Modifying Adaptive Therapy to Enhance Competitive Suppression
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
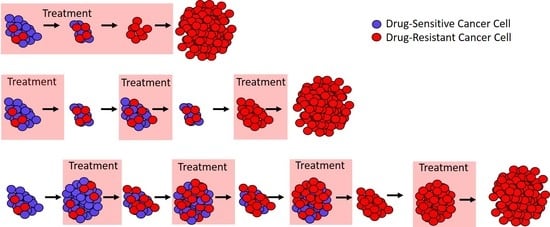
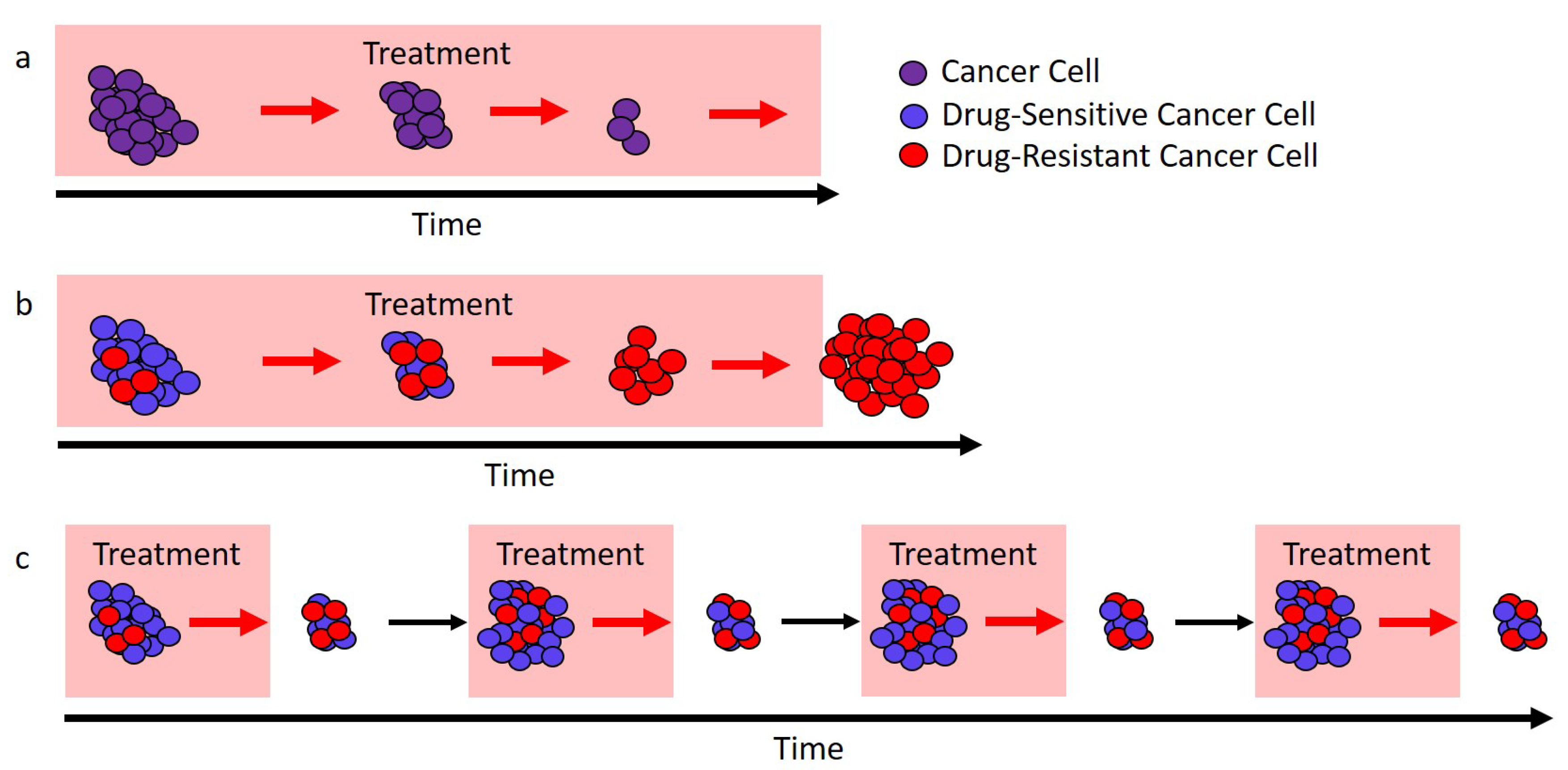
2.1. A Primer on Adaptive Therapy
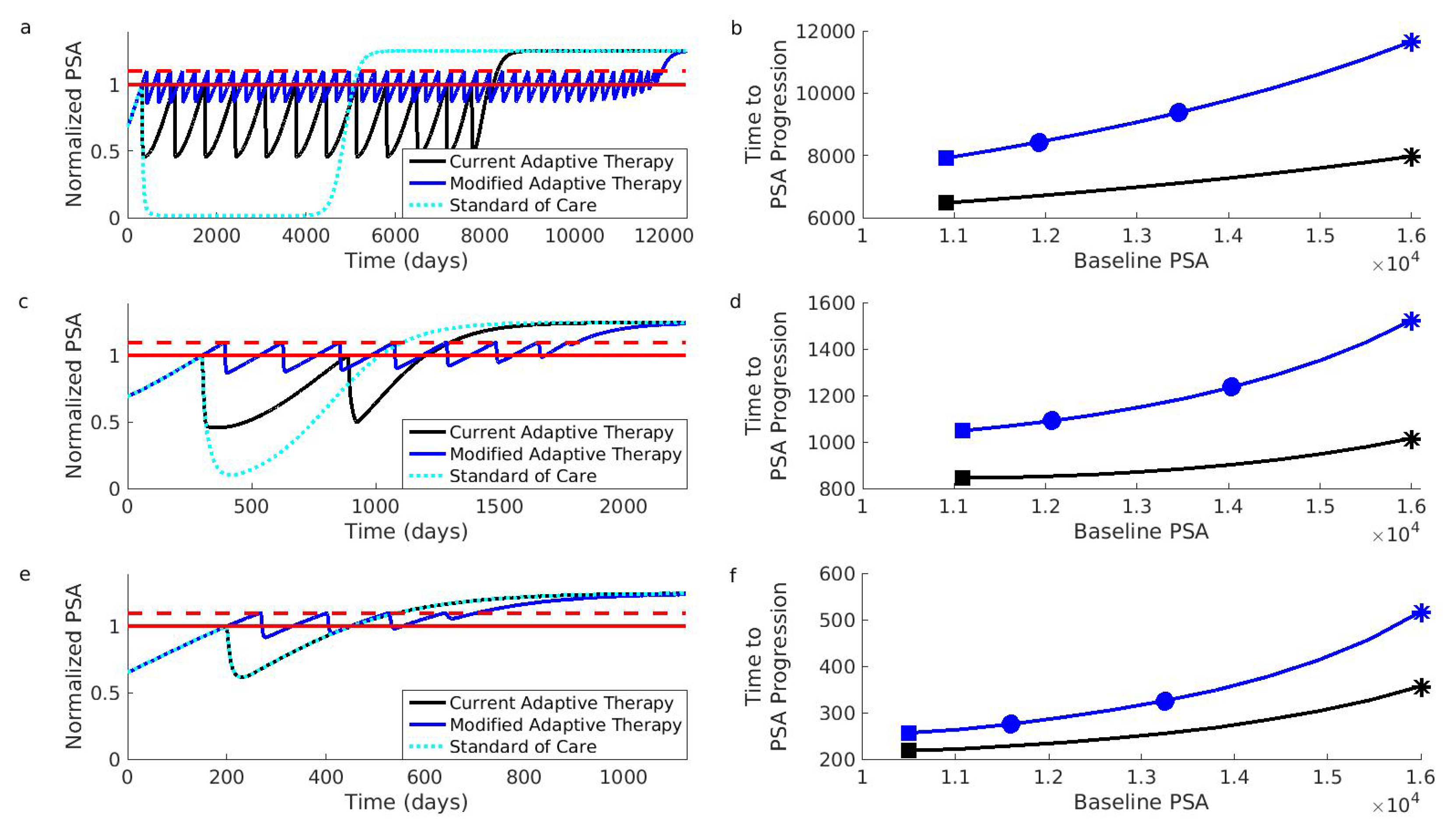
2.2. The Role of Tumor Size and Resistance Frequency in Adaptive Therapy
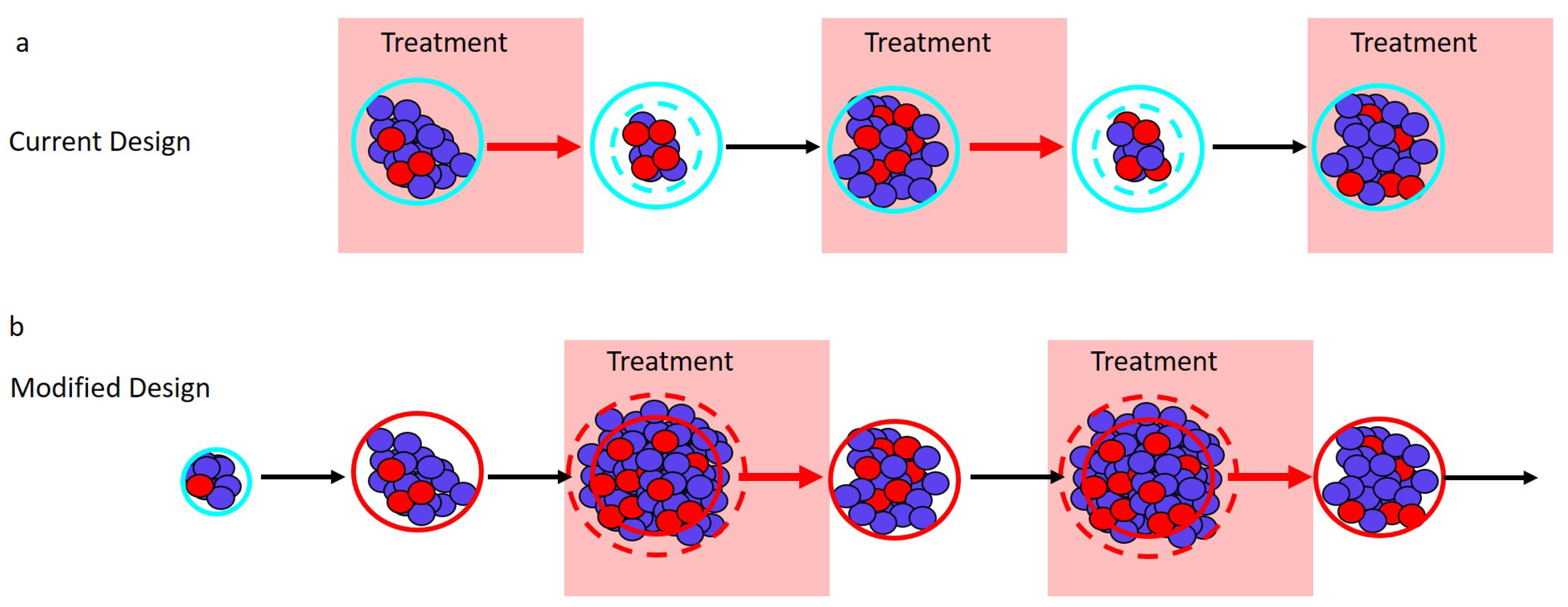
- Current design: Maximum tumor size is determined by a patient’s initial baseline burden. Different patients will have different initial burdens when they present for treatment. Despite this, the current adaptive therapy regimen always implements the “ rule” from the patient’s “initial baseline burden”. This limits the maximum size of the tumor and so lowers the amount of competitive suppression.Design modification: Can the patient’s tumor burden be safely increased? If yes, then the initiation of adaptive therapy should be delayed until this new larger “acceptable baseline burden” is