Simple Summary
Adaptive therapy is a new approach to cancer treatment that aims to forestall treatment resistance by controlling instead of minimizing tumor burdens. The underlying hypothesis is that drug-sensitive cancer cells can competitively suppress drug-resistant cells and greatly increase the amount of time that a therapy is effective. This approach is being tested in a clinical trial, and the preliminary results are extremely promising. If these promising results can indeed be attributed to the competitive suppression of resistance, then identifying simple ways to enhance competitive suppression should increase the benefits of this therapy. Here, we show that simple modifications to the design of adaptive therapy should greatly enhance its performance and also increase the range of patients who can benefit from it. Importantly, this investigation also highlights key assumptions underlying the design of adaptive therapy and emphasizes that these must be validated if adaptive therapy is to be optimized and used effectively.
Abstract
Adaptive therapy is a promising new approach to cancer treatment. It is designed to leverage competition between drug-sensitive and drug-resistant cells in order to suppress resistance and maintain tumor control for longer. Prompted by encouraging results from a recent pilot clinical trial, we evaluate the design of this initial test of adaptive therapy and identify three simple modifications that should improve performance. These modifications are designed to increase competition and are easy to implement. Using the mathematical model that supported the recent adaptive therapy trial, we show that the suggested modifications further delay time to tumor progression and also increase the range of patients who can benefit from adaptive therapy.
1. Introduction
Drug discovery and regimen design play complementary roles in advancing cancer therapy. Although drug discovery receives a disproportionate amount of attention [1,2,3], regimen design is critically important and has been responsible for dramatic advancements in patient care. Regimen design, for example, played a key role in making childhood acute lymphoblastic leukemia (ALL) an essentially curable disease [4,5,6]. Regimen design often involves identifying synergistic combinations [7,8,9] and sequences of drugs [10,11,12]. As discussed here, however, improving regimen design does not have to involve changing or adding therapeutics. It is possible to enhance patient outcomes simply by modifying how the current drugs are used.
We consider an innovative treatment regimen that is showing very promising results in a pilot clinical trial [13]. This novel regimen, called “adaptive therapy”, uses the same drug as the standard of care, but applies it differently. This emphasizes that simply changing how we use therapeutics can lead to dramatic improvements in patient care. Although these preliminary results must be interpreted with caution, they suggest that—in certain circumstances—adaptive therapy may represent a promising new paradigm for patient treatment.
Adaptive therapy has two important, but distinct features. First, it is “adaptive” [14]. Instead of using a fixed schedule, treatment decisions are based on how individual tumors respond to treatment. This is in contrast to the majority of regimens, which use predetermined treatment schedules. Second, it is designed to leverage competition between drug-sensitive and drug-resistant cells to improve tumor control (Figure 1). This differs from the common goal of achieving and/or sustaining large tumor responses. Rather than attempting to drive tumor cell populations to undetectable levels, it deliberately maintains a notable tumor burden in order to competitively suppress resistance [15]. Competitive suppression has been shown to work in theory and experiment (both in vitro and in vivo) for both cancer and infections [13,16,17,18,19,20,21,22,23,24]. The recent trial of adaptive therapy provides evidence that an easily implementable realization of competitive suppression may work in the clinic [13].
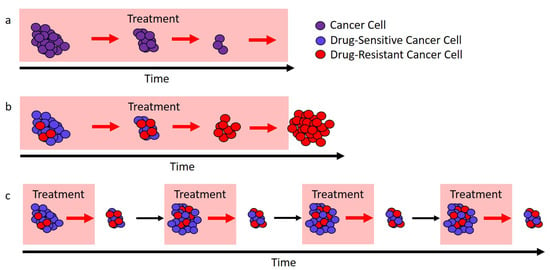
Figure 1.
Different approaches to treatment. (a) In ideal circumstances, continuous treatment (here, called the “standard of care”) will stop tumor cell growth and lead to cure. (b) If there are drug-resistant cells (red), the standard of care can only remove the drug-sensitive cells (blue), and this leaves a fully resistant tumor that can expand in size. (c) If treatment is modulated so that not all drug-sensitive cells (blue) are removed, then these cells can competitively suppress the expansion of the resistant cells (red). In ideal circumstances, this would lead to longer tumor control.
Although this particular trial tested adaptive therapy on metastatic castrate-resistant prostate cancer (mCRPC) [25,26], we are interested in adaptive therapy as a general approach to cancer treatment. Recent theoretical studies have considered the enhancement of adaptive therapy by using multiple drugs [27,28]. Here, we evaluate the design of adaptive therapy within the scope of a single drug and discuss how simple modifications to the design should improve patient outcomes. The modifications we suggest are not specific to prostate cancer, but are intended to enhance competition in general. This analysis rests on two important assumptions. First, we take as a basic tenet that “larger populations generate more competition”. In essence, drug-sensitive cells should competitively suppress the expansion of the resistant population, and the larger the sensitive population, the stronger this effect should be. Second, we assume that the success of adaptive therapy can be attributed to competitive suppression (see Box 1).
