Hürthle Cells on Fine-Needle Aspiration Cytology Are Important for Risk Assessment of Focally PET/CT FDG Avid Thyroid Nodules
Abstract
:Simple Summary
Abstract
1. Introduction
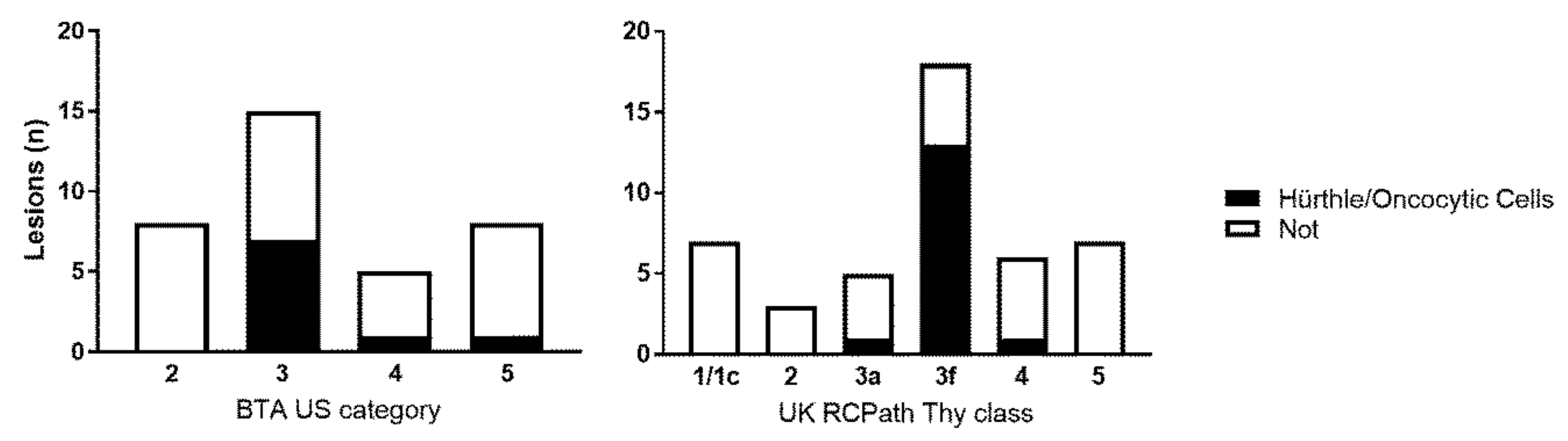
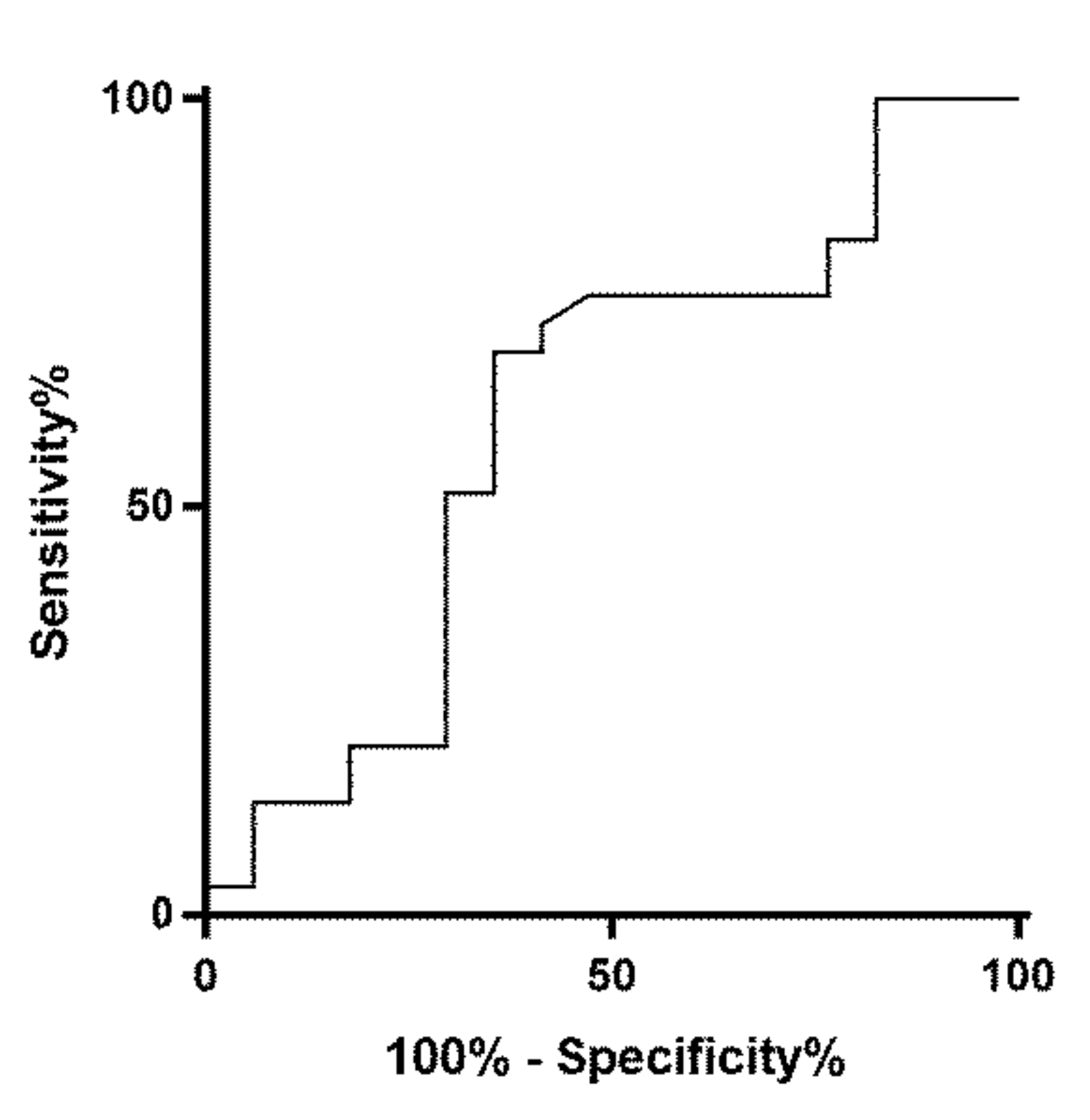
2. Results
3. Discussion
4. Methods
4.1. Patients and Clinical Characteristics
4.2. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Townsend, D. History and future technical innovation in positron emission tomography. J. Med. Imaging (Bellingham) 2017, 4, 011013. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Weaver, J.; Ngu, R.; Krishnamurthy Mohan, H. Clinical significance of patterns of incidental thyroid uptake at (18)F-FDG PET/CT. Clin. Radiol. 2015, 70, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L. My approach to oncocytic tumours of the thyroid. J. Clin. Pathol. 2004, 57, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maximo, V.; Lima, J.; Prazeres, H.; Soares, P.; Sobrinho-Simoes, M. The biology and the genetics of Hurthle cell tumors of the thyroid. Endocr. Relat. Cancer 2016, 23, X2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesland, J.M.; Sobrinho-Simoes, M.A.; Holm, R.; Sambade, M.C.; Johannessen, J.V. Hurthle-cell lesions of the thyroid: A combined study using transmission electron microscopy, scanning electron microscopy, and immunocytochemistry. Ultrastruct. Pathol. 1985, 8, 269–290. [Google Scholar] [CrossRef]
- Dardick, I.; Claude, A.; Parks, W.R.; Hoppe, D.; Stinson, J.; Burns, B.F.; Little, J.; Brown, D.L.; Dairkee, S.H. Warthin’s tumor: An ultrastructural and immunohistochemical study of basilar epithelium. Ultrastruct. Pathol. 1988, 12, 419–432. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Parsons, M.; Torigian, D.A.; Zhuang, H.; Alavi, A. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl. Med. Commun. 2009, 30, 240–244. [Google Scholar] [CrossRef]
- Nishimori, H.; Tabah, R.; Hickeson, M.; How, J. Incidental thyroid “PETomas”: Clinical significance and novel description of the self-resolving variant of focal FDG-PET thyroid uptake. Can. J. Surg. 2011, 54, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Soelberg, K.K.; Bonnema, S.J.; Brix, T.H.; Hegedus, L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: A systematic review. Thyroid 2012, 22, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard Ba, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81 (Suppl. S1), 1–122. [Google Scholar] [CrossRef] [PubMed]
