A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats
Abstract
:Simple Summary
Abstract
1. Introduction
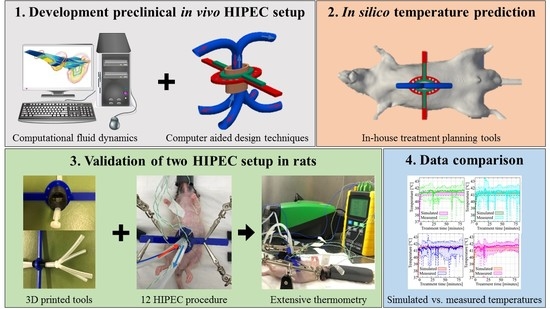
2. Material and Methods
2.1. Animal Model
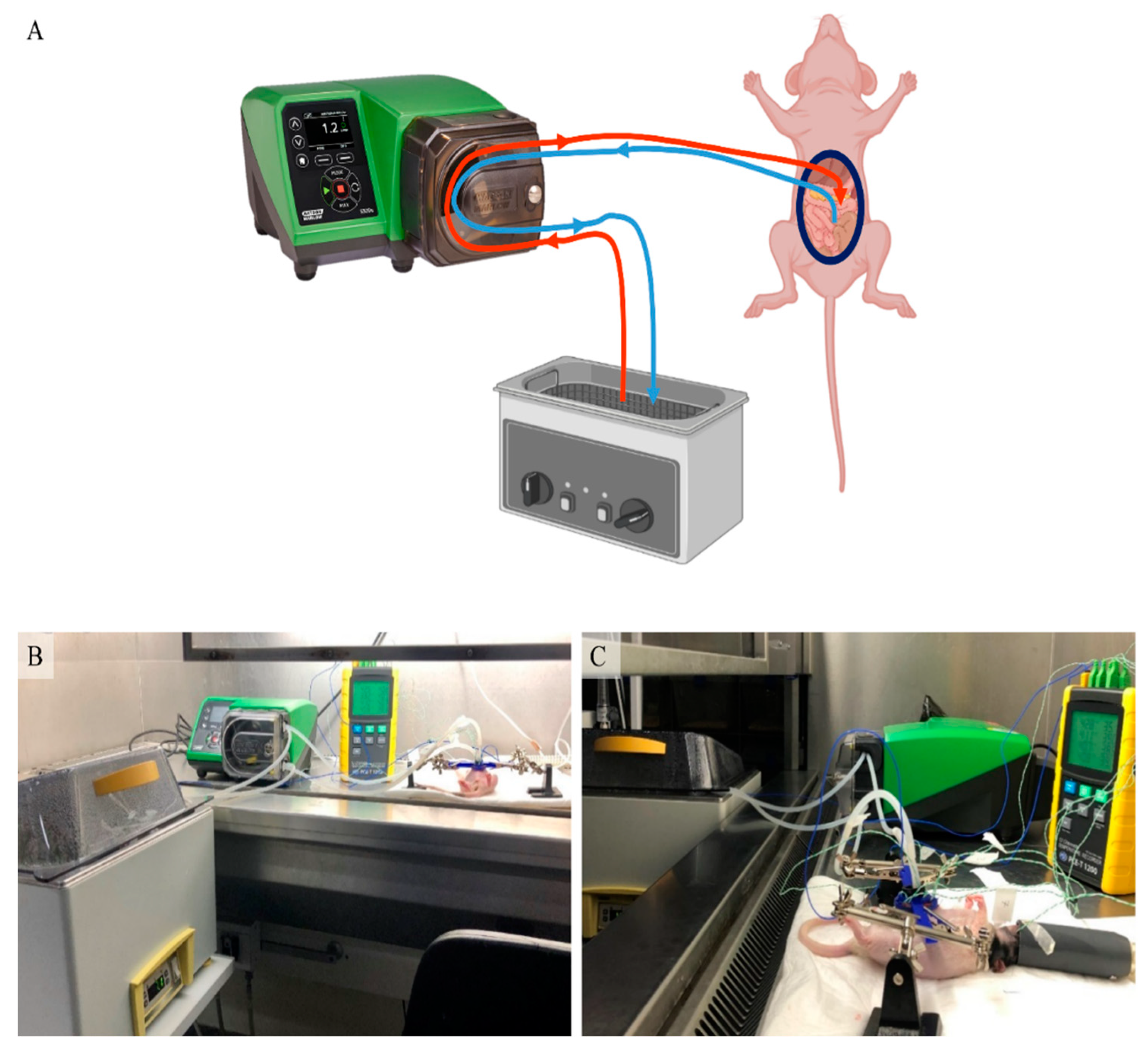
2.2. HIPEC Setup
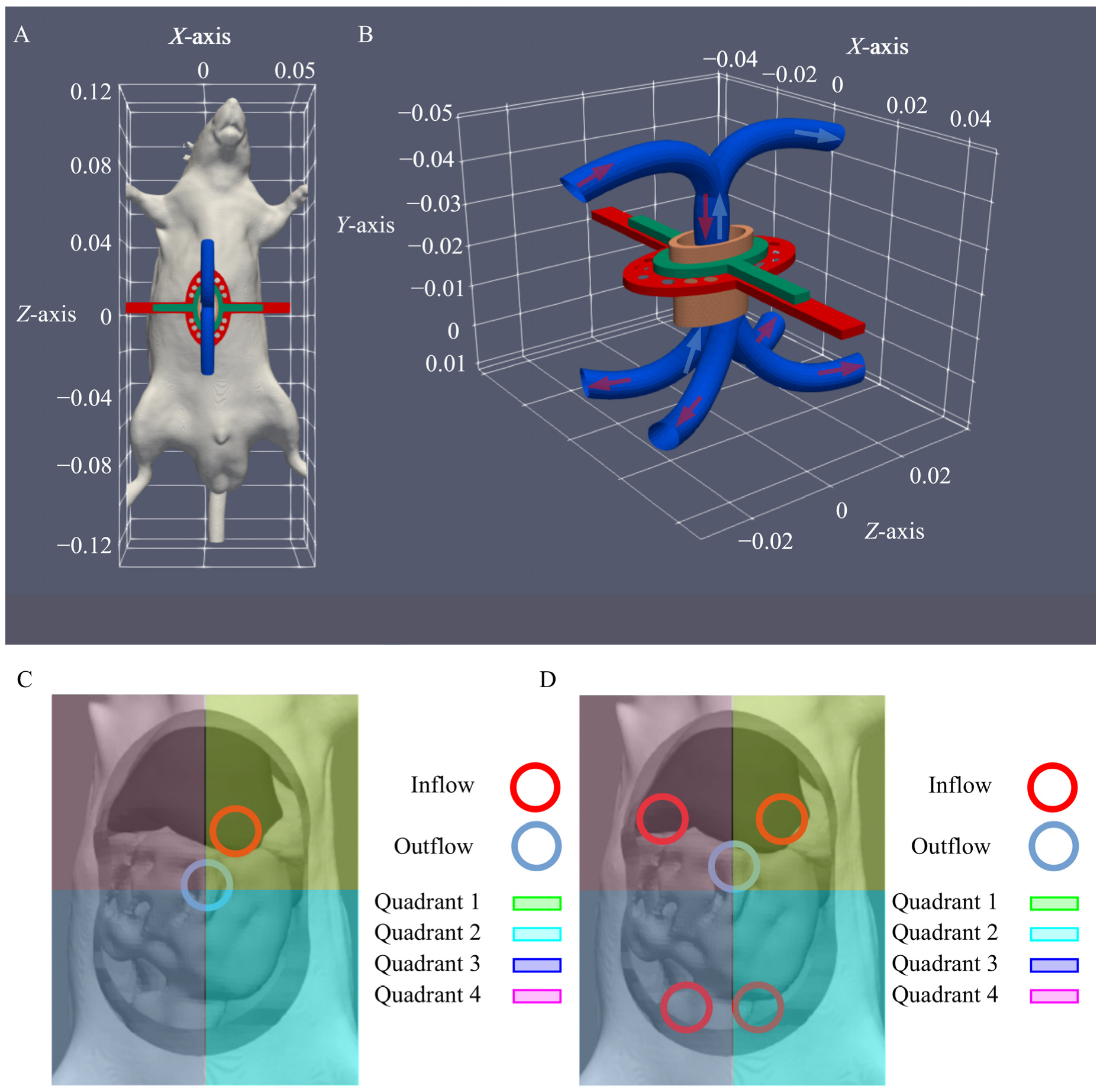
2.3. Inflow Apparatus
2.4. In Silico Flow Rate Exploration
2.5. HIPEC Procedure
2.6. Supportive Care
3. Results
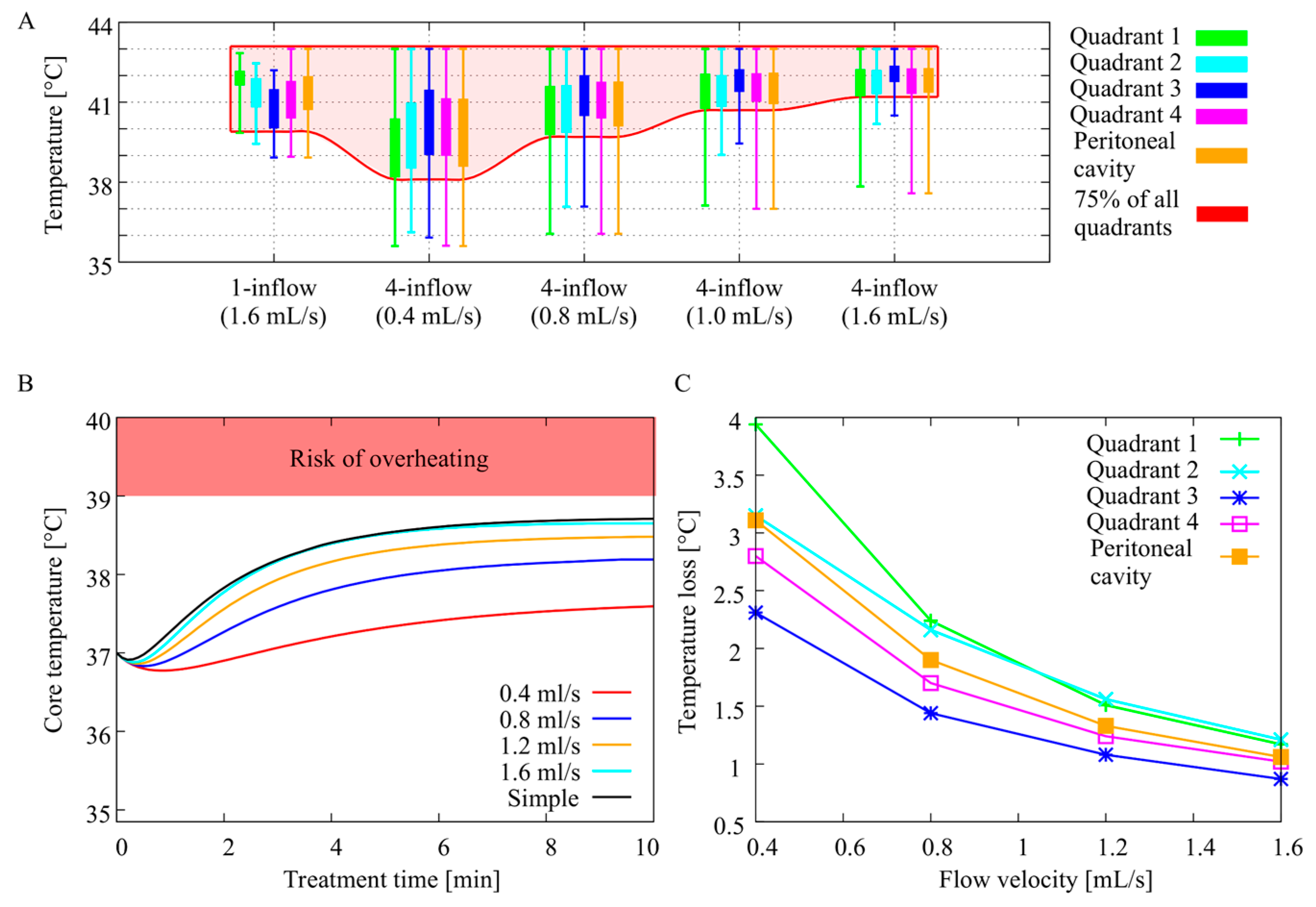
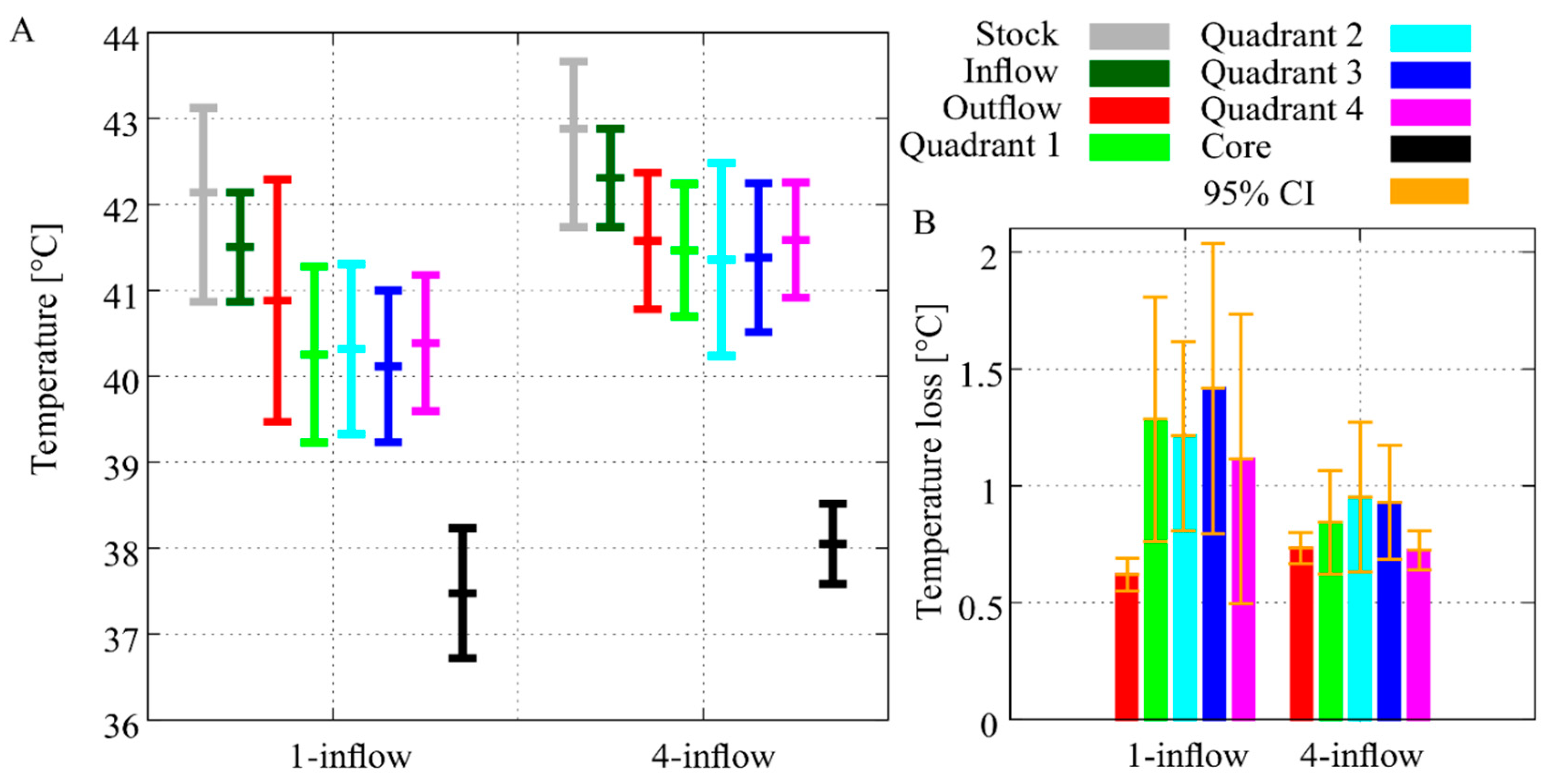
3.1. In Silico Flow Rate Exploration
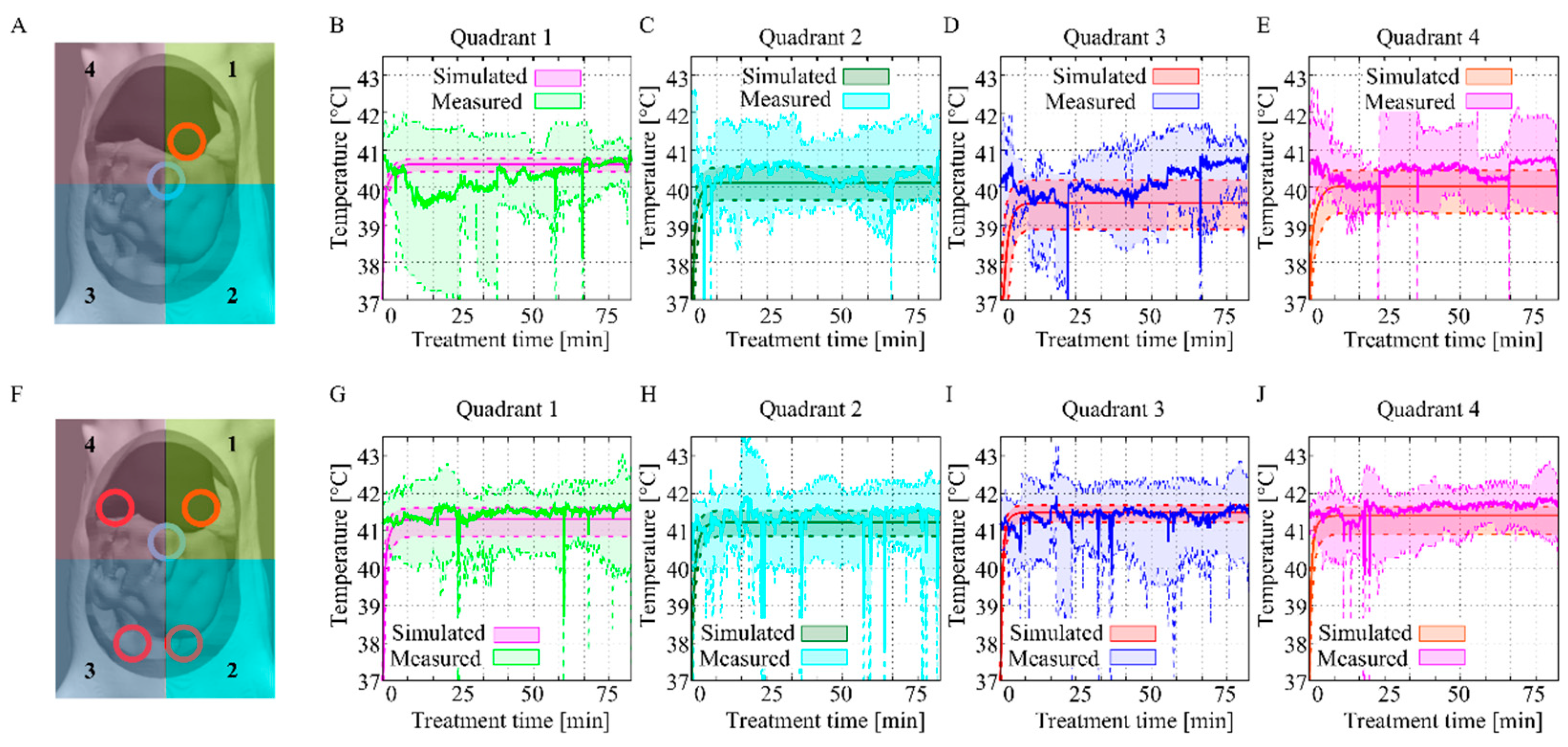
3.2. In Vivo Temperature Distribution
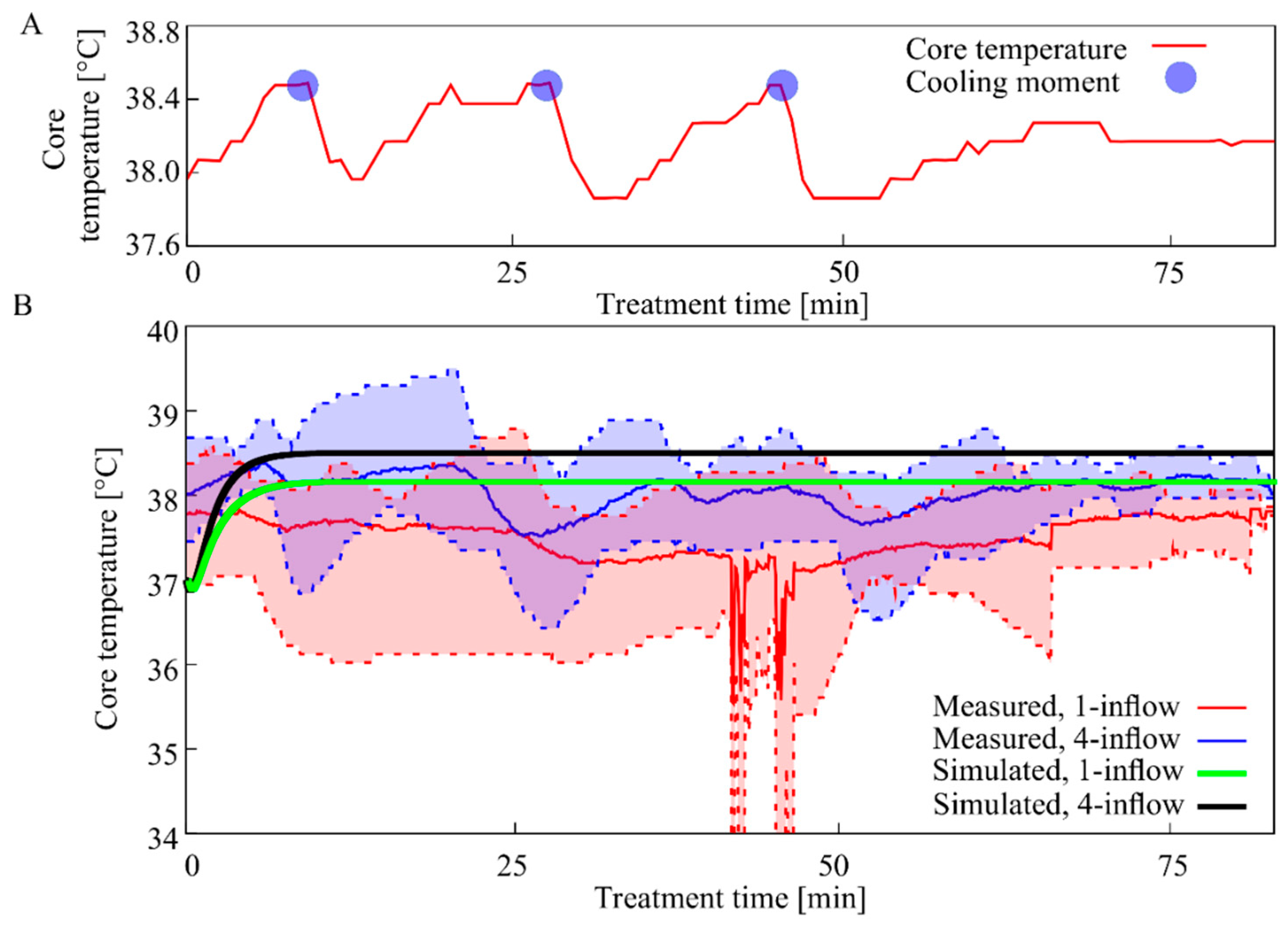
3.3. Core Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flanagan, M.; Solon, J.; Chang, K.; Deady, S.; Moran, B.; Cahill, R.; Shields, C.; Mulsow, J. Peritoneal metastases from extra-abdominal cancer—A population-based study. Eur. J. Surg. Oncol. 2018, 44, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.P.; Moustarah, F. Cancer, Peritoneal Metastasis; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Sugarbaker, P.H. Surgical management of peritoneal carcinosis: Diagnosis, prevention and treatment. Langenbeck’s