reached. Withholding treatment until the tumor has grown to a larger size should increase the amount of competition and enhance the performance of adaptive therapy. Whether it is acceptable to allow the tumor to grow before initiating treatment will depend on the specific details of the patient and the cancer, as well as the size of the initial baseline burden. In general, making this decision will require balancing the possible benefits (e.g., prolonged time to progression, reduced drug use) with the possible risks (e.g., increased metastasis, greater morbidity). The relationship between tumor size and these other factors is not straightforward [32,33,34,35,36]. In the original trial, however, the initial baseline PSAs ranged from 2.42 to 109.4 ng/mL, suggesting that there is a wide range of acceptable PSA levels [13]. Although an acceptable PSA level will be different for different patients, this wide range points to the possibility that the baselines of certain patients could be safely increased.
- Current design: The “ rule” reduces the average tumor size. In the current design, treatment begins whenever the tumor reaches the baseline burden and stops whenever it falls below 50 percent of the baseline burden. These successive reductions in tumor burden reduce the average size of the population that is generating competition.Design modification: What should trigger treatment? Since larger populations generate more competition, we suggest “inverting” what triggers treatment starts and stops. Treatment should start whenever the tumor burden exceeds the baseline level by a measurable amount (e.g., larger than the baseline burden), and treatment should stop whenever the burden returns to the baseline. Figure 2 shows how this modification shifts the timing of treatment (shaded blocks in Panel b are shifted relative to shaded blocks in Panel a). This should increase the average size of the population and enhance competition.