- De Koster, E.J.; de Geus-Oei, L.F.; Dekkers, O.M.; van Engen-van Grunsven, I.; Hamming, J.; Corssmit, E.P.M.; Morreau, H.; Schepers, A.; Smit, J.; Oyen, W.J.G.; et al. Diagnostic Utility of Molecular and Imaging Biomarkers in Cytological Indeterminate Thyroid Nodules. Endocr. Rev. 2018, 39, 154–191. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Puntoni, M.; Dezzana, M.; Bottoni, G.; Foppiani, L.; Marugo, A.; Catrambone, U.; Ugolini, M.; Sola, S.; Gatto, M.; et al. Indeterminate thyroid nodules. The role of (18)F-FDG PET/CT in the “era” of ultrasonography risk stratification systems and new thyroid cytology classifications. Endocrine 2020, 69, 553–561. [Google Scholar] [CrossRef]
- Ali, S.Z.; Cibas, E.S. The Bethesda System for Reporting Thyroid Cytopathology. Definitions, Criteria, and Explanatory Notes, 2nd ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Poller, D.N.; Baloch, Z.W.; Fadda, G.; Johnson, S.J.; Bongiovanni, M.; Pontecorvi, A.; Cochand-Priollet, B. Thyroid FNA: New classifications and new interpretations. Cancer Cytopathol. 2016, 124, 457–466. [Google Scholar] [CrossRef]
- Pathak, K.A.; Goertzen, A.L.; Nason, R.W.; Klonisch, T.; Leslie, W.D. A prospective cohort study to assess the role of FDG-PET in differentiating benign and malignant follicular neoplasms. Ann. Med. Surg. 2016, 12, 27–31. [Google Scholar] [CrossRef]
- Muñoz Pérez, N.; Villar del Moral, J.M.; Muros Fuentes, M.A.; López de la Torre, M.; Arcelus Martínez, J.I.; Becerra Massare, P.; Esteva Martínez, D.; Cañadas Garre, M.; Coll Del Rey, E.; Bueno Laraño, P.; et al. Could 18F-FDG-PET/CT avoid unnecessary thyroidectomies in patients with cytological diagnosis of follicular neoplasm? Langenbecks Arch. Surg. 2013, 398, 709–716. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Kloppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC WHO: Lyon, France, 2017. [Google Scholar]
- Granter, S.R.; Cibas, E.S. Cytologic findings in thyroid nodules after 131I treatment of hyperthyroidism. Am. J. Clin. Pathol. 1997, 107, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Montone, K.T.; Baloch, Z.W.; LiVolsi, V.A. The thyroid Hurthle (oncocytic) cell and its associated pathologic conditions: A surgical pathology and cytopathology review. Arch. Pathol. Lab. Med. 2008, 132, 1241–1250. [Google Scholar] [CrossRef]