Box 1. Is competition the reason adaptive therapy works?
The biology of prostate cancer and treatment is complicated. Even though adaptive therapy is designed to leverage competition, it is quite possible that it works for reasons other than competition. For example, improved outcomes may be due to adaptation to different local environments (similar to the hypothesized mechanism of success for bipolar androgen therapy [29,30]). If success is due to some factor other than competition, changes intended to enhance competition may actually reduce the effectiveness of adaptive therapy. This means that modifications should be made with care. Gaining a better understanding of what is causing the current success of adaptive therapy is critical if we are to (i) optimize regimen design and (ii) successfully apply adaptive therapy to other cancers.
2. Results
2.1. A Primer on Adaptive Therapy
Adaptive therapy maintains a measurable (but contained) tumor burden by modulating treatment according to tumor response. There are a variety of ways that this can be implemented [13,20], and different cancers will require using different methods to estimate tumor burden. Here, we focus on the specific approach taken in a recent pilot clinical trial for mCRPC (NCT02415621) [13]. In this trial, prostate-specific antigen (PSA) [31] is used as a measure of treatment response, and treatment is administered according to the “ rule” (Figure 2a). Since we are interested in the general application of adaptive therapy to a variety of cancers, we will treat PSA as a proxy for tumor burden. This allows us to readily generalize to other cancers where a different measure of tumor burden would be used.
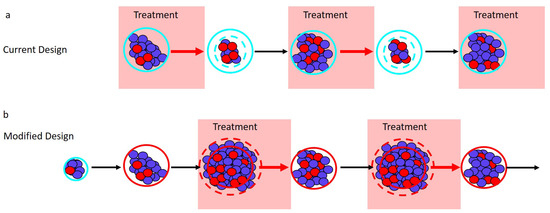
Figure 2.
Adaptive therapy designs. PSA is used as a proxy for tumor burden. (a) Current adaptive therapy design. Treatment decisions are based on the “ rule” using the patient’s initial pretreatment baseline PSA (solid blue circle). Treatment is stopped when the PSA falls to of the baseline PSA (dashed blue circle) and is re-initiated only once the PSA returns to its initial baseline. (b) Modified regimen design. If the initial baseline PSA (blue circle) is low, the PSA is allowed to increase to a larger acceptable baseline PSA (solid red circle). Treatment begins when the PSA exceeds the acceptable baseline by a measurable amount (dashed red circle) and stops once the PSA returns to the acceptable baseline (solid red circle).
Before adaptive therapy begins, a patient’s PSA is determined to establish a baseline from which all treatment decisions will be made. Treatment is then administered until the PSA is reduced to of the initial baseline. Once a reduction has been observed, treatment is halted and withheld until the PSA returns to the initial baseline. This completes a single cycle of adaptive therapy, and cycles are repeated until treatment can no longer prevent the PSA from exceeding the initial baseline (i.e., until PSA progression).
This approach differs markedly from the standard of care, which treats continuously until progression. Because adaptive therapy halts treatment every time a reduction in PSA is achieved, the expectation is that an appreciable drug-sensitive population is maintained and that this population will slow the expansion of the drug-resistant population—leading to prolonged tumor control (Figure 1). Preliminary results are extremely promising with median time to progression of at least 27 months [13] compared to a median of 9 months (for PSA progression) and 14 months (for radiographic progression) for a contemporaneous cohort that received the standard of care [13].
2.2. The Role of Tumor Size and Resistance Frequency in Adaptive Therapy
A curious feature of this initial test of adaptive therapy is that absolute tumor size plays no role in the current design. This is at odds with the general notion that “larger populations generate more competition”. Instead, the “ rule” bases treatment decisions on the tumor size relative to the initial baseline. An additional consequence of the “ rule” is that resistance frequency determines which patients have the opportunity to benefit from competitive suppression (as described below). Here, we describe how small modifications to adaptive therapy can make absolute tumor size central to the design and greatly enhance competitive suppression. Our suggested modifications are easily implementable and preserve the “adaptive” nature of the treatment regimen. Under these modifications, treatment would occur only when the tumor exceeds a predetermined acceptable burden (Figure 2b).
