Autoantibody Formation and Mapping of Immunogenic Epitopes against Cold-Shock-Protein YB-1 in Cancer Patients and Healthy Controls
Abstract
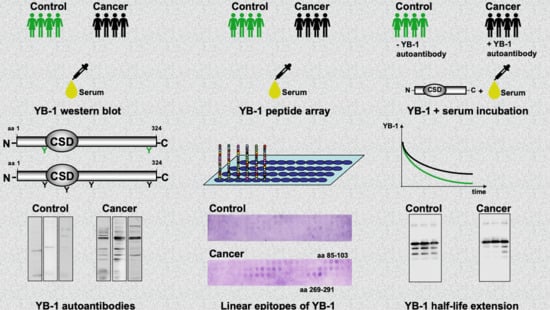
Simple Summary
Abstract
1. Introduction
2. Results
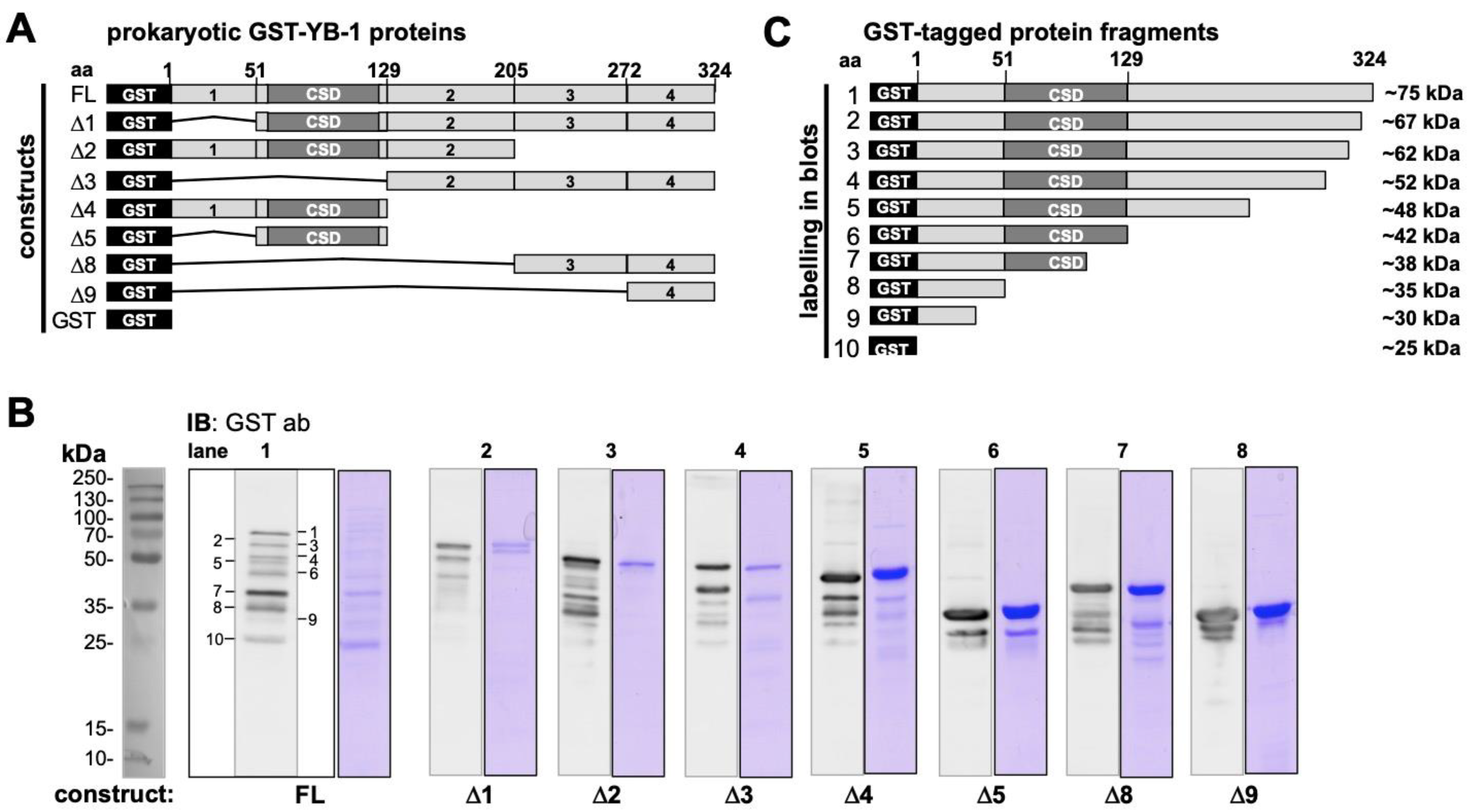
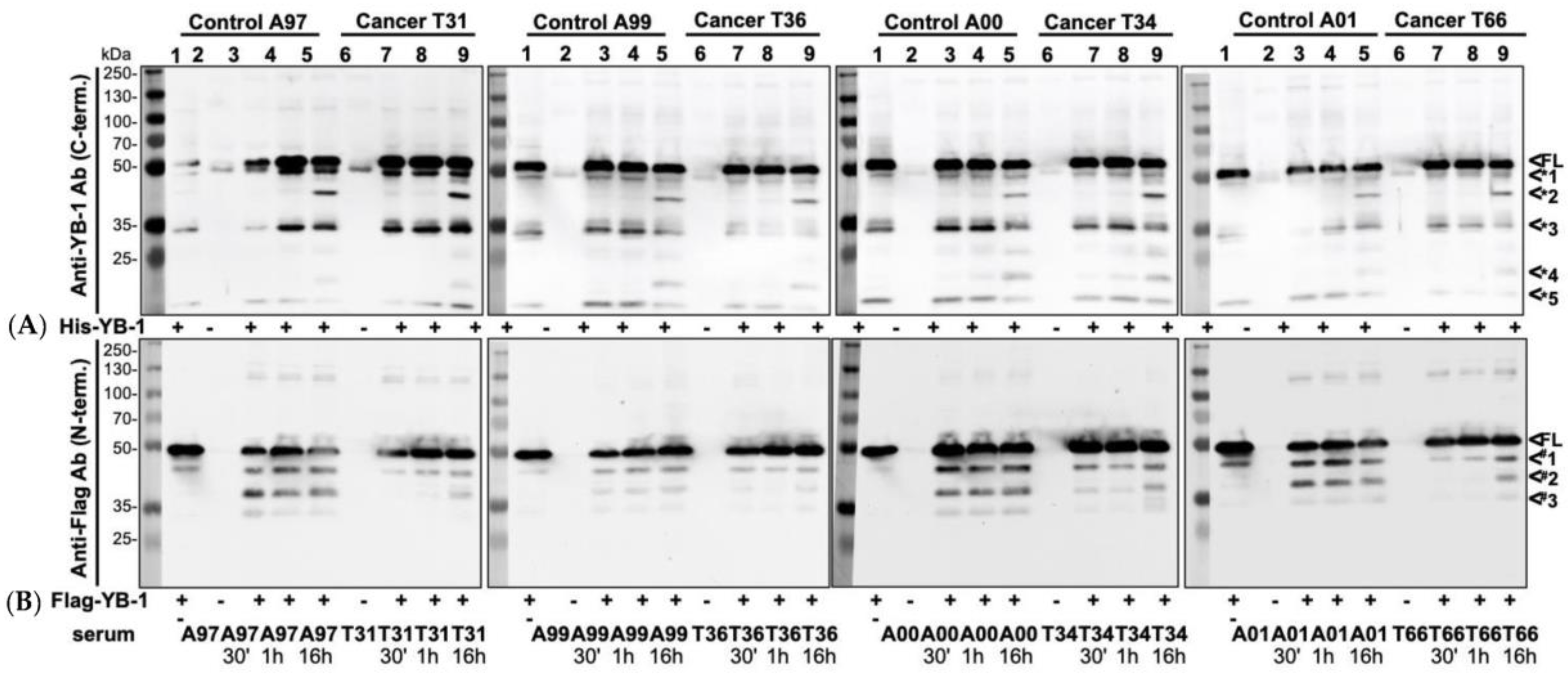
2.1. Recombinant YB-1 Protein Undergoes Spontaneous Cleavage
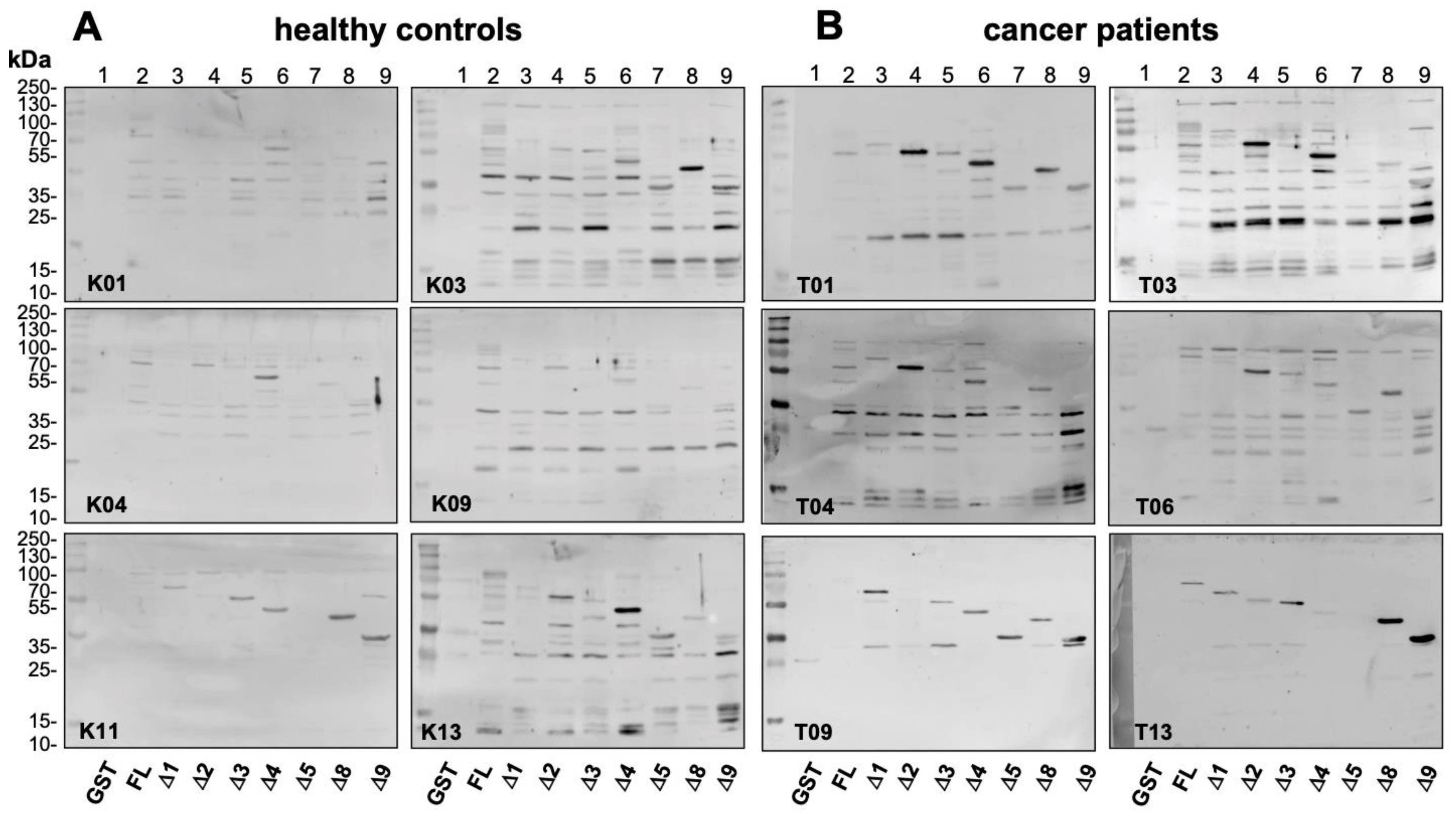
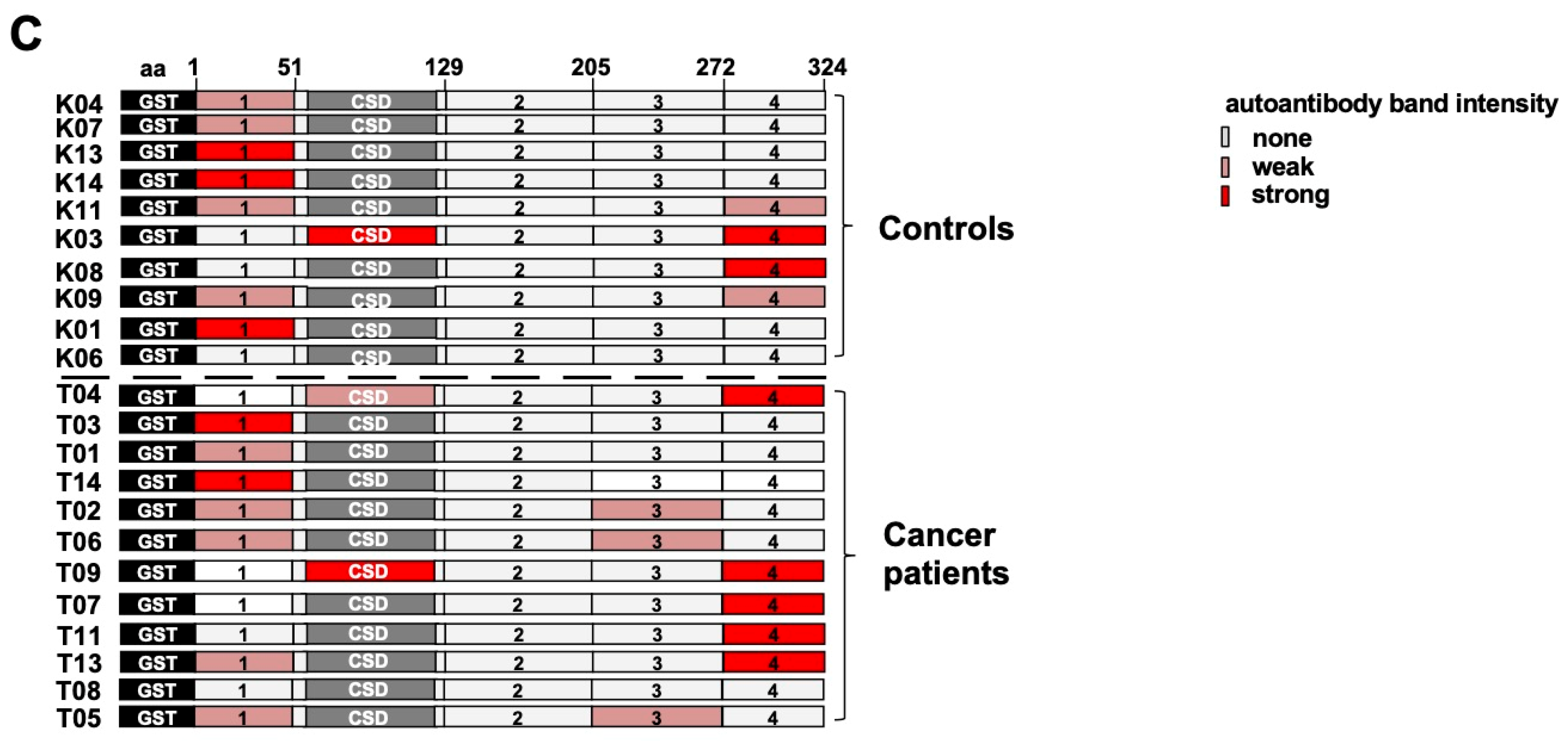
2.2. Autoantibody Pattern against Cold Shock Protein YB-1 Differs between Cancer Patients and Healthy Controls
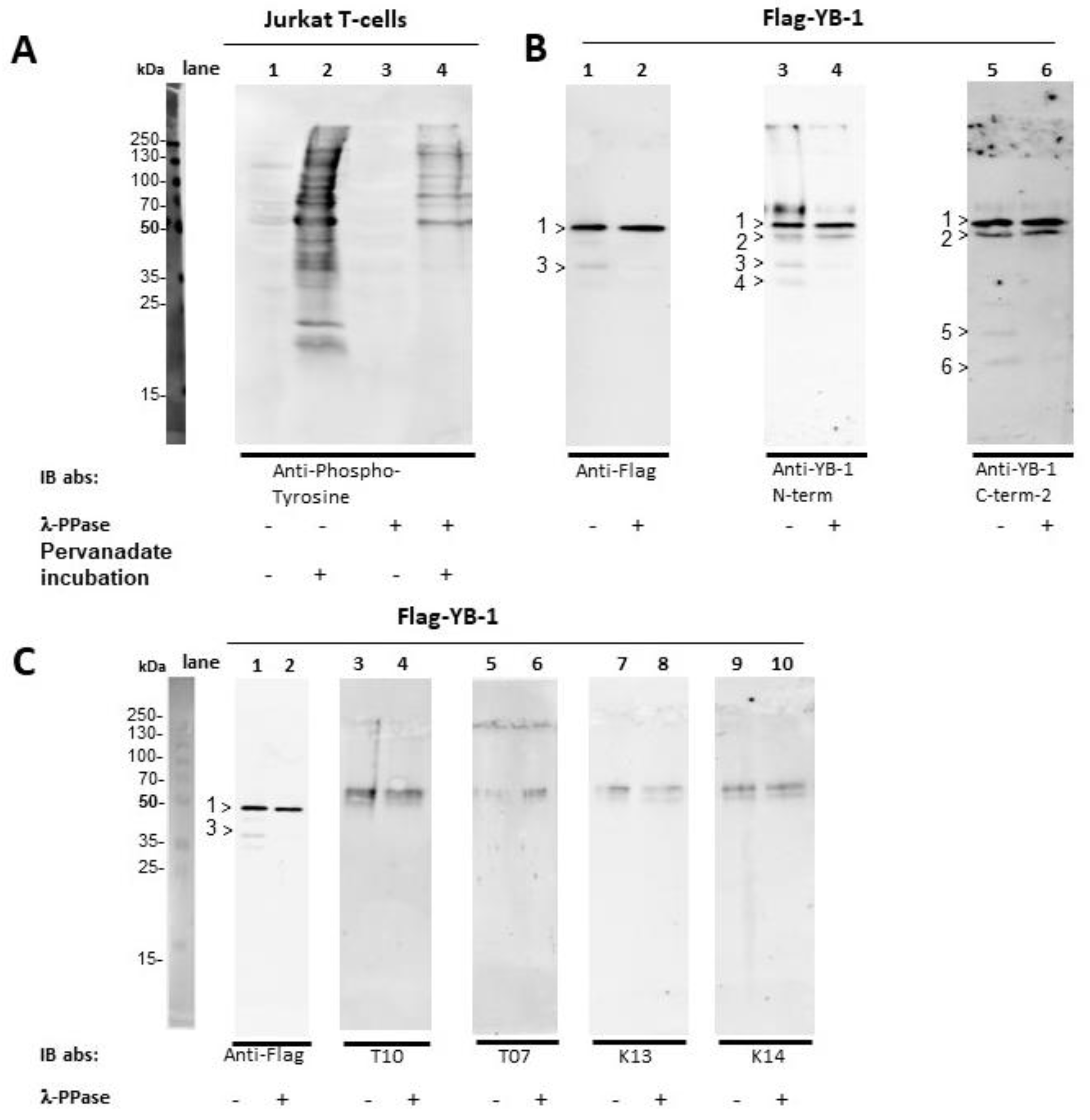
2.3. YB-1 Phosphorylation Does Not Interfere with YB-1 Autoantibody Binding
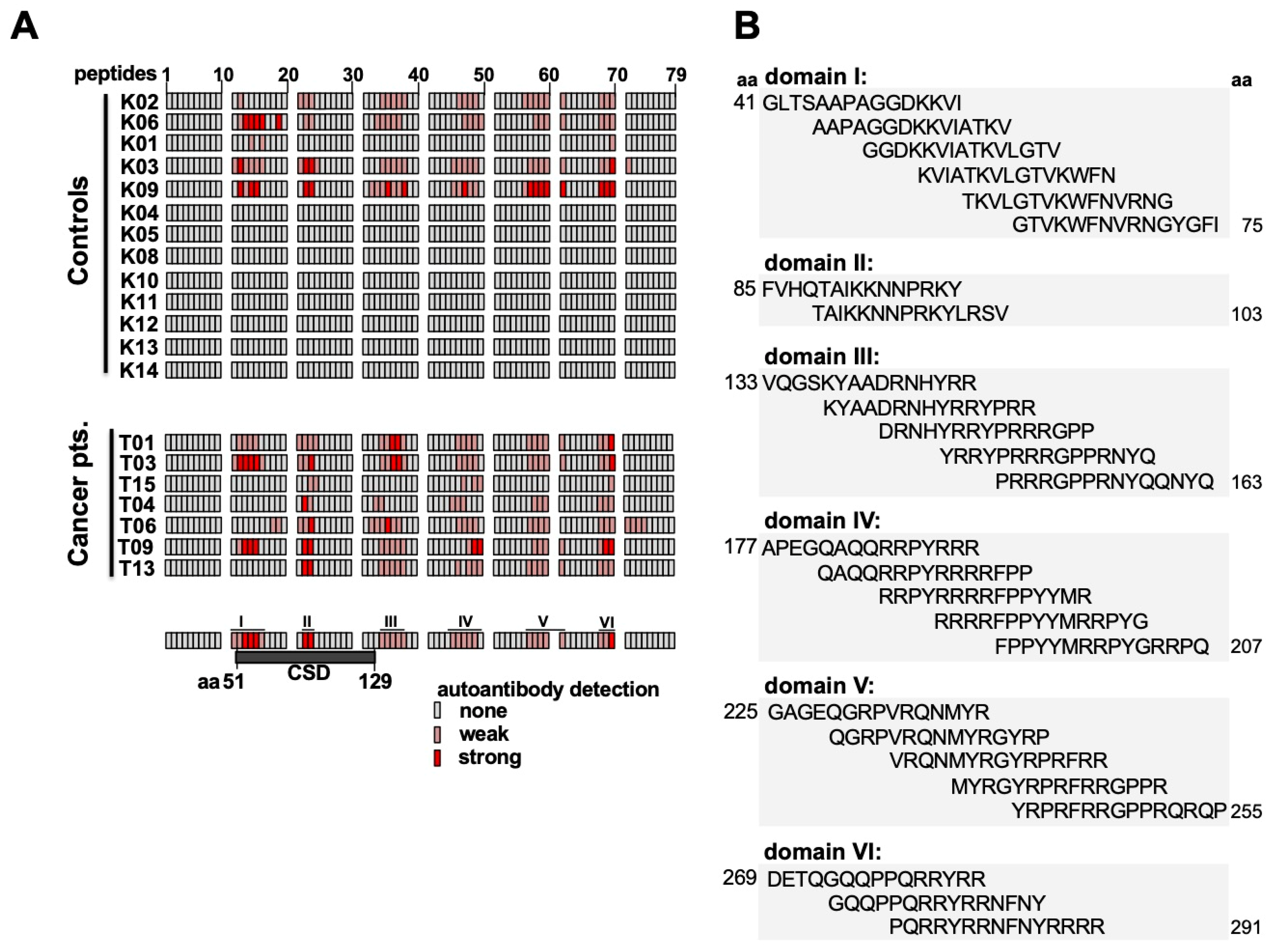
2.4. Epitope Mapping with Abbreviated YB-1 Protein Constructs and YB-1 Peptide Array
2.5. Endogenous YB-1 Serum Levels Do Not Differ between Healthy Controls and Cancer Patients; However, Addition of Recombinant Protein Reveals Retarded Degradation in Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Materials
4.2.1. Expression and Purification of Prokaryotic and Eukaryotic YB-1 Proteins
4.2.2. YB-1 Autoantibody Detection in Serum and Plasma Samples from Healthy Volunteers and Cancer Patients
4.2.3. YB-1 Dephosphorylation
4.2.4. Peptide Array
4.2.5. YB-1 Immunoblotting
4.2.6. YB-1 Cleavage Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maurya, P.K.; Mishra, A.; Yadav, B.S.; Singh, S.; Kumar, P.; Chaudhary, A.; Srivastava, S.; Murugesan, S.N.; Mani, A. Role of Y Box Protein-1 in cancer: As potential biomarker and novel therapeutic target. J. Cancer 2017, 8, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Brandt, S.; Bernhardt, A.; Gorny, X.; Schindele, D.; Jandrig, B.; Schostak, M.; Isermann, B.; Lindquist, J.A.; Mertens, P.R. Crosstalk between Akt signaling and cold shock proteins in mediating invasive cell phenotypes. Oncotarget 2018, 9, 19039–19049. [Google Scholar] [CrossRef] [PubMed]