Arch. Surg. 1988, 373, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Helderman, R.F.; Löke, D.R.; Kok, H.P.; Oei, A.L.; Tanis, P.J.; Franken, N.A.P.; Crezee, H. Variation in Clinical Application of Hyperthermic Intraperitoneal Chemotherapy: A Review. Cancers 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, S.; Yamamoto, H.; Shimizu, T.; Naitoh, H.; Yamaguchi, T.; Kaida, S.; Takebayashi, K.; Miyake, T.; Tani, T.; Tani, M. 5-fluorouracil combined with cisplatin and mitomycin C as an optimized regimen for hyperthermic intraperitoneal chemotherapy in gastric cancer. J. Surg. Oncol. 2018, 117, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Urano, M.; Ling, C.C. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int. J. Hyperth. 2002, 18, 307–315. [Google Scholar] [CrossRef]
- Kjellström, J.; Kjellén, E.; Johnsson, A. In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncol. 2005, 44, 687–693. [Google Scholar] [CrossRef]
- Ubink, I.; Bolhaqueiro, A.C.F.; Elias, S.G.; Raats, D.A.E.; Constantinides, A.; Peters, N.A.; Wassenaar, E.C.E.; De Hingh, I.H.J.T.; Rovers, K.P.; Van Grevenstein, W.M.U.; et al. Organoids from colorectal peritoneal metastases as a platform for improving hyperthermic intraperitoneal chemotherapy. BJS 2019, 106, 1404–1414. [Google Scholar] [CrossRef]