- Current design: Maximum tumor size is determined by a patient’s initial baseline burden. Different patients will have different initial burdens when they present for treatment. Despite this, the current adaptive therapy regimen always implements the “ rule” from the patient’s “initial baseline burden”. This limits the maximum size of the tumor and so lowers the amount of competitive suppression.Design modification: Can the patient’s tumor burden be safely increased? If yes, then the initiation of adaptive therapy should be delayed until this new larger “acceptable baseline burden” is reached. Withholding treatment until the tumor has grown to a larger size should increase the amount of competition and enhance the performance of adaptive therapy. Whether it is acceptable to allow the tumor to grow before initiating treatment will depend on the specific details of the patient and the cancer, as well as the size of the initial baseline burden. In general, making this decision will require balancing the possible benefits (e.g., prolonged time to progression, reduced drug use) with the possible risks (e.g., increased metastasis, greater morbidity). The relationship between tumor size and these other factors is not straightforward [32,33,34,35,36]. In the original trial, however, the initial baseline PSAs ranged from 2.42 to 109.4 ng/mL, suggesting that there is a wide range of acceptable PSA levels [13]. Although an acceptable PSA level will be different for different patients, this wide range points to the possibility that the baselines of certain patients could be safely increased.
- Current design: The “ rule” reduces the average tumor size. In the current design, treatment begins whenever the tumor reaches the baseline burden and stops whenever it falls below 50 percent of the baseline burden. These successive reductions in tumor burden reduce the average size of the population that is generating competition.Design modification: What should trigger treatment? Since larger populations generate more competition, we suggest “inverting” what triggers treatment starts and stops. Treatment should start whenever the tumor burden exceeds the baseline level by a measurable amount (e.g., larger than the baseline burden), and treatment should stop whenever the burden returns to the baseline. Figure 2 shows how this modification shifts the timing of treatment (shaded blocks in Panel b are shifted relative to shaded blocks in Panel a). This should increase the average size of the population and enhance competition.
- Current design: Patients with a high resistance frequency cannot benefit from adaptive therapy. If a patient’s initial resistance frequency is high, they will not be able to achieve a reduction in PSA during the first cycle of adaptive therapy. For these patients, treatment resembles the standard of care, and they are unable to benefit from adaptive therapy. The above suggested modifications of (i) increasing the baseline (whenever acceptable) and (ii) treating only when the burden exceeds this baseline help to ameliorate this shortcoming. With these modifications, only patients who begin with almost completely resistant tumors will be unable to complete multiple rounds of adaptive therapy.Design modification: Is the patient’s initial resistance frequency likely to be low? Although the previous modifications should allow patients with high resistance frequencies to benefit from adaptive therapy, special consideration should also be given to patients with very low resistance frequencies. Patients with low initial resistance frequencies may do better with the standard of care than with adaptive therapy (Box 2). For this reason, an effort should be made to identify and exclude patients with very low resistance frequencies. This may be difficult to do, but an evaluation of patient treatment history could help.
Box 2. How does resistance frequency impact the performance of adaptive therapy?
According to theory, resistance frequency impacts the performance of adaptive therapy in two distinct ways.
Absolute performance: Lowering the resistance frequency improves the absolute performance of adaptive therapy. This is not surprising since the lower the initial resistance frequency, the longer it will take for resistance to dominate and exceed the baseline burden. This observation does not rely on competitive suppression, and the same relationship also holds for the standard of care [37]. Both adaptive therapy and the standard of care perform better when the initial resistance frequency is low.
Performance relative to the standard of care: At very low resistance frequencies, adaptive therapy may not perform as well as the standard of care. Consider, for example, a completely sensitive tumor (i.e., resistance frequency of zero). In this case, the standard of care may even clear the tumor and result in cure. On the other hand, with adaptive therapy, there is an increased risk that resistance will be introduced de novo (e.g., via mutation or epigenetic changes [38,39,40,41]). If resistance is successfully introduced, then adaptive therapy will control the tumor only until resistance expands to dominate the cancer.
Extrapolating from this simple example suggests that if the resistance frequency is sufficiently small, the standard of care may be a better treatment option than adaptive therapy. This is precisely what theory predicts [23]. According to theory, the resistance frequency must be sufficiently large before adaptive therapy is the preferred treatment option.
Combining the roles of resistance frequency in the absolute and relative performance of adaptive therapy leads to the following rule: “The resistance frequency must exceed a minimum threshold, but beyond that, lower resistance frequencies are better”. This ensures that adaptive therapy is the preferred option while still maintaining the absolute performance of adaptive therapy. It is possible that this minimum threshold is so low as to be inconsequential (i.e., for tumors of interest, resistance frequencies might naturally exceed this minimum level) [42,43]. If this is the case, then resistance frequency need not play a role in adaptive therapy design. The essential observation, however, is that if resistance frequency is important, its role should be the opposite of what it is in the current adaptive therapy design. A low resistance frequency—not a high resistance frequency—should preclude the use of adaptive therapy. See Section 4.3 for possible exceptions and further discussion.