- Lovett, D.H.; Cheng, S.; Cape, L.; Pollock, A.S.; Mertens, P.R. YB-1 alters MT1-MMP trafficking and stimulates MCF-7 breast tumor invasion and metastasis. Biochem. Biophys. Res. Commun. 2010, 398, 482–488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- En-Nia, A.; Yilmaz, E.; Klinge, U.; Lovett, D.H.; Stefanidis, I.; Mertens, P.R. Transcription factor YB-1 mediates DNA polymerase alpha gene expression. J. Biol. Chem. 2005, 280, 7702–7711. [Google Scholar] [CrossRef] [PubMed]
- Ise, T.; Nagatani, G.; Imamura, T.; Kato, K.; Takano, H.; Nomoto, M.; Izumi, H.; Ohmori, H.; Okamoto, T.; Ohga, T.; et al. Transcription factor Y-box binding protein 1 binds preferentially to cisplatin modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 1999, 59, 342–346. [Google Scholar]
- Sakura, H.; Maekawa, T.; Imamoto, F.; Yasuda, K.; Ishii, S. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene 1988, 73, 499–507. [Google Scholar]
- Lyabin, D.N.; Eliseeva, I.A.; Ovchinnikov, L.P. YB-1 protein: Functions and regulation. Wiley Interdiscip. Rev. RNA 2014, 5, 95–110. [Google Scholar] [CrossRef]
- Rauen, T.; Raffetseder, U.; Frye, B.C.; Djudjaj, S.; Mühlenberg, P.J.; Eitner, F.; Lendahl, U.; Bernhagen, J.; Dooley, S.; Mertens, P.R. YB-1 acts as a ligand for Notch-3 receptors and modulates receptor activation. J. Biol. Chem. 2009, 284, 26928–26940. [Google Scholar] [CrossRef]
- Frye, B.C.; Halfter, S.; Djudjaj, S.; Muehlenberg, P.; Weber, S.; Raffetseder, U.; En-Nia, A.; Knott, H.; Baron, J.M.; Dooley, S.; et al. Y-box protein-1 is actively secreted through a non-classical pathway and acts as an extracellular mitogen. EMBO Rep. 2009, 10, 783–789. [Google Scholar] [CrossRef]