- Wakely, P.E., Jr. Oncocytic and oncocyte-like lesions of the head and neck. Ann. Diagn. Pathol. 2008, 12, 222–230. [Google Scholar] [CrossRef]
- Poller, D.N.; Johnson, S.J.; Bongiovanni, M. Measures to reduce diagnostic error and improve clinical decision making in thyroid FNA aspiration cytology: A proposed framework. Cancer Cytopathol. 2020. [Google Scholar] [CrossRef]
- Goffredo, P.; Roman, S.A.; Sosa, J.A. Hurthle cell carcinoma: A population-level analysis of 3311 patients. Cancer 2013, 119, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.; Kameyama, K.; Ito, K.; Nagahama, M.; Kitagawa, W.; Shibuya, H.; Ohkuwa, K.; Uruno, T.; Akaishi, J.; Suzuki, A.; et al. Does Hurthle cell carcinoma of the thyroid have a poorer prognosis than ordinary follicular thyroid carcinoma? Ann. Surg. Oncol. 2013, 20, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Shaha, A.R.; Loree, T.R.; Shah, J.P. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery 1995, 118, 1131–1136. [Google Scholar] [CrossRef]
- Samaan, N.A.; Maheshwari, Y.K.; Nader, S.; Hill, C.S., Jr.; Schultz, P.N.; Haynie, T.P.; Hickey, R.C.; Clark, R.L.; Goepfert, H.; Ibanez, M.L.; et al. Impact of therapy for differentiated carcinoma of the thyroid: An analysis of 706 cases. J. Clin. Endocrinol. Metab. 1983, 56, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 1998, 83, 2638–2648. [Google Scholar] [CrossRef]
- Kushchayeva, Y.; Duh, Q.Y.; Kebebew, E.; D’Avanzo, A.; Clark, O.H. Comparison of clinical characteristics at diagnosis and during follow-up in 118 patients with Hurthle cell or follicular thyroid cancer. Am. J. Surg. 2008, 195, 457–462. [Google Scholar] [CrossRef]
- Shaha, A.R.; Shah, J.P.; Loree, T.R. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am. J. Surg. 1996, 172, 692–694. [Google Scholar] [CrossRef]
- Poller, D.N.; Bongiovanni, M.; Trimboli, P. Risk of malignancy in the various categories of the UK Royal College of Pathologists Thy Terminology for Thyroid FNA cytology: A systematic review and meta-analysis. Cancer Cytopathol. 2019. [CrossRef]
- Cross, P.; Chandra, A.; Giles, T.; Johnson, S.; Kocjan, G.; Poller, D.; Stephenson, T. Guidance on the Reporting of Thyroid Cytology Specimens, 2nd ed.; Royal College of Pathologists: London, UK, 2016. [Google Scholar]