Accurately predicting the impact of these design modifications is difficult because the biology of prostate cancer is complicated and poorly understood. For example, it is known that prostate cancer can be heterogeneous with cell populations that vary in their (i) dependence on and (ii) ability to produce androgens [44,45,46]. How these different cell populations interact and compete may be more complicated than the simple “larger populations generate more competition” tenet that we use. Therefore, as an initial validation step, we assessed the impact of these modifications using a mathematical model that accounts for these interactions. This model is the same model that was used to design the current adaptive therapy regimen. According to this model, the design modifications we suggest will improve performance (Figure 3). This emphasizes that, at least for the biological understanding used to design the current regimen, the suggested modified regimen will more effectively prolong time to progression.
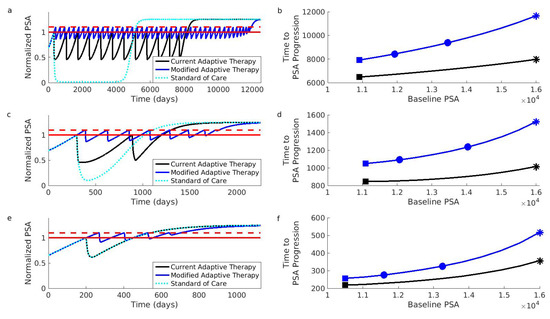
Figure 3.
Simulations comparing current and modified adaptive therapy designs. The comparison was performed for three different types of patients. These patients differed in their initial resistance frequencies: low (Patient 1, Panels (a,b)), medium (Patient 2, Panels (c,d)), and high (Patient 3, Panels (e,f)). (a) Comparison for Patient 1 assuming that the initial baseline (solid red) cannot be increased. The modified design (blue) controls the PSA for longer than the current design (black). Both adaptive therapy designs are better than the standard of care (dotted light blue curve). To facilitate comparison, PSA progression for both designs occurs when the PSA exceeds of the baseline (dashed red). The PSA is normalized relative to initial baseline PSA. (b) Time to PSA progression for Patient 1 under the modified (blue) and current (black) adaptive therapy design. If the current design is implemented at the initial baseline indicated by the black square, the modified design implemented at the same baseline would improve the results (blue square). However, increasing the baseline by (leftmost blue circle) or (rightmost blue circle) improves the results further. Asterisks correspond to the dynamics shown in (a). (c,d) Same as (a,b) except for Patient 2. (e,f) Same as (a,b) except for Patient 3. Notice that Patient 3 did not achieve a reduction in the PSA during the first cycle of adaptive therapy (the black curve in Panel (e) is always above 0.5). This means that the current adaptive therapy design coincides with the standard of care (the black curve and dotted light blue curve in Panel (e) lie on top of each other). Simulations use the same mathematical model as the original simulations that supported the design of the current adaptive therapy regimen [13,47]. See Section 4 for details on patient types and simulations.
The original simulations used to develop the current adaptive therapy regimen [13] focused on two types of patients. These patient types differ in how the different cell populations compete, and this leads to different initial resistance frequencies. The first patient type (represented by “Patient 1” in Figure 3) exhibits competition dynamics that make resistant cells initially rare. The second patient type (represented by “Patient 2”) is characterized by competition dynamics that promote moderate pretreatment levels of resistant cells. We also considered a third patient type (“Patient 3”) with an initially high resistance frequency. This third patient type demonstrates that patients can benefit from the modified regimen even if they would have been excluded from the current adaptive therapy trial (see Section 4 for additional details on patient types).
Figure 3 shows that the suggested modifications should improve the performance of adaptive therapy for all three types of patients. Even if adaptive therapy must be implemented from a patient’s initial baseline, the modified design results in significant improvements (compare the black curves to blue curves). Additionally, these improvements will be enhanced if a larger acceptable baseline can be used. For example, if the current adaptive therapy design results in the performance indicated by the black square (Panel b), then the modified design (implemented at the initial baseline) will increase the length of tumor control (blue square, Panel b). This improvement can be further enhanced if the baseline can be increased even by modest amounts (the leftmost blue square shows improvement for a increase in the baseline; the rightmost blue square for a increase, Panel b). Further increases will result in further improvements (the blue curve is increasing).
3. Discussion
Adaptive therapy is a novel treatment paradigm that has shown very promising results in a small clinical trial for mCRPC. Its novelty partly lies in that it is designed to competitively suppress resistance as opposed to minimizing tumor burden. Here, we outlined simple modifications that should enhance the performance of adaptive therapy.