- Tacke, F.; Galm, O.; Kanig, N.; Yagmur, E.; Brandt, S.; Lindquist, J.A.; Eberhardt, C.S.; Raffetseder, U.; Mertens, P.R. High prevalence of Y-box protein-1/p18 fragment in plasma of patients with malignancies of different origin. BMC Cancer 2014, 14. [Google Scholar] [CrossRef]
- Tacke, F.; Kanig, N.; En-Nia, A.; Kaehne, T.; Eberhardt, C.S.; Shpacovitch, V.; Trautwein, C.; Mertens, P.R. Y-box protein-1/p18 fragment identifies malignancies in patients with chronic liver disease. BMC Cancer 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Fehr, A.; Brandt, S.; Jerchel, S.; Ballhause, T.M.; Philipsen, L.; Stolze, S.; Geffers, R.; Wenig, H.; Fischer, K.D.; et al. Inflammatory cell infiltration and resolution of kidney inflammation is orchestrated by the cold-shock protein Y-box binding protein-1. Kidney Int. 2017, 92, 1157–1177. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; En-Nia, A.; Wiesmann, F.; Krings, R.; Djudjaj, S.; Breuer, E.; Fuchs, T.; Wild, P.J.; Hartmann, A.; Dunn, S.E.; et al. Nuclear detection of Y-box protein-1 (YB-1) closely associates with progesterone receptor negativity and is a strong adverse survival factor in human breast cancer. BMC Cancer 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Janz, M.; Harbeck, N.; Dettmar, P.; Berger, U.; Schmidt, A.; Jürchott, K.; Schmitt, M.; Royer, H.D. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI1. Int. J. Cancer 2002, 97, 278–282. [Google Scholar] [CrossRef]
- Jeoung, D.; Lee, E.B.; Lee, S.; Lim, Y.; Lee, D.Y.; Kim, J.; Kim, H.Y.; Song, Y.W. Autoantibody to DNA binding protein B as a novel serologic marker in systemic sclerosis. Biochem. Biophys. Res. Commun. 2002, 299, 549–555. [Google Scholar] [CrossRef]
