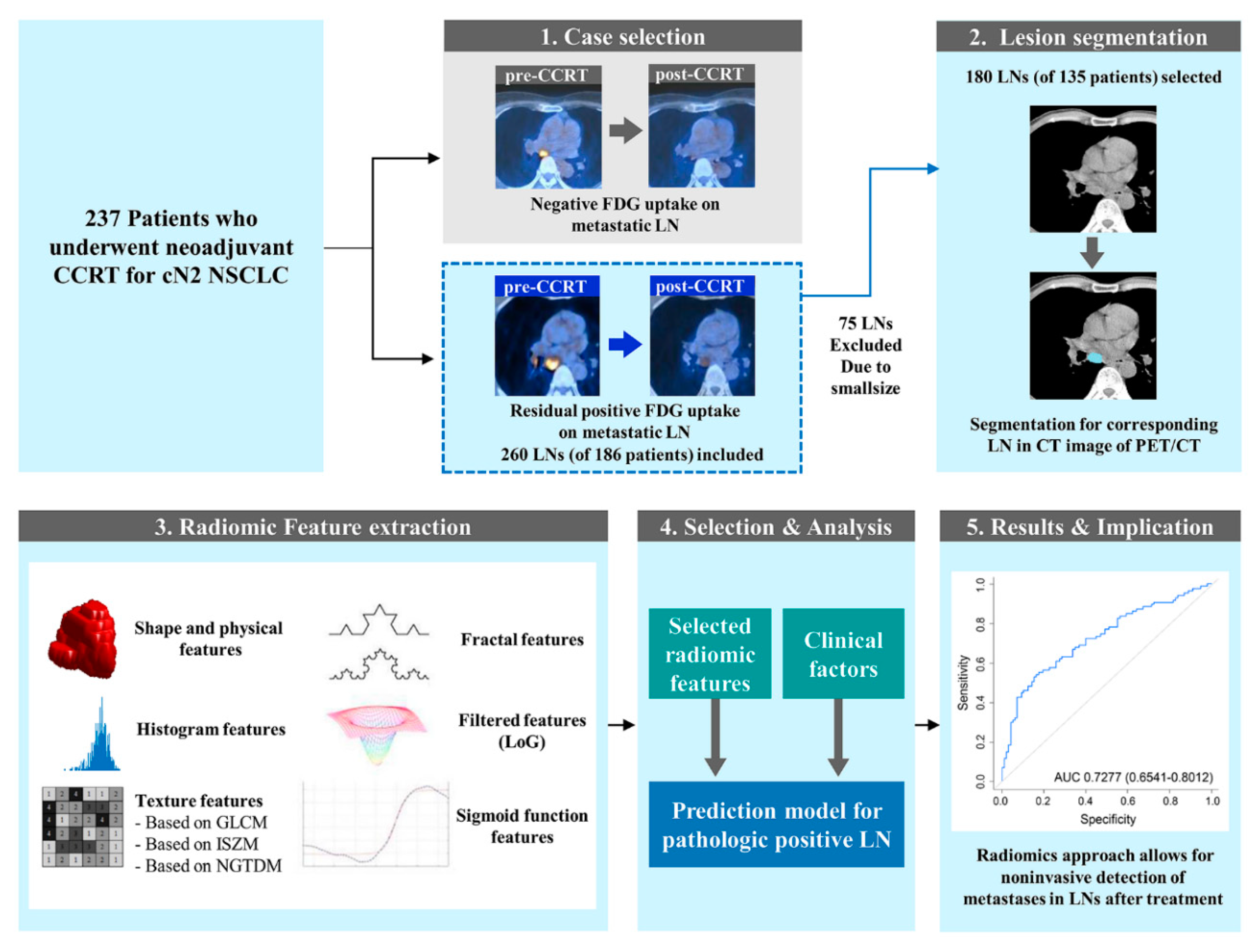
4. Materials and Methods
4.1. Patients
4.2. Image Analysis and Region of Interest Segmentation
4.3. CT Radiomics Feature Extraction
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability
Appendix A
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, M.; Hatabu, H.; Johnson, B.E.; McLoud, T.C. State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology 2014, 271, 6–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2009, 50 (Suppl. 1), 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- de Cabanyes Candela, S.; Detterbeck, F.C. A systematic review of restaging after induction therapy for stage IIIa lung cancer: Prediction of pathologic stage. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Bak, S.H.; Lee, H.Y. CT Radiomics in Thoracic Oncology: Technique and Clinical Applications. Nucl. Med. Mol. Imaging 2018, 52, 91–98. [Google Scholar] [CrossRef]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef]
- Chong, Y.; Kim, J.H.; Lee, H.Y.; Ahn, Y.C.; Lee, K.S.; Ahn, M.J.; Kim, J.; Shim, Y.M.; Han, J.; Choi, Y.L. Quantitative CT variables enabling response prediction in neoadjuvant therapy with EGFR-TKIs: Are they different from those in neoadjuvant concurrent chemoradiotherapy? PLoS ONE 2014, 9, e88598. [Google Scholar] [CrossRef]
- Son, J.Y.; Lee, H.Y.; Kim, J.H.; Han, J.; Jeong, J.Y.; Lee, K.S.; Kwon, O.J.; Shim, Y.M. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: The added value of using iodine mapping. Eur. Radiol. 2016, 26, 43–54. [Google Scholar] [CrossRef]
- Coroller, T.P.; Agrawal, V.; Narayan, V.; Hou, Y.; Grossmann, P.; Lee, S.W.; Mak, R.H.; Aerts, H.J. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2016, 119, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, G.J.; O′Brien, M.E.; Siddique, M.; Chicklore, S.; Loi, H.Y.; Sharma, B.; Punwani, R.; Bassett, P.; Goh, V.; Chua, S. Non-Small Cell Lung Cancer Treated with Erlotinib: Heterogeneity of (18)F-FDG Uptake at PET-Association with Treatment Response and Prognosis. Radiology 2015, 276, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Grossmann, P.; Tan, Y.; Oxnard, G.R.; Rizvi, N.; Schwartz, L.H.; Zhao, B. Defining a Radiomic Response Phenotype: A Pilot Study using targeted therapy in NSCLC. Sci. Rep. 2016, 6, 33860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, G.A.; Tanoue, L.T.; Margolis, M.L.; Barker, J.; Detterbeck, F.; American College of Chest, P. The noninvasive staging of non-small cell lung cancer: The guidelines. Chest 2003, 123, 147S–156S. [Google Scholar] [CrossRef] [Green Version]
- Kramer, H.; Groen, H.J. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann. Surg. 2003, 238, 180–188. [Google Scholar] [CrossRef]