- Jung, H. Interaction of thermotolerance and thermosensitization induced in CHO cells by combined hyperthermic treatments at 40 and 43 degrees C. Radiat. Res. 1982, 91, 433–446. [Google Scholar] [CrossRef]
- Van Der Heijden, A.G.; Jansen, C.F.; Verhaegh, G.; O’Donnell, M.A.; Schalken, J.A.; Witjes, J.A. The Effect of Hyperthermia on Mitomycin-C Induced Cytotoxicity in Four Human Bladder Cancer Cell Lines. Eur. Urol. 2004, 46, 670–674. [Google Scholar] [CrossRef]
- Helderman, R.F.; Löke, D.R.; Verhoeff, J.; Rodermond, H.M.; Van Bochove, G.G.; Boon, M.; Van Kesteren, S.; Vallejo, J.J.G.; Kok, H.P.; Tanis, P.J.; et al. The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines. Cells 2020, 9, 1775. [Google Scholar] [CrossRef]
- Lemoine, L.; Thijssen, E.; Carleer, R.; Cops, J.; Lemmens, V.; Van Eyken, P.; Sugarbaker, P.; Van Der Speeten, K. Body surface area-based versus concentration-based intraperitoneal perioperative chemotherapy in a rat model of colorectal peritoneal surface malignancy: Pharmacologic guidance towards