- Braunschweig, D.; Krakowiak, P.; Duncanson, P.; Boyce, R.; Hansen, R.L.; Ashwood, P.; Hertz-Picciotto, I.; Pessah, I.N.; Van de Water, J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl. Psychiatry 2013, 3, e277. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Shaheen, A.A.; Baeza, N.; Lytvyak, E.; Urbanski, S.J.; Mason, A.L.; Norman, G.L.; Fritzler, M.J.; Swain, M.G. Evaluation of classical and novel autoantibodies for the diagnosis of Primary Biliary Cholangitis-Autoimmune Hepatitis Overlap Syndrome (PBC-AIH OS). PLoS ONE 2018, 13, e0193960. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Günther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat. Commun. 2018, 9, 5224. [Google Scholar] [CrossRef]
- Silverman, G.J. Regulatory natural autoantibodies to apoptotic cells: Pallbearers and protectors. Arthritis Rheum. 2012, 63, 597–602. [Google Scholar] [CrossRef]
- Evdokimova, V.; Ruzanov, P.; Imataka, H.; Raught, B.; Svitkin, Y.; Ovchinnikov, L.P.; Sonenberg, N. The Major mRNA-associated Protein YB-1 Is a Potent 5′ Cap-Dependent mRNA Stabilizer. EMBO J. 2001, 20, 5491–5502. [Google Scholar] [CrossRef]
- Kim, E.R.; Selyutina, A.A.; Buldakov, I.A.; Evdokimova, V.M.; Ovchinnikov, L.P.; Sorokin, A.V. The proteolytic YB-1 fragment interacts with DNA repair machinery and enhances survival during DNA damaging stress. Cell Cycle 2013, 12, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, J.-S.; Li, S.; Yang, Y.; Sun, P.; Zhu, O.; Wang, J.; Jiang, B.; Yang, D.; Liu, M. Structural basis of DNA binding to human YB-1 cold shock domain regulated by phosphorylation. Nucleic Acids Res. 2020, 48, 9361–9371. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, H.; Jiang, W.; Inouye, M.; Heinemann, U. Crystal structure of CspA, the major cold shock protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 1994, 91, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Evdokimova, V.M.; Wei, C.L.; Sitikov, A.S.; Simonenko, P.N.; Lazarev, O.A.; Vasilenko, K.S.; Ustinov, V.A.; Hershey, J.W.B.; Ovchinnikov, L.P. The Major Protein of Messenger Ribonucleoprotein Particles in Somatic Cells Is a Member of the Y-box Binding Transcription Factor Family. J. Biol. Chem. 1995, 270, 3186–3192. [Google Scholar] [CrossRef]