- Mateu-Navarro, M.; Rami-Porta, R.; Bastus-Piulats, R.; Cirera-Nogueras, L.; Gonzalez-Pont, G. Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann. Thorac. Surg. 2000, 70, 391–395. [Google Scholar] [CrossRef]
- Daneshmand, S.; Ahmadi, H.; Huynh, L.N.; Dobos, N. Preoperative staging of invasive bladder cancer with dynamic gadolinium-enhanced magnetic resonance imaging: Results from a prospective study. Urology 2012, 80, 1313–1318. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S.; Ojha, B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: A prospective study. J. Thorac. Cardiovasc. Surg. 2006, 131, 1229–1235. [Google Scholar] [CrossRef] [Green Version]
- Davnall, F.; Yip, C.S.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, R.; Higashiyama, M.; Fujiwara, A.; Tokunaga, T.; Maeda, J.; Okami, J.; Kozuka, T.; Hosoki, T.; Hasegawa, Y.; Takami, M.; et al. Occult mediastinal lymph node metastasis in NSCLC patients diagnosed as clinical N0-1 by preoperative integrated FDG-PET/CT and CT: Risk factors, pattern, and histopathological study. Lung Cancer (Amst. Neth.) 2011, 71, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Port, J.L.; Korst, R.J.; Liss, Y.; Meherally, D.N.; Altorki, N.K. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann. Thorac. Surg. 2007, 84, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Caro, A.; Garcia, S.; Reguart, N.; Arguis, P.; Sanchez, M.; Gimferrer, J.M.; Marrades, R.; Lomena, F. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2010, 37, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- McNitt-Gray, M.F.; Wyckoff, N.; Sayre, J.W.; Goldin, J.G.; Aberle, D.R. The effects of co-occurrence matrix based texture parameters on the classification of solitary pulmonary nodules imaged on computed tomography. Comput. Med. Imaging Graph. Off. J. Comput. Med. Imaging Soc. 1999, 23, 339–348. [Google Scholar] [CrossRef]
- Chen, C.H.; Chang, C.K.; Tu, C.Y.; Liao, W.C.; Wu, B.R.; Chou, K.T.; Chiou, Y.R.; Yang, S.N.; Zhang, G.; Huang, T.C. Radiomic features analysis in computed tomography images of lung nodule classification. PLoS ONE 2018, 13, e0192002. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Hansen, M.; Baldwin, D.R.; Hasler, E.; Zamora, J.; Abraira, V.; Roque, I.F.M. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Song, S.H.; Park, H.; Lee, G.; Lee, H.Y.; Sohn, I.; Kim, H.S.; Lee, S.H.; Jeong, J.Y.; Kim, J.; Lee, K.S.; et al. Imaging Phenotyping Using Radiomics to Predict Micropapillary Pattern within Lung Adenocarcinoma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [Green Version]
- Welcome to pyradiomics documentation! Available online: https://pyradiomics.readthedocs.io/en/latest/ (accessed on 27 November 2020).
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 27 November 2020).
