Multimodal Treatment in Metastatic Colorectal Cancer (mCRC) Improves Outcomes—The University College London Hospital (UCLH) Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Systemic Therapy
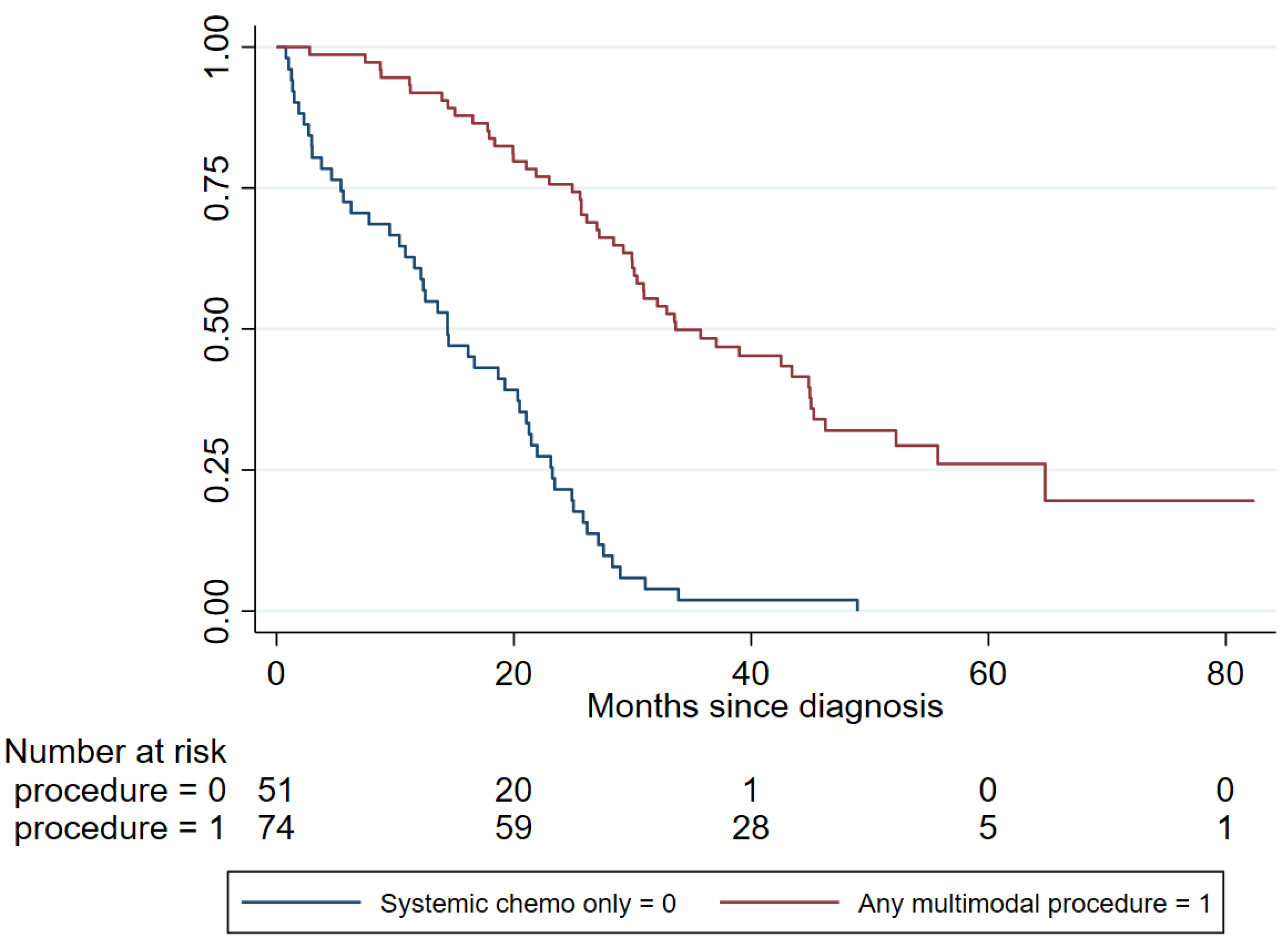
2.3. Main Outcomes of Multimodal Therapy
2.4. Liver-Specific Outcomes
2.5. Lung-Specific Outcomes
2.6. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. Data Collection
4.3. Statistical Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Research UK. Available online: https://cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer (accessed on 30 March 2020).
- Office for National Statistics. Cancer Survival by Stage at Diagnosis for England. Available online: https://www.ons.gov.uk/ (accessed on 30 March 2020).