- Guryanov, S.G.; Filimonov, V.V.; Timchenko, A.A.; Melnik, B.S.; Kihara, H.; Kutyshenko, V.P.; Ovchinnikov, L.P.; Semisotnov, G.V. The major mRNP protein YB-1: Structural and association properties in solution. Biochim. Biophys. Acta 2013, 1834, 559–567. [Google Scholar] [CrossRef]
- Willis, W.L.; Hariharan, S.; David, J.J.; Strauch, A.R. Transglutaminase-2 mediates calcium-regulated crosslinking of the Y-Box 1 (YB-1) translation-regulatory protein in TGFβ1-activated myofibroblasts. J. Cell. Biochem. 2013, 114, 2753–2769. [Google Scholar] [CrossRef]
- Yang, X.-J.; Zhu, H.; Mu, S.-R.; Wei, W.-J.; Yuan, X.; Wang, M.; Liu, Y.; Hui, J.; Huang, Y. Crystal structure of a Y-box binding protein 1 (YB-1)-RNA complex reveals key features and residues interacting with RNA. J. Biol. Chem. 2019. [Google Scholar] [CrossRef]
- Alemasova, E.E.; Pestryakov, P.E.; Sukhanova, M.V.; Kretov, D.A.; Moor, N.A.; Curmi, P.A.; Ovchinnikov, L.P.; Lavrik, O.L. Poly(ADP-ribosyl)ation as a new posttranslational modification of YB-1. Biochimie 2015, 119, 36–44. [Google Scholar] [CrossRef]
- Frank, R. Spot-Synthesis: An easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 1992, 48, 9217–9232. [Google Scholar] [CrossRef]
- Sutherland, B.W.; Kucab, J.; Wu, J.; Lee, C.; Cheang, M.C.; Yorida, E.; Turbin, D.; Dedhar, S.; Nelson, C.; Pollak, M.; et al. Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene 2005, 24, 4281–4292. [Google Scholar] [CrossRef]
- Kloks, C.P.A.M.; Tessari, M.; Vuister, G.W.; Hilbers, C.W. Cold Shock Domain of the Human Y-Box Protein YB-1. Backbone Dynamics and Equilibrium between the Native State and a Partially Unfolded State. Biochemistry 2004, 43, 10237–10246. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.K.; Parsy-Kowalska, C.B.; Chapman, C.J. Autoantibodies: Opportunities for Early Cancer Detection. Trends Cancer 2017, 3, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Zaenker, P.; Gray, E.S.; Ziman, M.R. Autoantibody Production in Cancer—The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun. Rev. 2016, 15, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.J.; Murray, A.; McElveen, J.E.; Sahin, U.; Luxemburger, U.; Türeci, Ö.; Wiewrodt, R.; Barnes, A.C.; Robertson, J.F. Autoantibodies in lung cancer: Possibilities for early detection and subsequent cure. Thorax 2008, 63, 228–233. [Google Scholar] [CrossRef]
- Kobayashi, M.; Katayama, H.; Fahrmann, J.F.; Hanash, S.M. Development of autoantibody signatures for common cancers. Semin. Immunol. 2020, 47, e101388. [Google Scholar] [CrossRef]
- Liu, S.; Tan, Q.; Song, Y.; Shi, Y.; Han, X. Anti-p53 autoantibody in blood as a diagnostic biomarker for colorectal cancer: A meta-analysis. Scand. J. Immunol. 2019, 91, e12829. [Google Scholar] [CrossRef]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Gil, J.; Ramirez-Torres, A.; Encarnación-Guevara, S. Lysine acetylation and cancer: A proteomics perspective. J. Proteom. 2017, 150, 297–309. [Google Scholar] [CrossRef]
- Ming-Jer, Y.; Kai-Cheng, H.; Tony, E.L.; Wen-Chang, C.; Jan-Jong, H. The Role of Ubiquitin-Specific Peptidases in Cancer Progression. J. Biomed. Sci. 2019, 26, 42. [Google Scholar]
- Ewert, L.; Fischer, A.; Brandt, S.; Scurt, F.G.; Philipsen, L.; Müller, A.J.; Girndt, M.; Zenclussen, A.C.; Lindquist, J.A.; Gorny, X.; et al. Cold Shock Y-box Binding protein-1 Acetylation Status in Monocytes Is Associated With Systemic Inflammation and Vascular Damage. Atherosclerosis 2018, 278, 156–165. [Google Scholar] [CrossRef]