According to the tenet “larger populations generate more competition”, our modified design should increase the amount of competitive suppression by (i) increasing the baseline tumor burden whenever possible and (ii) only treating when the tumor exceeds the baseline. An additional benefit of the modified design is that it does not exclude patients with high resistance frequencies. This means that more patients have the opportunity to benefit from adaptive therapy.
The main risk of using adaptive therapy is that some patients may do worse than if they had received the standard of care [23]. This is most likely to occur when there is little to no resistance. To mitigate this risk, emphasis should be placed on identifying and excluding patients who are unlikely to harbor much resistance. Here, we did not explicitly consider the possibility of cure or additional dynamics, which could make adaptive therapy perform worse than the standard of care (e.g., mutation or epigenetic changes [38,39,40,41]). In general, these may be possibilities and will complicate the choice between the standard of care and adaptive therapy [48].
In addition to our assumption that larger tumor cell populations generate more competition and adaptive therapy works because of competition (see Box 1), there are two further assumptions underpinning our analysis: (i) the PSA is a good proxy for tumor burden [49] and (ii) when PSA progression occurs, it can actually be linked to resistance [50]. The comparison we presented uses the same mathematical model that supported the original development of the current adaptive therapy regimen. This model makes very specific assumptions about how cell populations interact (see Section 4). For example, it focuses on the competitive interactions between different cell populations and not the effects of mutation or epigenetic changes [38,39,40,41]. As knowledge of cancer biology and treatment improves, there should be a continuing effort to account for all interactions that will influence performance and inform regimen design.
Our discussion uses mCRPC as an illustrative example because this was adaptive therapy’s first clinical application. We are, however, interested in adaptive therapy as a general approach to cancer treatment. Indeed, adaptive therapy is being trialed for other cancers (Table 1). At the time of writing, results for these trials are not available, but it is essential to emphasize that the performance of adaptive therapy will depend on the cancer, drug, and patient population. Pre-clinical models have also shown the effectiveness of adaptive therapy in breast and ovarian cancers [14,20]. The modifications we suggest are not specific to prostate cancer, but are intended to enhance competition in general. We are certainly not claiming that our design modifications lead to the optimal design. However, they are simple, easily implementable modifications that maintain the adaptive nature of adaptive therapy. Regardless of why adaptive therapy is working or whether it can be improved, the results from the current clinical trial make one thing clear: having effective therapeutics is not enough; how you use them matters.

Table 1.
Examples of clinical trials for adaptive therapy.
4. Materials and Methods
4.1. Mathematical Model
Prostate cancer cells often rely on androgens to grow. Treatment normally aims to exploit this dependence by employing some form of androgen deprivation therapy (ADT). Even though ADT is initially successful, resistance almost inevitably develops, and the cancer progresses. There are a variety of mechanisms that can contribute to this resistance [40,51,52], but variation in androgen dependence is often a contributing factor. The mathematical model used to conceptualize the current adaptive therapy design assumes that certain cells are less dependent on androgens [13,47]. These cells (called androgen independent and denoted by I) are resistant to ADT and are responsible for tumor progression during treatment. We use the same model here to compare the current and modified adaptive therapy designs.
This model involves three different populations of cancer cells: (i) androgen dependent (D), (ii) androgen independent (I), and (iii) androgen producing (P). The mathematical equations describing the interactions of these populations are:
where (i) , , and are the per capita growth rates of the respective populations in the absence of competition, (ii) , , and are the carrying capacities of the respective populations, and (iii) the are constants describing how competition between populations impacts growth.
According to these equations, the different populations exhibit logistic growth with both intra- and inter-population competition. Consider, for example, the dynamics of the androgen dependent population (D) described by Equation (1). In the absence of any competition, growth would simply be proportional to the size of the population (i.e., Equation (1) would become ). Intra-population competition, however, reduces this growth by a factor of . Accounting for competition from all populations reduces growth even further (i.e., by a factor of ).
An essential feature of this model is that the different cell populations interact with each other in two ways. The first is competition (as described through the coefficients ). Competition reduces the growth capacity of each population. The second interaction occurs between the androgen dependent (D) and androgen producing cells (P). Increasing the androgen producing population (P) increases the amount of available androgen, and this increases the carrying capacity of the androgen dependent cells (D). Mathematically, this is encoded by making the carrying capacity of the androgen dependent population () proportional to the size of the androgen producing population (i.e., ). Increasing reduces the effect of competition (from all populations) on the androgen dependent population (D). Other types of interactions are not included in the model. For example, cells cannot move between populations through processes like mutation or epigenetic changes [38,39,40,41].
This model assumes that treatment modulates dynamics by changing the carrying capacities of the androgen dependent population () and the androgen producing population (). When there is no treatment, these carrying capacities are and . During treatment, these carrying capacities are reduced to and . Treatment has no direct effect on the androgen independent population (I).