standardization. Oncotarget 2019, 10, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Pelz, J.; Doerfer, J.; Hohenberger, W.; Meyer, T. A new survival model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing rats in the treatment of peritoneal carcinomatosis. BMC Cancer 2005, 5, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, K.; Rickenbacher, A.; Jang, J.-H.; Oberkofler, C.E.; Vonlanthen, R.; Von Boehmer, L.; Humar, B.; Graf, R.; Gertsch, P.; Clavien, P.-A. New Insight into Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. 2012, 256, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.B.; Hendriks, T.; Lomme, R.M.L.M.; Rutten, H.J.T.; Bleichrodt, R.P.; De Hingh, I.H.J.T. Intraoperative versus Early Postoperative Intraperitoneal Chemotherapy after Cytoreduction for Colorectal Peritoneal Carcinomatosis: An Experimental Study. Ann. Surg. Oncol. 2011, 19, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Maeta, M.; Koga, S. Influence of local hyperthermia on the healing of small intestinal anastomoses in the rat. BJS 1991, 78, 57–59. [Google Scholar] [CrossRef]
- Sánchez-García, S.; Padilla-Valverde, D.; Villarejo-Campos, P.; García-Santos, E.P.; Martín-Fernández, J. Hyperthermic chemotherapy intra-abdominal laparoscopic approach: Development of a laparoscopic model using CO2 recirculation system and clinical translation in peritoneal carcinomatosis. Int. J. Hyperth. 2017, 33, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-García, S.; Padilla, D.; Villarejo-Campos, P.; Martín-Fernández, J.; García-Rojo, M.; Rodríguez-Martínez, M. Experimental development of an intra-abdominal chemohyperthermia model using a closed abdomen technique and a PRS-1.0 Combat CO2 recirculation system. Surgery 2014, 155, 719–725. [Google Scholar] [CrossRef]
- Gesson-Paute, A.; Ferron, G.; Thomas, F.; De Lara, E.C.; Chatelut, E.; Querleu, D. Pharmacokinetics of Oxaliplatin During Open Versus Laparoscopically Assisted Heated Intraoperative Intraperitoneal Chemotherapy (HIPEC): An Experimental Study. Ann. Surg. Oncol. 2007, 15, 339–344. [Google Scholar] [CrossRef]
- Rettenmaier, M.A.; Mendivil, A.A.; Gray, C.M.; Chapman, A.P.; Stone, M.K.; Tinnerman, E.J.; Goldstein, B.H. Intra-abdominal temperature distribution during consolidation hyperthermic intraperitoneal chemotherapy with carboplatin in the treatment of advanced stage ovarian carcinoma. Int. J. Hyperth. 2015, 31, 396–402. [Google Scholar] [CrossRef]