- Palicharla, V.R.; Maddika, S. HACE1 Mediated K27 Ubiquitin Linkage Leads to YB-1 Protein Secretion. Cell. Signal. 2015, 27, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Nagele, E.P.; Han, M.; Acharya, N.K.; DeMarshall, C.; Kosciuk, M.C.; Nagele, R.G. Natural IgG Autoantibodies are Abundant and Ubiquitous in Human Sera, and their Number s influenced by Age, Gender, and Disease. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Aburjania, Z.; Jang, S.; Whitt, J.; Jaskula-Stzul, R.; Chen, H.; Rose, J.B. The Role of Notch3 in Cancer. Oncologist 2018, 23, 900–911. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Selyutina, A.A.; Skabkin, M.A.; Guryanov, S.G.; Nazimov, I.V.; Richard, C.; Th’ng, J.; Yau, J.; Sorensen, P.H.B.; Ovchinnikov, L.P.; et al. Proteasome-mediated cleavage of the Y-box-binding protein 1 is linked to DNA-damage stress response. EMBO J. 2005, 24, 3602–3612. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Jing, S.; Bianqin, G.; Ping, L.; Qindong, L.; Chenggui, L.; Feng, C.; Wenbin, K.; Qin, W.; Jinyu, D.; et al. Development of a Chemiluminescence Immunoassay for Serum YB-1 and its Clinical Application as a Potential Diagnostic Marker for Hepatocellular Carcinoma. Hepat. Mon. 2013, 13, e8918. [Google Scholar] [CrossRef]
- Max, K.E.; Zeeb, M.; Bienert, R.; Balbach, J.; Heinemann, U. Common mode of DNA binding to cold shock domains. Crystal structure of hexathymidine bound to the domain-swapped form of a major cold shock protein from Bacillus caldolyticus. FEBS J. 2007, 274, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Budkina, K.S.; Zlobin, N.E.; Kononova, S.V.; Ovchinnikov, L.P.; Babakov, A.V. Cold Shock Domain Proteins: Structure and Interaction with Nucleic Acids. Biochemistry 2020, 85. [Google Scholar] [CrossRef]
- Lasham, A.; Print, C.G.; Woolley, A.G.; Dunn, S.E.; Braithwaite, A.W. YB-1: Oncoprotein, prognostic marker and therapeutic target? Biochem. J. 2013, 449, 11–23. [Google Scholar] [CrossRef]