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Feo, L.; Polcino, M.; Nash, G.M. Resection of the Primary Tumor in Stage IV Colorectal Cancer: When Is It Necessary? Surg. Clin. N. Am. 2017, 97, 657–669. [Google Scholar] [CrossRef]
- Adam, R.; Vinet, E. Regional treatment of metastasis: Surgery of colorectal liver metastases. Ann Oncol. 2004, 15 (Suppl. 4), iv103–iv106. [Google Scholar] [CrossRef]
- Marshall, J.L. Managing potentially resectable metastatic colon cancer. Gastrointest. Cancer Res. 2008, 2 (Suppl. 4), S23–S26. [Google Scholar]
- Walasek, T.; Reinfuss, M.; Kurzynski, M. Brain metastasis from colorectal carcinoma. Clinical picture, treatment and prognosis. Oncol. Radiother. 2018, 2, 11–16. [Google Scholar]
- Santini, D.; Tampellini, M.; Vincenzi, B. Natural history of bone metastasis in colorectal cancer: Final results of a large Italian bone metastases study. Ann. Oncol. 2012, 23, 2072–2077. [Google Scholar] [CrossRef]
- Weiser, M.R.; Jarnagin, W.R.; Saltz, L.B. Colorectal Cancer Patients with Oligometastatic Liver Disaese: What is the Optimal Approach? Oncology 2013, 27, 1074–1078. [Google Scholar]
- Zhang, G.Q.; Taylor, J.P.; Stem, M.; Almaazmi, H.; Efron, J.E.; Atallah, C.; Safar, B. Aggressive Multimodal Treatment and Metastatic Colorectal Cancer Survival. J. Am. Coll. Surg. 2020, 230, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Lowes, M.; Kleiss, M.; Lueck, R.; Detken, S.; Koenig, A.; Nietert, M.; Beissbarth, T.; Stanek, K.; Langer, C.; Ghadimi, M.; et al. The utilization of multidisciplinary tumor boards (MDT) in clinical routine: Results of a health care research study focusing on patients with metastasized colorectal cancer. Int. J. Colorectal Dis. 2017, 32, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Afrăsânie, V.A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Paduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer—Practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Office for National Statistics. Cancer Survival by Stage at Diagnosis for England (Experimental Statistics): Adults Diagnosed 2012, 2013 and 2014 and Followed up to 2015. 2016. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalbystageatdiagnosisforenglandexperimentalstatistics/adultsdiagnosed20122013and2014andfollowedupto2015 (accessed on 13 October 2020).
- Weiss, M.J.; D’Angelica, M.I. Patient selection for hepatic resection for metastatic colorectal cancer. J. Gastrointest. Oncol. 2012, 3, 3–10. [Google Scholar] [PubMed]
- Clarke, C.N.; Kopetz, E.S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest. Oncol. 2015, 6, 660–667. [Google Scholar] [PubMed]
- Villeneuve, P.J.; Sundaresan, R.S. Surgical management of colorectal lung metastasis. Clin. Colon Rectal Surg. 2009, 22, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef]
- Pfannschmidt, J.; Dienemann, H.; Hoffmann, H. Surgical resection of pulmonary metastases from colorectal cancer: A systematic review of published series. Ann. Thorac. Surg. 2007, 84, 324–338. [Google Scholar] [CrossRef]
- Serrano, P.E.; Gu, C.-S.; Husien, M.; Jalink, D.; Ritter, A.; Martel, G.; Tsang, M.E.; Law, C.H.; Hallet, J.; McAlistter, V.; et al. Risk factors for survival following recurrence after first liver resection for colorectal cancer liver metastases. J. Surg. Oncol. 2019, 120, 1420–1426. [Google Scholar] [CrossRef]
- Adam, R.; Pascal, G.; Azoulay, D.; Tanaka, K.; Castaing, D.; Bismuth, H. Liver resection for colorectal metastases: The third hepatectomy. Ann. Surg. 2003, 238, 871–883; discussion 83–84. [Google Scholar] [CrossRef]
- Pędziwiatr, M.; Mizera, M.; Witowski, J.; Major, P.; Torbicz, G.; Gajewska, N.; Budzynski, A. Primary tumor resection in stage IV unresectable colorectal cancer: What has changed? Med. Oncol. 2017, 34, 188. [Google Scholar]
- Lam-Boer, J.; Mol, L.; Verhoef, C.; de Haan, A.F.; Yilmaz, M.; Punt, C.J.; de Wilt, J.H.W.; Koopman, M. The CAIRO4 study: The role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer—A randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer 2014, 14, 741. [Google Scholar]