- Lee, G.; Park, H.; Bak, S.H.; Lee, H.Y. Radiomics in Lung Cancer from Basic to Advanced: Current Status and Future Directions. Korean J. Radiol. 2020, 21, 159–171. [Google Scholar] [CrossRef]
- Niu, L.; Qian, M.; Yang, W.; Meng, L.; Xiao, Y.; Wong, K.K.; Abbott, D.; Liu, X.; Zheng, H. Surface roughness detection of arteries via texture analysis of ultrasound images for early diagnosis of atherosclerosis. PLoS ONE 2013, 8, e76880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennon, F.E.; Cianci, G.C.; Cipriani, N.A.; Hensing, T.A.; Zhang, H.J.; Chen, C.T.; Murgu, S.D.; Vokes, E.E.; Vannier, M.W.; Salgia, R. Lung cancer-a fractal viewpoint. Nat. Rev. Clin. Oncol. 2015, 12, 664–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Subashi, E.; Yin, F.F.; Chang, Z. Dynamic fractal signature dissimilarity analysis for therapeutic response assessment using dynamic contrast-enhanced MRI. Med. Phys. 2016, 43, 1335–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeshan, B.; Skogen, K.; Pressney, I.; Coutroubis, D.; Miles, K. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: Preliminary evidence of an association with tumour metabolism, stage, and survival. Clin. Radiol. 2012, 67, 157–164. [Google Scholar] [CrossRef]
| Characteristics | Patients with Pathologic Negative LNs (n = 65) | Patients with Pathologic Positive LNs (n = 70) | p-Value |
|---|---|---|---|
| Mean age ± SD (years) | 61.83 ± 7.063 | 60.66 ± 7.549 | 0.480 |
| Gender | 0.037 | ||
| Male | 56 (86.2%) | 50 (71.4%) | |
| Female | 9 (13.8%) | 20 (28.6%) | |
| Tumor size, mm (mean ± SD) | 49.8 ± 23.2 | 47.2 ± 21.3 | 0.675 |
| SUVmax of tumor (mean ± SD) | 13.6 ± 4.1 | 12.3 ± 4.8 | 0.127 |
| Number of lymph nodes involved in PET-CT (median) | 2 (1–6) | 2 (1–5) | 0.130 |
| pT stage | 0.872 | ||
| 1a | 6 (9.2%) | 7 (10.0%) | |
| 1b | 9 (13.8%) | 7 (10.0%) | |
| 2a | 28 (43.1%) | 36 (51.4%) | |
| 2b | 11 (16.9%) | 10 (14.3%) | |
| 3 | 11 (16.9%) | 10 (14.3%) | |
| Histopathology | 0.011 | ||
| Adenocarcinoma | 32 (49.2%) | 52 (74.3%) | |
| Squamous cell carcinoma | 30 (46.2%) | 16 (22.9%) | |
| Non-small cell lung cancer * | 3 (4.6%) | 2 (2.9%) |
| Category | Variable | Reference | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Demographic factors | Sex | Male | 2.286 | 1.095–4.770 | 0.028 |
| Age | 0.258 | 0.043–1.552 | 0.139 | ||
| Pathologic factors | pT | pT1 | 0.667 | 0.237–1.873 | 0.442 |
| Cell type | Adenocarcinoma | 0.406 | 0.219–0.755 | 0.004 |
| Variable | Reference | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Sex | Male | 2.02 | 0.88–4.62 | 0.096 |
| Cell type | Adenocarcinoma | 0.39 | 0.19–0.77 | 0.0073 |
| Maximal 3D diameter | 9.80 | 3.14–30.61 | <0.0001 | |
| Cluster tendency | 2.36 | 1.23–4.57 | 0.0099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.H.; Park, H.; Lee, H.Y.; Ahn, J.H.; Lee, S.H.; Sohn, I.; Choi, J.Y.; Kim, H.K. Computed Tomography Radiomics for Residual Positron Emission Tomography-Computed Tomography Uptake in Lymph Nodes after Treatment. Cancers 2020, 12, 3564. https://doi.org/10.3390/cancers12123564
Kim CH, Park H, Lee HY, Ahn JH, Lee SH, Sohn I, Choi JY, Kim HK. Computed Tomography Radiomics for Residual Positron Emission Tomography-Computed Tomography Uptake in Lymph Nodes after Treatment. Cancers. 2020; 12(12):3564. https://doi.org/10.3390/cancers12123564
Chicago/Turabian StyleKim, Chu Hyun, Hyunjin Park, Ho Yun Lee, Joong Hyun Ahn, Seung Hak Lee, Insuk Sohn, Joon Young Choi, and Hong Kwan Kim. 2020. "Computed Tomography Radiomics for Residual Positron Emission Tomography-Computed Tomography Uptake in Lymph Nodes after Treatment" Cancers 12, no. 12: 3564. https://doi.org/10.3390/cancers12123564
APA StyleKim, C. H., Park, H., Lee, H. Y., Ahn, J. H., Lee, S. H., Sohn, I., Choi, J. Y., & Kim, H. K. (2020). Computed Tomography Radiomics for Residual Positron Emission Tomography-Computed Tomography Uptake in Lymph Nodes after Treatment. Cancers, 12(12), 3564. https://doi.org/10.3390/cancers12123564








