Contrast-Enhanced Mammography for Screening Women after Breast Conserving Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Contrast-Enhanced Mammography Technique
2.3. Chart Review and Reference Standard
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
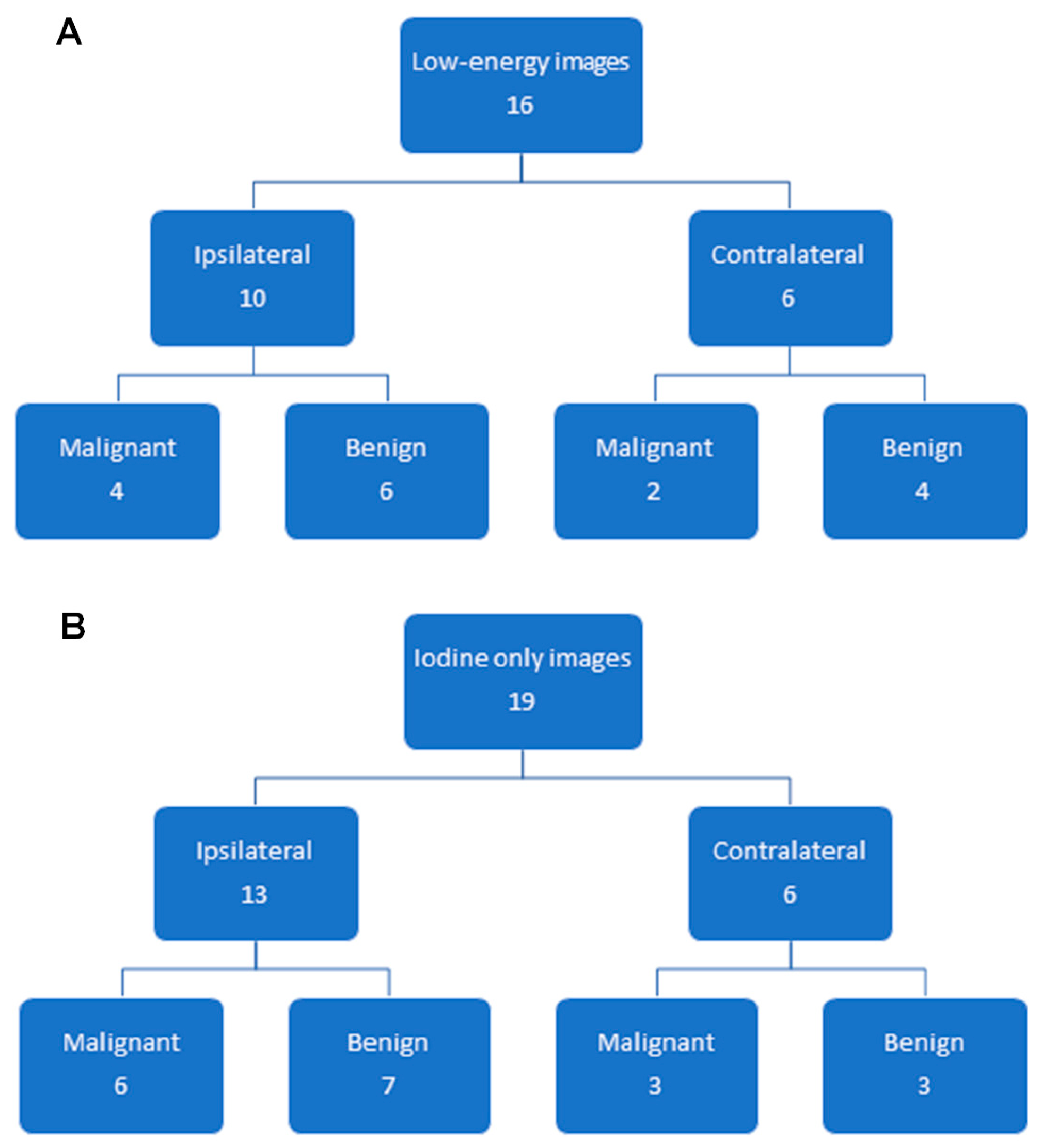
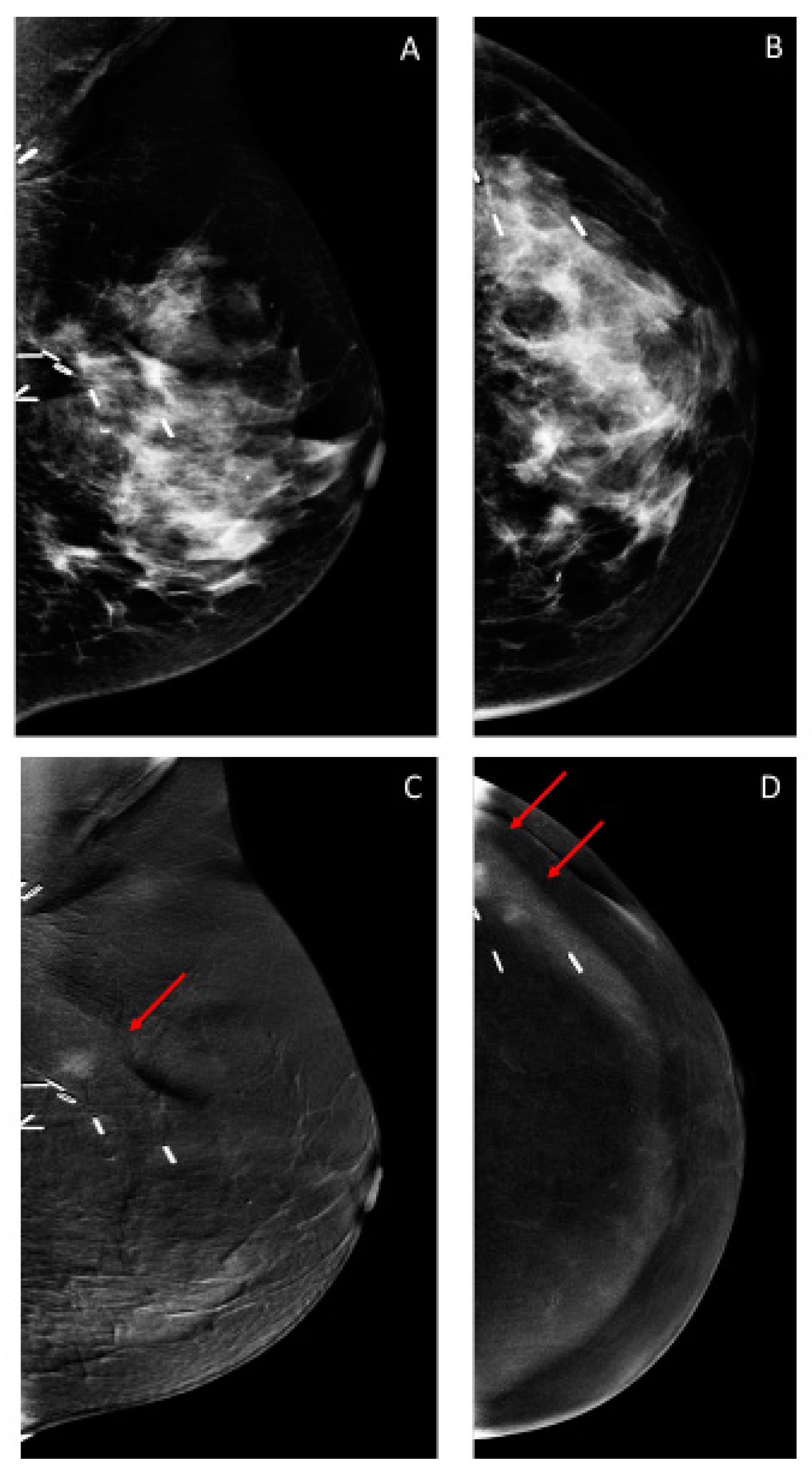
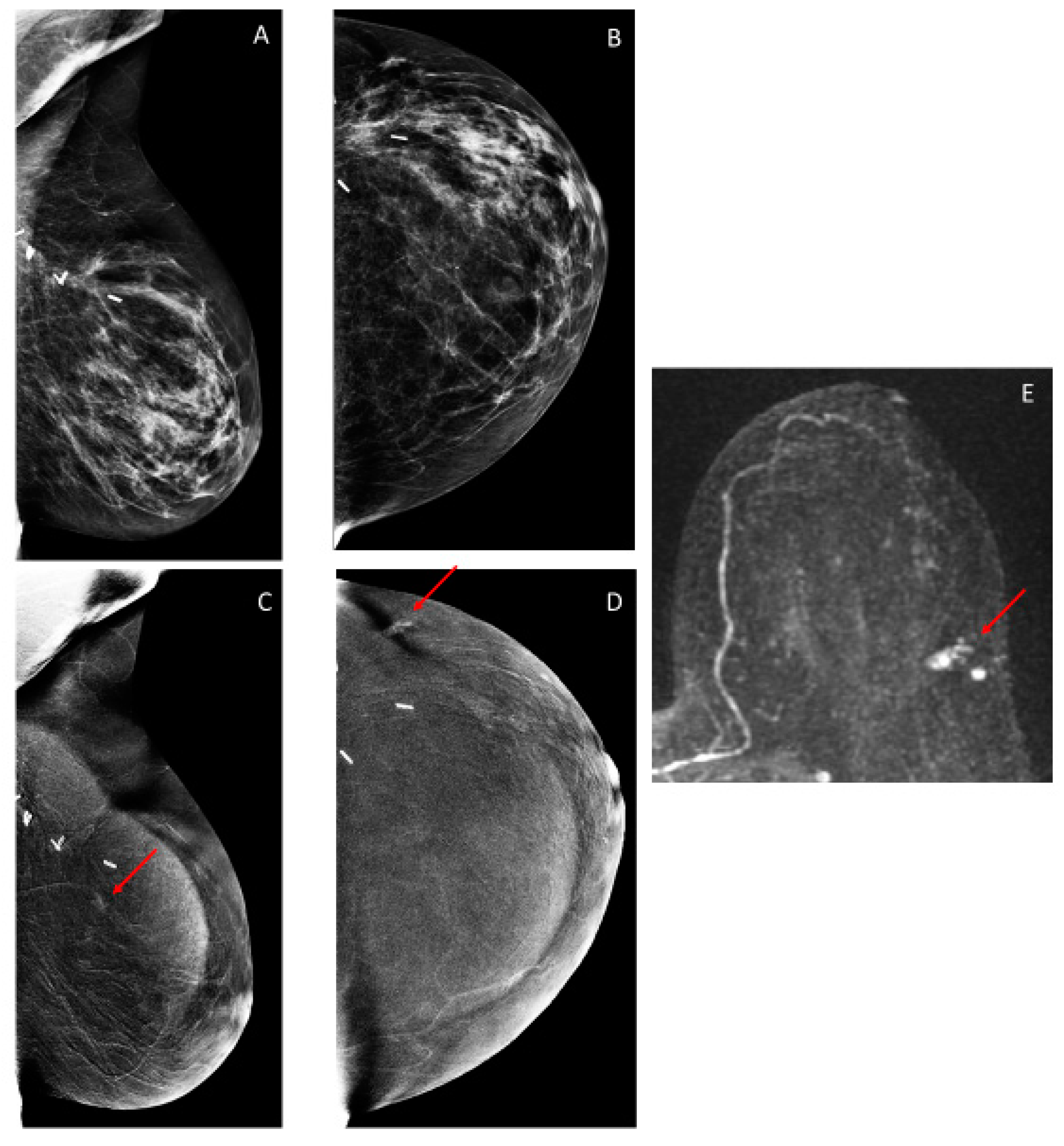
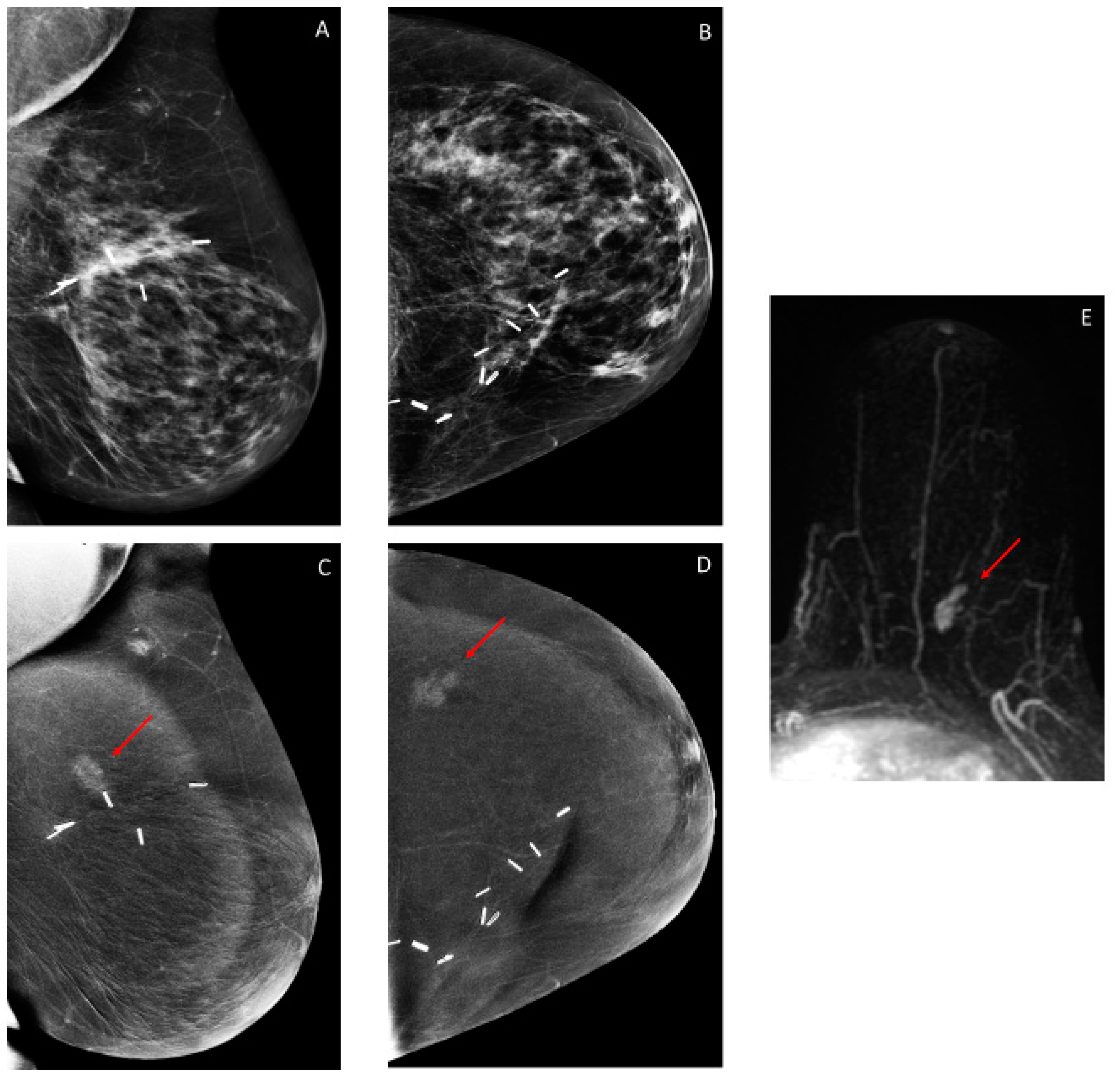
3.2. Findings on Low-Energy Images, With or Without Enhancement
3.3. Additional Findings on Iodine Images
3.4. Overall CEM Results
3.5. Follow-up
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Mannino, M.; Yarnold, J.R. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: Can radiotherapy ever be safely withheld? Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 90, 14–22. [Google Scholar] [CrossRef]
- Kiess, A.P.; McArthur, H.L.; Mahoney, K.; Patil, S.; Morris, P.G.; Ho, A.; Hudis, C.A.; McCormick, B. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 2012, 118, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Holleczek, B.; Stegmaier, C.; Radosa, J.C.; Solomayer, E.F.; Brenner, H. Risk of loco-regional recurrence and distant metastases of patients with invasive breast cancer up to ten years after diagnosis-results from a registry-based study from Germany. BMC Cancer 2019, 19, e520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowery, A.J.; Kell, M.R.; Glynn, R.W.; Kerin, M.J.; Sweeney, K.J. Locoregional recurrence after breast cancer surgery: A systematic review by receptor phenotype. Breast Cancer Res. Treat. 2012, 133, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J. Am. Coll. Radiol. 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Lu, W.L.; Jansen, L.; Post, W.J.; Bonnema, J.; Van de Velde, J.C.; De Bock, G.H. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2009, 114, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houssami, N.; Ciatto, S.; Martinelli, F.; Bonardi, R.; Duffy, S.W. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 1505–1510. [Google Scholar] [CrossRef]