4.2. Parameter Values and Simulation Details
The parameter values used to produce Figure 3 are the same as the ones used in [13]. The intrinsic growth parameters are , and the carrying capacity for the androgen independent population is 10,000. Two different sets of competition coefficients are used to simulate the dynamics of the two patient types described in [13]. We also included a third patient type that does not respond well to the current adaptive therapy design. Coefficients for this third patient type were chosen to be identical to the “non-responder” patient type described by Cunningham et al. in an extended analysis of the original mathematical model [47]. The competition coefficients for the different patient types are given in Table 2.

Table 2.
Competition coefficients for different patient types.
Changes in the PSA are described by (). Initial conditions for the simulations were determined in the same way as in [13]. Cell populations were initialized to the values shown in Table 3. The populations were then allowed to grow in the absence of treatment until the PSA reached the desired baseline. Once the PSA reached the desired baseline, adaptive therapy (either the current or modified version) was initiated. The range of PSA baselines used in Figure 3 was . Code S1 provides the MATLAB code used to generate Figure 3.

Table 3.
Initial conditions for different patient types.
4.3. The Role of Resistance Frequency: Additional Considerations
A central assumption underlying our analysis is that larger populations generate more competition. This means that for high resistance frequencies, the sensitive population may be so small that its ability to competitively suppress the resistant population is negligible. Because the mathematical model used in this analysis assumes that drug-resistant cells are completely resistant to treatment, this simply means that adaptive therapy will have diminishing returns as the resistance frequency increases. In reality, however, treatment may be partly effective at directly controlling the resistant cells. In this case, when the resistant frequency is sufficiently high (and hence, the sensitive population small), the direct effect of treatment on the resistant cells may exceed the effect of competitive suppression from the sensitive population. This means that there may be situations where an initially high resistance frequency means that the standard of care outperforms an adaptive technique that leverages competition. This also suggests that a hybrid approach of leveraging competition when the resistance frequency is initially low and then switching to the standard of care once the resistance frequency has become sufficiently large would lead to even further gains.
5. Conclusions
Adaptive therapy is a promising new approach to treating cancer. It is designed to forestall treatment resistance and prolong tumor control by competitively suppressing drug-resistant cancer cells. If its improved performance over the standard of care is due to competitive suppression, then certain design modifications should enhance its performance. Here, we propose three design modifications aimed at increasing competition and improving the selection of patients who receive adaptive therapy. Using the same mathematical model that supported the original adaptive therapy design, we demonstrate that these modifications substantially improve the performance of adaptive therapy.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/12/3556/s1, Code S1: MATLAB code to generate Figure 3.
Author Contributions
Conceptualization, E.H. and A.F.R.; software, E.H.; writing, original draft preparation, E.H.; writing, review and editing, E.H and A.F.R.; funding acquisition, A.F.R. All authors read and agreed to the published version of the manuscript.
Funding
This research was funded by Eberly Family Trust.
Acknowledgments
The MATLAB code for the simulations in Figure 3 was modeled after the code provided in the Supplementary Information of [13]. Landon vom Steeg, Jeanette Hansen, Valerie Morley, Don Hansen, and Yannick Viossat provided valuable and insightful comments on the original manuscript draft.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; nor in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ALL | Acute lymphoblastic leukemia |
| mCRPC | metastatic castrate-resistant prostate cancer |
| PSA | Prostate-specific antigen |
| GnRH | Gonadotropin-releasing hormone |
| ADT | Androgen deprivation therapy |
References
- Aggarwal, S. Targeted cancer therapies. Nat. Rev. Drug Discov. 2010, 9, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Kanavos, P.; Sullivan, R.; Lewison, G.; Schurer, W.; Eckhouse, S.; Vlachopioti, Z. The role of funding and policies on innovation in cancer drug development. Ecancermedicalscience 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Cleary, E.G.; Ledley, F.D. NIH funding for research underlying new cancer therapies. Lancet Oncol. 2020, 21, 755–757. [Google Scholar] [CrossRef]