- Al-Chalabi, H.; Karthik, S.; Vaidyanathan, S. Radiological-pathological correlation of the British Thyroid Association ultrasound classification of thyroid nodules: A real-world validation study. Clin. Radiol. 2019, 74, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Castellana, M.; Virili, C.; Paone, G.; Scappaticcio, L.; Piccardo, A.; Giovanella, L.; Trimboli, P. Ultrasound systems for risk stratification of thyroid nodules prompt inappropriate biopsy in autonomously functioning thyroid nodules. Clin. Endocrinol. 2020, 93, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, A.; Di Stasio, E.; Guglielmi, R.; Bizzarri, G.; Taccogna, S.; Misischi, I.; Graziano, F.; Petrucci, L.; Bianchini, A.; Papini, E. Predictive Value of Malignancy of Thyroid Nodule Ultrasound Classification Systems: A Prospective Study. J. Clin. Endocrinol. Metab. 2018, 103, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Fulciniti, F.; Zilioli, V.; Ceriani, L.; Giovanella, L. Accuracy of international ultrasound risk stratification systems in thyroid lesions cytologically classified as indeterminate. Diagn. Cytopathol. 2017, 45, 113–117. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poller, D.N.; Megadmi, H.; Ward, M.J.A.; Trimboli, P. Hürthle Cells on Fine-Needle Aspiration Cytology Are Important for Risk Assessment of Focally PET/CT FDG Avid Thyroid Nodules. Cancers 2020, 12, 3544. https://doi.org/10.3390/cancers12123544
Poller DN, Megadmi H, Ward MJA, Trimboli P. Hürthle Cells on Fine-Needle Aspiration Cytology Are Important for Risk Assessment of Focally PET/CT FDG Avid Thyroid Nodules. Cancers. 2020; 12(12):3544. https://doi.org/10.3390/cancers12123544
Chicago/Turabian StylePoller, David N., Hakim Megadmi, Matthew J. A. Ward, and Pierpaolo Trimboli. 2020. "Hürthle Cells on Fine-Needle Aspiration Cytology Are Important for Risk Assessment of Focally PET/CT FDG Avid Thyroid Nodules" Cancers 12, no. 12: 3544. https://doi.org/10.3390/cancers12123544
APA StylePoller, D. N., Megadmi, H., Ward, M. J. A., & Trimboli, P. (2020). Hürthle Cells on Fine-Needle Aspiration Cytology Are Important for Risk Assessment of Focally PET/CT FDG Avid Thyroid Nodules. Cancers, 12(12), 3544. https://doi.org/10.3390/cancers12123544