- Current design: Patients with a high resistance frequency cannot benefit from adaptive therapy. If a patient’s initial resistance frequency is high, they will not be able to achieve a reduction in PSA during the first cycle of adaptive therapy. For these patients, treatment resembles the standard of care, and they are unable to benefit from adaptive therapy. The above suggested modifications of (i) increasing the baseline (whenever acceptable) and (ii) treating only when the burden exceeds this baseline help to ameliorate this shortcoming. With these modifications, only patients who begin with almost completely resistant tumors will be unable to complete multiple rounds of adaptive therapy.Design modification: Is the patient’s initial resistance frequency likely to be low? Although the previous modifications should allow patients with high resistance frequencies to benefit from adaptive therapy, special consideration should also be given to patients with very low resistance frequencies. Patients with low initial resistance frequencies may do better with the standard of care than with adaptive therapy (Box 2). For this reason, an effort should be made to identify and exclude patients with very low resistance frequencies. This may be difficult to do, but an evaluation of patient treatment history could help.
3. Discussion
4. Materials and Methods
4.1. Mathematical Model
4.2. Parameter Values and Simulation Details
4.3. The Role of Resistance Frequency: Additional Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| mCRPC | metastatic castrate-resistant prostate cancer |
| PSA | Prostate-specific antigen |
| GnRH | Gonadotropin-releasing hormone |
| ADT | Androgen deprivation therapy |
References
- Aggarwal, S. Targeted cancer therapies. Nat. Rev. Drug Discov. 2010, 9, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Kanavos, P.; Sullivan, R.; Lewison, G.; Schurer, W.; Eckhouse, S.; Vlachopioti, Z. The role of funding and policies on innovation in cancer drug development. Ecancermedicalscience 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Cleary, E.G.; Ledley, F.D. NIH funding for research underlying new cancer therapies. Lancet Oncol. 2020, 21, 755–757. [Google Scholar] [CrossRef]
- Pui, C.H.; Evans, W.E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006, 354, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Evans, W.E. A 50-Year journey to cure childhood acute lymphoblastic leukemia. Semin. Hematol. 2013, 50, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Greaves, M. Leukaemia ‘firsts’ in cancer research and treatment. Nat. Rev. Cancer 2016, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dry, J.R.; Yang, M.; Saez-Rodriguez, J. Looking beyond the cancer cell for effective drug combinations. Genome Med. 2016, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Hoang, T.T.; Tanrikulu, I.C.; Vatland, Q.A.; Hoang, T.M.; Raines, R.T. A human ribonuclease variant and ERK-pathway inhibitors exhibit highly synergistic toxicity for cancer cells. Mol. Cancer Ther. 2018, 17, 2622–2632. [Google Scholar] [CrossRef] [Green Version]
- Zagidullin, B.; Aldahdooh, J.; Zheng, S.; Wang, W.; Wang, Y.; Saad, J.; Malyutina, A.; Jafari, M.; Tanoli, Z.; Pessia, A.; et al. DrugComb: An integrative cancer drug combination data portal. Nucleic Acids Res. 2019, 47, W43–W51. [Google Scholar] [CrossRef]
- Parikh, R.C.; Du, X.L.; Morgan, R.O.; Lairson, D.R. Patterns of treatment sequences in chemotherapy and targeted biologics for metastatic colorectal cancer: Findings from a large community-based cohort of elderly patients. Drugs Real World Outcomes 2016, 3, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Topham, J.T.; Marra, M.A. Sequencing strategies to guide decision making in cancer treatment. PLoS Med. 2016, 13, e1002189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffo, O. Treatment sequencing in oncology: Balancing clinical trial and real-world evidence. Future Oncol. 2019, 15, 2887–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Cunningham, J.J.; Brown, J.S.; Gatenby, R.A. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat. Commun. 2017, 8, 1816. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Silva, A.S.; Gillies, R.J.; Frieden, B.R. Adaptive therapy. Cancer Res. 2009, 69, 4894–4903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatenby, R.A. A change of strategy in the war on cancer. Nature 2009, 459, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Read, A.F.; Day, T.; Huijben, S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S2), 10871–10877. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.S.; Kam, Y.; Khin, Z.P.; Minton, S.E.; Gillies, R.J.; Gatenby, R.A. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res. 2012, 72, 6362–6370. [Google Scholar] [CrossRef] [Green Version]