- Ruers, T.; Punt, C.; Van Coevorden, F.; Pierie, J.P.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.A.; Mauer, M.; et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: A randomized EORTC Intergroup phase II study (EORTC 40004). Ann. Oncol. 2012, 23, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Ruers, T.; Punt, C.J.A.; van Coevorden, F.; Pierie, J.-P.; Borel Rinkes, I.; Ledermann, J.A.; Poston, G.J.; Bechstein, W.O.; Lentz, M.-A.; Mauer, M.E.; et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): Long-term survival results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). J. Clin. Oncol. 2015, 33 (Suppl. 15), 3501. [Google Scholar] [CrossRef]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Characteristic | Number (Percentage) |
|---|---|
| Gender | |
| Male | 70 (56%) |
| Female | 55 (44%) |
| Age, years | |
| Median + IQR | 62 (55–68) |
| Range | 19–89 |
| Side of Primary | |
| Left | 82 (66%) |
| Right | 43 (34%) |
| KRAS Mutation | |
| Mutant | 48 (47%) |
| Wild Type | 54 (53%) |
| Missing | 23 |
| BRAF Mutation | |
| Mutant | 10 (10%) |
| Wild Type | 86 (90%) |
| Missing | 29 |
| Carcinoembryonic Antigen CEA | |
| Elevated (range) | 49 (77%) (5.4–28326) |
| Normal | 15 (13%) |
| Missing | 61 |
| Location of Metastases | |
| Liver-Only | 55 (44%) |
| Lung-Only | 9 (7%) |
| Multiple Sites (>1) | 61 (49%) |
| Adjuvant therapy | |
| Yes | 23 (180%) |
| No | 102 (82%) |
| Neoadjuvant therapy | |
| Yes | 27 (22%) |
| No | 98 (78%) |
| Number of Lines Palliative Chemotherapy | |
| 0 | 23 (18%) |
| 1 | 37 (30%) |
| 2 | 17 (14%) |
| 3 | 24 (19%) |
| ≥4 | 24 (19%) |
| Number of Multimodal Procedures | |
| 0 | 51 (40.8%) |
| 1 | 35 (28%) |
| 2 | 17 (13.6%) |
| ≥3 | 22 (17.6%) |
| Characteristic | Number of Events/Subjects | Adjusted Hazard Ratio (95% CI) ** | p-Value | |
|---|---|---|---|---|
| Age | ≤70 years | 102/125 | 0.94 (0.58–1.52) | 0.79 |
| >70 years | 23/125 | |||
| Gender | Female | 55/125 | 1.00 (0.67–1.49) | 0.99 |
| Male | 70/125 | |||
| Sidedness | Right | 43/125 | 0.77 (0.51–1.15) | 0.51 |
| Left | 82/125 | |||
| Liver-only metastases | No | 70/125 | 0.84 (0.56–1.27) | 0.41 |
| Yes | 55/125 | |||
| Lung-only metastases | No | 116/125 | 0.195 (0.06–0.60) | 0.005 |
| Yes | 9/125 | |||
| KRAS/NRAS | Wild-type | 46/98 | 0.79 (0.49–1.27) | 0.33 |
| mutant | 52/98 | |||
| BRAF status | Wild-type | 86/96 | 1.47 (0.70–3.11) | 0.31 |
| mutant | 10/96 | |||
| Neo+/−adjuvant chemo received | No | 83/125 | 0.26 (0.16–0.42) | <0.0001 |
| Yes | 42/125 | |||
| Systemic chemotherapy only | No | 74/125 | 5.46 (3.49–8.54) | <0.001 |
| Yes | 51/125 | |||
| Primary procedure | No | 8/74 | 0.50 (0.23–1.08) | 0.08 |
| Yes | 66/74 ¥ | |||
| Metastatic surgery | No | 32/74 | 0.36 (0.20–0.63) | <0.001 |
| Yes | 42/74 ¥ | |||
| Liver surgery | No | 41/74 | 0.54 (0.30–0.97) | <0.05 |
| Yes | 33/74 ¥ | |||
| Lung surgery | No | 65/74 | 0.14 (0.03–0.61) | 0.008 |
| Yes | 9/74 ¥ | |||
| Other non-surgical metastatic procedure * | No | 54/70 | 0.66 (0.33–1.32) | 0.24 |
| Yes | 20/74 ¥ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joharatnam-Hogan, N.; Wilson, W.; Shiu, K.K.; Fusai, G.K.; Davidson, B.; Hochhauser, D.; Bridgewater, J.; Khan, K. Multimodal Treatment in Metastatic Colorectal Cancer (mCRC) Improves Outcomes—The University College London Hospital (UCLH) Experience. Cancers 2020, 12, 3545. https://doi.org/10.3390/cancers12123545
Joharatnam-Hogan N, Wilson W, Shiu KK, Fusai GK, Davidson B, Hochhauser D, Bridgewater J, Khan K. Multimodal Treatment in Metastatic Colorectal Cancer (mCRC) Improves Outcomes—The University College London Hospital (UCLH) Experience. Cancers. 2020; 12(12):3545. https://doi.org/10.3390/cancers12123545
Chicago/Turabian StyleJoharatnam-Hogan, Nalinie, William Wilson, Kai Keen Shiu, Giuseppe Kito Fusai, Brian Davidson, Daniel Hochhauser, John Bridgewater, and Khurum Khan. 2020. "Multimodal Treatment in Metastatic Colorectal Cancer (mCRC) Improves Outcomes—The University College London Hospital (UCLH) Experience" Cancers 12, no. 12: 3545. https://doi.org/10.3390/cancers12123545
APA StyleJoharatnam-Hogan, N., Wilson, W., Shiu, K. K., Fusai, G. K., Davidson, B., Hochhauser, D., Bridgewater, J., & Khan, K. (2020). Multimodal Treatment in Metastatic Colorectal Cancer (mCRC) Improves Outcomes—The University College London Hospital (UCLH) Experience. Cancers, 12(12), 3545. https://doi.org/10.3390/cancers12123545