- Brennan, S.; Liberman, L.; Dershaw, D.D.; Morris, E. Breast MRI screening of women with a personal history of breast cancer. Ajr. Am. J. Roentgenol. 2010, 195, 510–516. [Google Scholar] [CrossRef]
- Cho, N.; Han, W.; Han, B.K.; Bae, M.S.; Ko, E.S.; Nam, S.J.; Chae, E.Y.; Lee, J.W.; Kim, S.H.; Kang, B.J.; et al. Breast Cancer Screening With Mammography Plus Ultrasonography or Magnetic Resonance Imaging in Women 50 Years or Younger at Diagnosis and Treated With Breast Conservation Therapy. Jama Oncol. 2017, 3, 1495–1502. [Google Scholar] [CrossRef]
- Weinstock, C.; Campassi, C.; Goloubeva, O.; Wooten, K.; Kesmodel, S.; Bellevance, E.; Feigenberg, S.; Ioffe, O.; Tkaczuk, K.H. Breast magnetic resonance imaging (MRI) surveillance in breast cancer survivors. SpringerPlus 2015, 4, e459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehman, C.D.; Lee, J.M.; DeMartini, W.B.; Hippe, D.S.; Rendi, M.H.; Kalish, G.; Porter, P.; Gralow, J.; Partridge, S.C. Screening MRI in Women with a Personal History of Breast Cancer. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Kobrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- Francescone, M.A.; Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Hughes, M.C.; Zheng, J.; Moskowitz, C.; Morris, E.A. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur. J. Radiol. 2014, 83, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Lobbes, M.B.; Lalji, U.; Houwers, J.; Nijssen, E.C.; Nelemans, P.J.; van Roozendaal, L.; Smidt, M.L.; Heuts, E.; Wildberger, J.E. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur. Radiol. 2014, 24, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Fallenberg, E.M.; Dromain, C.; Diekmann, F.; Engelken, F.; Krohn, M.; Singh, J.M.; Ingold-Heppner, B.; Winzer, K.J.; Bick, U.; Renz, D.M. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur. Radiol. 2014, 24, 256–264. [Google Scholar] [CrossRef]
- Lalji, U.C.; Houben, I.P.; Prevos, R.; Gommers, S.; van Goethem, M.; Vanwetswinkel, S.; Pijnappel, R.; Steeman, R.; Frotscher, C.; Mok, W.; et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: Validation of results in a large multireader, multicase study. Eur. Radiol. 2016, 26, 4371–4379. [Google Scholar] [CrossRef] [Green Version]
- Tagliafico, A.S.; Bignotti, B.; Rossi, F.; Signori, A.; Sormani, M.P.; Valdora, F.; Calabrese, M.; Houssami, N. Diagnostic performance of contrast-enhanced spectral mammography: Systematic review and meta-analysis. Breast 2016, 28, 13–19. [Google Scholar] [CrossRef]
- Tardivel, A.M.; Balleyguier, C.; Dunant, A.; Delaloge, S.; Mazouni, C.; Mathieu, M.C.; Dromain, C. Added Value of Contrast-Enhanced Spectral Mammography in Postscreening Assessment. Breast J. 2016, 22, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.L.; James, J.J.; Cornford, E.J.; Chen, Y.; Burrell, H.C.; Hamilton, L.J.; Girio-Fragkoulakis, C. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin. Radiol. 2016, 71, 1148–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, J.S.; Lebron, L.; Keating, D.; D’Alessio, D.; Comstock, C.E.; Lee, C.H.; Pike, M.C.; Ayhan, M.; Moskowitz, C.S.; Morris, E.A.; et al. Performance of Dual-Energy Contrast-enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology 2019, 293, 81–88. [Google Scholar] [CrossRef]