- Colijn, C.; Cohen, T. How competition governs whether moderate or aggressive treatment minimizes antibiotic resistance. eLife 2015, 4, e10559. [Google Scholar] [CrossRef] [Green Version]
- Day, T.; Read, A.F. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLoS Comput. Biol. 2016, 12, e1004689. [Google Scholar] [CrossRef]
- Enriquez-Navas, P.M.; Kam, Y.; Das, T.; Hassan, S.; Silva, A.; Foroutan, P.; Ruiz, E.; Martinez, G.; Minton, S.; Gillies, R.J.; et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci. Transl. Med. 2016, 8, 327ra24. [Google Scholar] [CrossRef] [Green Version]
- Gjini, E.; Brito, P.H. Integrating antimicrobial therapy with host immunity to fight drug-resistant infections: Classical vs. adaptive treatment. PLoS Comput. Biol. 2016, 12, e1004857. [Google Scholar] [CrossRef] [Green Version]
- Bacevic, K.; Noble, R.; Soffar, A.; Wael Ammar, O.; Boszonyik, B.; Prieto, S.; Vincent, C.; Hochberg, M.E.; Krasinska, L.; Fisher, D. Spatial competition constrains resistance to targeted cancer therapy. Nat. Commun. 2017, 8, 1995. [Google Scholar] [CrossRef]
- Hansen, E.; Woods, R.J.; Read, A.F. How to use a chemotherapeutic agent when resistance to it threatens the patient. PLoS Biol. 2017, 15, e2001110. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Karslake, J.; Woods, R.J.; Read, A.F.; Wood, K.B. Antibiotics can be used to contain drug-resistant bacteria by maintaining sufficiently large sensitive populations. PLoS Biol. 2020, 18, e3000713. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Higano, C.S.; Shore, N.D.; Hussain, M.; Petrylak, D.P. Treating patients with metastatic castration resistant prostate cancer: A comprehensive review of available therapies. J. Urol. 2015, 194, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, P.; De Bono, J.S.; Fizazi, K.; Freedland, S.J.; Grilli, M.; Kantoff, P.W.; Sonpavde, G.; Sternberg, C.N.; Yegnasubramanian, S.; Antonarakis, E.S. Update on systemic prostate cancer therapies: Management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur. Urol. 2019, 75, 88–99. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Dinh, M.N.; Brown, J.S.; Zhang, J.; Anderson, A.R.; Gatenby, R.A. Multidrug cancer therapy in metastatic castrate-resistant prostate cancer: An evolution-based strategy. Clin. Cancer Res. 2019, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, J.; You, L.; Zhang, J.; Gatenby, R.A.; Brown, J.S.; Newton, P.K.; Anderson, A.R.A. Towards Multidrug Adaptive Therapy. Cancer Res. 2020, 80, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Teply, B.A.; Wang, H.; Luber, B.; Sullivan, R.; Rifkind, I.; Bruns, A.; Spitz, A.; DeCarli, M.; Sinibaldi, V.; Pratz, C.F.; et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: An open-label, phase 2, multicohort study. Lancet Oncol. 2018, 19, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Leone, G.; Buttigliero, C.; Pisano, C.; Di Stefano, R.F.; Tabbò, F.; Turco, F.; Vignani, F.; Scagliotti, G.V.; Di Maio, M.; Tucci, M. Bipolar androgen therapy in prostate cancer: Current evidences and future perspectives. Crit. Rev. Oncol. Hematol. 2020, 152, 102994. [Google Scholar] [CrossRef]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Jiang, Y.Z.; Chen, S.; Cao, Z.G.; Wu, J.; Shen, Z.Z.; Shao, Z.M. Effect of large tumor size on cancer-specific mortality in node-negative breast cancer. Mayo Clin. Proc. 2012, 87, 1171–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colzani, E.; Johansson, A.L.V.; Liljegren, A.; Foukakis, T.; Clements, M.; Adolfsson, J.; Hall, P.; Czene, K. Time-dependent risk of developing distant metastasis in breast cancer patients according to treatment, age and tumour characteristics. Br. J. Cancer 2014, 110, 1378–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidhar, V.; Nipp, R.D.; Ryan, D.P.; Hong, T.S.; Nguyen, P.L.; Wo, J.Y. Association between very small tumor size and increased cancer-specific mortality in node-positive colon cancer. Dis. Colon Rectum 2016, 59, 187–193. [Google Scholar] [CrossRef]