- Kok, H.P.; Beck, M.; Löke, D.R.; Helderman, R.F.C.P.A.; Van Tienhoven, G.; Ghadjar, P.; Wust, P.; Crezee, H. Locoregional peritoneal hyperthermia to enhance the effectiveness of chemotherapy in patients with peritoneal carcinomatosis: A simulation study comparing different locoregional heating systems. Int. J. Hyperth. 2020, 37, 76–88. [Google Scholar] [CrossRef]
- Schooneveldt, G.; Löke, D.R.; Zweije, R.; Helderman, R.F.; Kok, H.P.; Crezee, H. Experimental validation of a thermophysical fluid model for use in a hyperthermia treatment planning system. Int. J. Heat Mass Transf. 2020, 152, 119495. [Google Scholar] [CrossRef]
- Löke, D.R.; Helderman, R.F.C.P.A.; Franken, N.A.P.; Oei, A.L.; Crezee, J.; Kok, H.P. Simulating drug penetration during hyperthermic intraperitoneal chemotherapy. Drug Deliv. 2020. under review. [Google Scholar]
- Löke, D.R.; Helderman, R.F.C.P.A.; Rodermond, H.M.; Tanis, P.J.; Streekstra, G.J.; Franken, N.A.P.; Oei, A.L.; Crezee, J.; Kok, H.P. Demonstration of treatment planning software for hyperthermic intraperitoneal chemotherapy in a rat model. Int. J. Hyperth. 2020, in press. [Google Scholar]