- Fallenberg, E.M.; Schmitzberger, F.F.; Amer, H.; Ingold-Heppner, B.; Balleyguier, C.; Diekmann, F.; Engelken, F.; Mann, R.M.; Renz, D.M.; Bick, U.; et al. Contrast-enhanced spectral mammography vs. mammography and MRI-clinical performance in a multi-reader evaluation. Eur. Radiol. 2017, 27, 2752–2764. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Lin, Y.C.; Wan, Y.L.; Yeow, K.M.; Huang, P.C.; Lo, Y.F.; Tsai, H.P.; Ueng, S.H.; Chang, C.J. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: Interobserver blind-reading analysis. Eur. Radiol. 2014, 24, 2394–2403. [Google Scholar] [CrossRef]
- Sorin, V.; Yagil, Y.; Yosepovich, A.; Shalmon, A.; Gotlieb, M.; Neiman, O.H.; Sklair-Levy, M. Contrast-Enhanced Spectral Mammography in Women with Intermediate Breast Cancer Risk and Dense Breasts. Ajr. Am. J. Roentgenol. 2018, 211, W267–W274. [Google Scholar] [CrossRef]
- Mori, M.; Akashi-Tanaka, S.; Suzuki, S.; Daniels, M.I.; Watanabe, C.; Hirose, M.; Nakamura, S. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2017, 24, 104–110. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Pinker, K.; Dershaw, D.D.; Hughes, M.; Gibbons, G.F.; Rahbar, K.; Robson, M.E.; Mangino, D.A.; Goldman, D.; Moskowitz, C.S.; et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: A pilot study. Eur. J. Radiol. 2017, 97, 37–43. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Heerdt, A.S.; Thornton, C.; Moskowitz, C.S.; Ferrara, J.; Morris, E.A. Bilateral contrast-enhanced dual-energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013, 266, 743–751. [Google Scholar] [CrossRef] [Green Version]