- Bornhorst, J.A.; Falke, J.J. Purification of Proteins Using Polyhistidine Affinity Tags. Methods Enzmol. 2000, 326, 245–254. [Google Scholar]
- Harper, S.; Speicher, D.W. Purification of proteins fused to glutathione S-tranferase. Methods Mol. Biol. 2011, 681, 259–280. [Google Scholar]
- Kwon, M.; Firestein, B.L. DNA transfection: Calcium phosphate method. Methods Mol. Biol. 2013, 1018, 107–110. [Google Scholar] [PubMed]
- Beutling, U.; Frank, R. Epitope analysis using synthetic peptide repertoires prepared by SPOT synthesis technology. In Antibody Engineering; Kontermann, R.S., Dübel, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 537–572. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgenroth, R.; Reichardt, C.; Steffen, J.; Busse, S.; Frank, R.; Heidecke, H.; Mertens, P.R. Autoantibody Formation and Mapping of Immunogenic Epitopes against Cold-Shock-Protein YB-1 in Cancer Patients and Healthy Controls. Cancers 2020, 12, 3507. https://doi.org/10.3390/cancers12123507
Morgenroth R, Reichardt C, Steffen J, Busse S, Frank R, Heidecke H, Mertens PR. Autoantibody Formation and Mapping of Immunogenic Epitopes against Cold-Shock-Protein YB-1 in Cancer Patients and Healthy Controls. Cancers. 2020; 12(12):3507. https://doi.org/10.3390/cancers12123507
Chicago/Turabian StyleMorgenroth, Ronnie, Charlotte Reichardt, Johannes Steffen, Stefan Busse, Ronald Frank, Harald Heidecke, and Peter R. Mertens. 2020. "Autoantibody Formation and Mapping of Immunogenic Epitopes against Cold-Shock-Protein YB-1 in Cancer Patients and Healthy Controls" Cancers 12, no. 12: 3507. https://doi.org/10.3390/cancers12123507
APA StyleMorgenroth, R., Reichardt, C., Steffen, J., Busse, S., Frank, R., Heidecke, H., & Mertens, P. R. (2020). Autoantibody Formation and Mapping of Immunogenic Epitopes against Cold-Shock-Protein YB-1 in Cancer Patients and Healthy Controls. Cancers, 12(12), 3507. https://doi.org/10.3390/cancers12123507