- Ribes, A.; Caremoli, C. Salomé platform component model for numberical simulation. In Proceedings of the 31st Annual International Computer Software and Applications Confernce (COMPSAC 2007), Beijing, China, 24–27 July 2007; pp. 553–564. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; Van Slooten, G.; Van Tinteren, H. 8-Year Follow-up of Randomized Trial: Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy in Patients with Peritoneal Carcinomatosis of Colorectal Cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J. Clin. Oncol. 2018, 36, LBA3503. [Google Scholar] [CrossRef]
- Ceelen, W.P. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur. J. Surg. Oncol. 2019, 45, 400–402. [Google Scholar] [CrossRef]
- Nagourney, R.A.; Evans, S.; Tran, P.H.; Nagourney, A.J.; Sugarbaker, P.H. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Yonemura, Y.; De Aretxabala, X.; Fujimura, T.; Fushida, S.; Katayama, K.; Bandou, E.; Sugiyama, K.; Kawamura, T.; Kinoshita, K.; Endou, Y.; et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: Final results of a randomized controlled study. Hepatogastroenterology 2002, 48, 1776–1782. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löke, D.R.; Helderman, R.F.C.P.A.; Sijbrands, J.; Rodermond, H.M.; Tanis, P.J.; Franken, N.A.P.; Oei, A.L.; Kok, H.P.; Crezee, J. A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats. Cancers 2020, 12, 3516. https://doi.org/10.3390/cancers12123516
Löke DR, Helderman RFCPA, Sijbrands J, Rodermond HM, Tanis PJ, Franken NAP, Oei AL, Kok HP, Crezee J. A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats. Cancers. 2020; 12(12):3516. https://doi.org/10.3390/cancers12123516
Chicago/Turabian StyleLöke, Daan R., Roxan F. C. P. A. Helderman, Jan Sijbrands, Hans M. Rodermond, Pieter J. Tanis, Nicolaas A. P. Franken, Arlene L. Oei, H. Petra Kok, and Johannes Crezee. 2020. "A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats" Cancers 12, no. 12: 3516. https://doi.org/10.3390/cancers12123516
APA StyleLöke, D. R., Helderman, R. F. C. P. A., Sijbrands, J., Rodermond, H. M., Tanis, P. J., Franken, N. A. P., Oei, A. L., Kok, H. P., & Crezee, J. (2020). A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats. Cancers, 12(12), 3516. https://doi.org/10.3390/cancers12123516