- James, J.R.; Pavlicek, W.; Hanson, J.A.; Boltz, T.F.; Patel, B.K. Breast Radiation Dose with CESM Compared With 2D FFDM and 3D Tomosynthesis Mammography. Ajr Am. J. Roentgenol. 2017, 208, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Jeukens, C.R.L.P.N.; Lalji, U.C.; Meijer, E.; Bakija, B.; Theunissen, R.; Wildberger, J.E.; Lobbes, M.B.I. Radiation Exposure of Contrast-Enhanced Spectral Mammography Compared With Full-Field Digital Mammography. Investig. Radiol. 2014, 49, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Canale, S.; Saab-Puong, S.; Carton, A.K.; Muller, S.; Fallenberg, E.M. Optimization of contrast-enhanced spectral mammography depending on clinical indication. J. Med. Imaging 2014, 1, e033506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, e76. [Google Scholar] [CrossRef] [PubMed]
- Sogani, J.; Morris, E.A.; Kaplan, J.B.; D’Alessio, D.; Goldman, D.; Moskowitz, C.S.; Jochelson, M.S. Comparison of Background Parenchymal Enhancement at Contrast-enhanced Spectral Mammography and Breast MR Imaging. Radiology 2017, 282, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Leisenring, W.; Alonzo, T.; Pepe, M.S. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics 2000, 56, 345–351. [Google Scholar] [CrossRef]
- Trajman, A.; Luiz, R.R. McNemar chi2 test revisited: Comparing sensitivity and specificity of diagnostic examinations. Scand. J. Clin. Lab. Investig. 2008, 68, 77–80. [Google Scholar] [CrossRef]
- Heywang-Kobrunner, S.H.; Hacker, A.; Sedlacek, S. Advantages and Disadvantages of Mammography Screening. Breast Care 2011, 6, 199–207. [Google Scholar] [CrossRef]
- Vourtsis, A.; Berg, W.A. Breast density implications and supplemental screening. Eur. Radiol. 2019, 29, 1762–1777. [Google Scholar] [CrossRef]
- Starikov, A.; Drotman, M.; Hentel, K.; Katzen, J.; Min, R.J.; Arleo, E.K. 2D mammography, digital breast tomosynthesis, and ultrasound: Which should be used for the different breast densities in breast cancer screening? Clin. Imaging 2016, 40, 68–71. [Google Scholar] [CrossRef]