- Han, K.; Kim, E.K.; Kwak, J.Y. 1.5–2 cm tumor size was not associated with distant metastasis and mortality in small thyroid cancer: A population-based study. Sci. Rep. 2017, 7, 46298. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Foo, J.; Michor, F. Evolution of resistance to anti-cancer therapy during general dosing schedules. J. Theor. Biol. 2010, 263, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, S.J.; Haendler, B. Exploiting epigenetic alterations in prostate cancer. Int. J. Mol. Sci. 2017, 18, 1017. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, M.; Paller, C.; Kyprianou, N. Mechanisms of therapeutic resistance in prostate cancer. Curr. Oncol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef] [Green Version]
- Ruggero, K.; Farran-Matas, S.; Martinez-Tebar, A.; Aytes, A. Epigenetic regulation in prostate cancer progression. Curr. Mol. Biol. Rep. 2018, 4, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bozic, I.; Nowak, M.A. Timing and heterogeneity of mutations associated with drug resistance in metastatic cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15964–15968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, J.; Michor, F. Evolution of acquired resistance to anti-cancer therapy. J. Theor. Biol. 2014, 355, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pienta, K.J.; Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1665–1671. [Google Scholar] [CrossRef] [Green Version]
- Green, S.M.; Mostaghel, E.A.; Nelson, P.S. Androgen action and metabolism in prostate cancer. Mol. Cell. Endocrinol. 2012, 360, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.J.; Brown, J.S.; Gatenby, R.A.; Staňková, K. Optimal control to develop therapeutic strategies for metastatic castrate resistant prostate cancer. J. Theor. Biol. 2018, 459, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Hansen, E.; Read, A.F. Cancer therapy: Attempt cure or manage drug resistance? Evol. Appl. 2020, 13, 1660–1672. [Google Scholar] [CrossRef]
- Karzai, F.H.; Madan, R.A.; Figg, W.D. Beyond PSA: Managing modern therapeutic options in metastatic castration-resistant prostate cancer. South. Med. J. 2015, 108, 224–228. [Google Scholar] [CrossRef]
- Studer, U.E.; Whelan, P.; Wimpissinger, F.; Casselman, J.; de Reijke, T.M.; Knönagel, H.; Loidl, W.; Isorna, S.; Sundaram, S.K.; Collette, L. Differences in time to disease progression do not predict for cancer-specific survival in patients receiving immediate or deferred androgen-deprivation therapy for prostate cancer: Final results of EORTC randomized trial 30891 with 12 years of follow-up. Eur. Urol. 2014, 66, 829–838. [Google Scholar] [CrossRef]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Drabovich, A.P.; Jarvi, K.A.; Diamandis, E.P. Mechanisms of androgen-independent prostate cancer. Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2014, 25, 42–54. [Google Scholar]
| Clinical Trial Identifier | Cancer Type | Therapeutic |
|---|---|---|
| NCT02415621 | prostate cancer | abiraterone |
| (metastatic castrate resistant) | ||
| NCT03511196 | prostate cancer | GnRH agonist and/or |
| (stage IV castration sensitive) | abiraterone plus prednisone | |
| NCT03543969 | melanoma | vemurafenib and cobimetinib |
| (advanced BRAF mutant) | ||
| NCT03630120 | thyroid cancer | tyrosine kinase inhibitor |
| (advanced progressive 131I-refractory | ||
| differentiated or medullary) |
| Patient 1 | 1 | 0.8 | 0.7 | 0.6 | 1 | 0.9 | 0.4 | 0.5 | 1 |
| Patient 2 | 1 | 0.8 | 0.6 | 0.5 | 1 | 0.9 | 0.4 | 0.7 | 1 |
| Patient 3 | 1 | 0.9 | 0.7 | 0.5 | 1 | 0.8 | 0.4 | 0.6 | 1 |
| Patient 1 | |||
| Patient 2 | |||
| Patient 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, E.; Read, A.F. Modifying Adaptive Therapy to Enhance Competitive Suppression. Cancers 2020, 12, 3556. https://doi.org/10.3390/cancers12123556
Hansen E, Read AF. Modifying Adaptive Therapy to Enhance Competitive Suppression. Cancers. 2020; 12(12):3556. https://doi.org/10.3390/cancers12123556
Chicago/Turabian StyleHansen, Elsa, and Andrew F. Read. 2020. "Modifying Adaptive Therapy to Enhance Competitive Suppression" Cancers 12, no. 12: 3556. https://doi.org/10.3390/cancers12123556