- Pui, C.H.; Evans, W.E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 2006, 354, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Evans, W.E. A 50-Year journey to cure childhood acute lymphoblastic leukemia. Semin. Hematol. 2013, 50, 185–196. [Google Scholar] [CrossRef]
- Greaves, M. Leukaemia ‘firsts’ in cancer research and treatment. Nat. Rev. Cancer 2016, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dry, J.R.; Yang, M.; Saez-Rodriguez, J. Looking beyond the cancer cell for effective drug combinations. Genome Med. 2016, 8, 125. [Google Scholar] [CrossRef]
- Hoang, T.T.; Tanrikulu, I.C.; Vatland, Q.A.; Hoang, T.M.; Raines, R.T. A human ribonuclease variant and ERK-pathway inhibitors exhibit highly synergistic toxicity for cancer cells. Mol. Cancer Ther. 2018, 17, 2622–2632. [Google Scholar] [CrossRef]
- Zagidullin, B.; Aldahdooh, J.; Zheng, S.; Wang, W.; Wang, Y.; Saad, J.; Malyutina, A.; Jafari, M.; Tanoli, Z.; Pessia, A.; et al. DrugComb: An integrative cancer drug combination data portal. Nucleic Acids Res. 2019, 47, W43–W51. [Google Scholar] [CrossRef]
- Parikh, R.C.; Du, X.L.; Morgan, R.O.; Lairson, D.R. Patterns of treatment sequences in chemotherapy and targeted biologics for metastatic colorectal cancer: Findings from a large community-based cohort of elderly patients. Drugs Real World Outcomes 2016, 3, 69–82. [Google Scholar] [CrossRef]
- Topham, J.T.; Marra, M.A. Sequencing strategies to guide decision making in cancer treatment. PLoS Med. 2016, 13, e1002189. [Google Scholar] [CrossRef] [PubMed]
- Caffo, O. Treatment sequencing in oncology: Balancing clinical trial and real-world evidence. Future Oncol. 2019, 15, 2887–2889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cunningham, J.J.; Brown, J.S.; Gatenby, R.A. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat. Commun. 2017, 8, 1816. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Silva, A.S.; Gillies, R.J.; Frieden, B.R. Adaptive therapy. Cancer Res. 2009, 69, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A. A change of strategy in the war on cancer. Nature 2009, 459, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Read, A.F.; Day, T.; Huijben, S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S2), 10871–10877. [Google Scholar] [CrossRef]
- Silva, A.S.; Kam, Y.; Khin, Z.P.; Minton, S.E.; Gillies, R.J.; Gatenby, R.A. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res. 2012, 72, 6362–6370. [Google Scholar] [CrossRef]
- Colijn, C.; Cohen, T. How competition governs whether moderate or aggressive treatment minimizes antibiotic resistance. eLife 2015, 4, e10559. [Google Scholar] [CrossRef]
- Day, T.; Read, A.F. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLoS Comput. Biol. 2016, 12, e1004689. [Google Scholar] [CrossRef]
- Enriquez-Navas, P.M.; Kam, Y.; Das, T.; Hassan, S.; Silva, A.; Foroutan, P.; Ruiz, E.; Martinez, G.; Minton, S.; Gillies, R.J.; et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci. Transl. Med. 2016, 8, 327ra24. [Google Scholar] [CrossRef]
- Gjini, E.; Brito, P.H. Integrating antimicrobial therapy with host immunity to fight drug-resistant infections: Classical vs. adaptive treatment. PLoS Comput. Biol. 2016, 12, e1004857. [Google Scholar] [CrossRef]
- Bacevic, K.; Noble, R.; Soffar, A.; Wael Ammar, O.; Boszonyik, B.; Prieto, S.; Vincent, C.; Hochberg, M.E.; Krasinska, L.; Fisher, D. Spatial competition constrains resistance to targeted cancer therapy. Nat. Commun. 2017, 8, 1995. [Google Scholar] [CrossRef]
- Hansen, E.; Woods, R.J.; Read, A.F. How to use a chemotherapeutic agent when resistance to it threatens the patient. PLoS Biol. 2017, 15, e2001110. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Karslake, J.; Woods, R.J.; Read, A.F.; Wood, K.B. Antibiotics can be used to contain drug-resistant bacteria by maintaining sufficiently large sensitive populations. PLoS Biol. 2020, 18, e3000713. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Higano, C.S.; Shore, N.D.; Hussain, M.; Petrylak, D.P. Treating patients with metastatic castration resistant prostate cancer: A comprehensive review of available therapies. J. Urol. 2015, 194, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, P.; De Bono, J.S.; Fizazi, K.; Freedland, S.J.; Grilli, M.; Kantoff, P.W.; Sonpavde, G.; Sternberg, C.N.; Yegnasubramanian, S.; Antonarakis, E.S. Update on systemic prostate cancer therapies: Management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur. Urol. 2019, 75, 88–99. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Dinh, M.N.; Brown, J.S.; Zhang, J.; Anderson, A.R.; Gatenby, R.A. Multidrug cancer therapy in metastatic castrate-resistant prostate cancer: An evolution-based strategy. Clin. Cancer Res. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- West, J.; You, L.; Zhang, J.; Gatenby, R.A.; Brown, J.S.; Newton, P.K.; Anderson, A.R.A. Towards Multidrug Adaptive Therapy. Cancer Res. 2020, 80, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Teply, B.A.; Wang, H.; Luber, B.; Sullivan, R.; Rifkind, I.; Bruns, A.; Spitz, A.; DeCarli, M.; Sinibaldi, V.; Pratz, C.F.; et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: An open-label, phase 2, multicohort study. Lancet Oncol. 2018, 19, 76–86. [Google Scholar] [CrossRef]
- Leone, G.; Buttigliero, C.; Pisano, C.; Di Stefano, R.F.; Tabbò, F.; Turco, F.; Vignani, F.; Scagliotti, G.V.; Di Maio, M.; Tucci, M. Bipolar androgen therapy in prostate cancer: Current evidences and future perspectives. Crit. Rev. Oncol. Hematol. 2020, 152, 102994. [Google Scholar] [CrossRef]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Jiang, Y.Z.; Chen, S.; Cao, Z.G.; Wu, J.; Shen, Z.Z.; Shao, Z.M. Effect of large tumor size on cancer-specific mortality in node-negative breast cancer. Mayo Clin. Proc. 2012, 87, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Colzani, E.; Johansson, A.L.V.; Liljegren, A.; Foukakis, T.; Clements, M.; Adolfsson, J.; Hall, P.; Czene, K. Time-dependent risk of developing distant metastasis in breast cancer patients according to treatment, age and tumour characteristics. Br. J. Cancer 2014, 110, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, V.; Nipp, R.D.; Ryan, D.P.; Hong, T.S.; Nguyen, P.L.; Wo, J.Y. Association between very small tumor size and increased cancer-specific mortality in node-positive colon cancer. Dis. Colon Rectum 2016, 59, 187–193. [Google Scholar] [CrossRef]
- Han, K.; Kim, E.K.; Kwak, J.Y. 1.5–2 cm tumor size was not associated with distant metastasis and mortality in small thyroid cancer: A population-based study. Sci. Rep. 2017, 7, 46298. [Google Scholar] [CrossRef]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Foo, J.; Michor, F. Evolution of resistance to anti-cancer therapy during general dosing schedules. J. Theor. Biol. 2010, 263, 179–188. [Google Scholar] [CrossRef][Green Version]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Baumgart, S.J.; Haendler, B. Exploiting epigenetic alterations in prostate cancer. Int. J. Mol. Sci. 2017, 18, 1017. [Google Scholar] [CrossRef]
- Nakazawa, M.; Paller, C.; Kyprianou, N. Mechanisms of therapeutic resistance in prostate cancer. Curr. Oncol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef]
- Ruggero, K.; Farran-Matas, S.; Martinez-Tebar, A.; Aytes, A. Epigenetic regulation in prostate cancer progression. Curr. Mol. Biol. Rep. 2018, 4, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bozic, I.; Nowak, M.A. Timing and heterogeneity of mutations associated with drug resistance in metastatic cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15964–15968. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.; Michor, F. Evolution of acquired resistance to anti-cancer therapy. J. Theor. Biol. 2014, 355, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1665–1671. [Google Scholar] [CrossRef]
- Green, S.M.; Mostaghel, E.A.; Nelson, P.S. Androgen action and metabolism in prostate cancer. Mol. Cell. Endocrinol. 2012, 360, 3–13. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef]
- Cunningham, J.J.; Brown, J.S.; Gatenby, R.A.; Staňková, K. Optimal control to develop therapeutic strategies for metastatic castrate resistant prostate cancer. J. Theor. Biol. 2018, 459, 67–78. [Google Scholar] [CrossRef]
- Hansen, E.; Read, A.F. Cancer therapy: Attempt cure or manage drug resistance? Evol. Appl. 2020, 13, 1660–1672. [Google Scholar] [CrossRef]
- Karzai, F.H.; Madan, R.A.; Figg, W.D. Beyond PSA: Managing modern therapeutic options in metastatic castration-resistant prostate cancer. South. Med. J. 2015, 108, 224–228. [Google Scholar] [CrossRef]
- Studer, U.E.; Whelan, P.; Wimpissinger, F.; Casselman, J.; de Reijke, T.M.; Knönagel, H.; Loidl, W.; Isorna, S.; Sundaram, S.K.; Collette, L. Differences in time to disease progression do not predict for cancer-specific survival in patients receiving immediate or deferred androgen-deprivation therapy for prostate cancer: Final results of EORTC randomized trial 30891 with 12 years of follow-up. Eur. Urol. 2014, 66, 829–838. [Google Scholar] [CrossRef]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Drabovich, A.P.; Jarvi, K.A.; Diamandis, E.P. Mechanisms of androgen-independent prostate cancer. Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2014, 25, 42–54. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).