- Skaane, P.; Gullien, R.; Bjorndal, H.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Jahr, G.; Jebsen, I.N.; Krager, M. Digital breast tomosynthesis (DBT): Initial experience in a clinical setting. Acta 2012, 53, 524–529. [Google Scholar] [CrossRef]
- Diekmann, F.; Diekmann, S.; Jeunehomme, F.; Muller, S.; Hamm, B.; Bick, U. Digital mammography using iodine-based contrast media: Initial clinical experience with dynamic contrast medium enhancement. Investig. Radiol. 2005, 40, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Luczynska, E.; Heinze-Paluchowska, S.; Dyczek, S.; Blecharz, P.; Rys, J.; Reinfuss, M. Contrast-Enhanced Spectral Mammography: Comparison with Conventional Mammography and Histopathology in 152 Women. Korean J. Radiol. 2014, 15, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.S.; Rubbert, C.; Mathys, B.; Antoch, G.; Mohrmann, S.; Obenauer, S. Use of contrast-enhanced spectral mammography for intramammary cancer staging: Preliminary results. Acad. Radiol. 2014, 21, 1363–1369. [Google Scholar] [CrossRef]
- Fallenberg, E.M.; Dromain, C.; Diekmann, F.; Renz, D.M.; Amer, H.; Ingold-Heppner, B.; Neumann, A.U.; Winzer, K.J.; Bick, U.; Hamm, B.; et al. Contrast-enhanced spectral mammography: Does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res. Treat. 2014, 146, 371–381. [Google Scholar] [CrossRef]
- Dromain, C.; Balleyguier, C.; Muller, S.; Mathieu, M.C.; Rochard, F.; Opolon, P.; Sigal, R. Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. Ajr. Am. J. Roentgenol. 2006, 187, W528–W537. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, F.; Freyer, M.; Diekmann, S.; Fallenberg, E.M.; Fischer, T.; Bick, U.; Pollinger, A. Evaluation of contrast-enhanced digital mammography. Eur. J. Radiol. 2011, 78, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.H.; Mansour, S.M.; Ahmed, H.A.; Abdel Ghany, A.F.; Kamel, O.F.; Elkholy, N.G. The role of contrast-enhanced spectral mammography in the evaluation of the postoperative breast cancer. Clin. Radiol. 2019, 74, 771–781. [Google Scholar] [CrossRef] [Green Version]
- Sippo, D.A.; Burk, K.S.; Mercaldo, S.F.; Rutledge, G.M.; Edmonds, C.; Guan, Z.; Hughes, K.S.; Lehman, C.D. Performance of Screening Breast MRI across Women with Different Elevated Breast Cancer Risk Indications. Radiology 2019, 292, 51–59. [Google Scholar] [CrossRef]
- Lehman, C.D.; Arao, R.F.; Sprague, B.L.; Lee, J.M.; Buist, D.S.; Kerlikowske, K.; Henderson, L.M.; Onega, T.; Tosteson, A.N.; Rauscher, G.H.; et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017, 283, 49–58. [Google Scholar] [CrossRef]
- Carney, P.A.; Parikh, J.; Sickles, E.A.; Feig, S.A.; Monsees, B.; Bassett, L.W.; Smith, R.A.; Rosenberg, R.; Ichikawa, L.; Wallace, J.; et al. Diagnostic mammography: Identifying minimally acceptable interpretive performance criteria. Radiology 2013, 267, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.A.; Talati, N.; Oudsema, R.; Steinberger, S.; Margolies, L.R. BI-RADS 3: Current and Future Use of Probably Benign. Curr. Radiol. Rep. 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, M.C.; Gatsonis, C.; Hanna, L.; DeMartini, W.B.; Lehman, C. Positive predictive value of BI-RADS MR imaging. Radiology 2012, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]
| Imaging Modality | Findings on Low-Energy Images, with or without Enhancement (n = 54) | Findings on Iodine Images Only (n = 46) |
|---|---|---|
| Mammographic views | 50 | 13 |
| Ultrasound | 13 | 42 |
| MRI | 12 | 36 |
| Biopsied Lesions | n | % |
|---|---|---|
| Malignant | 15/35 | 42.9 |
| Invasive ductal carcinoma | 8/15 | 53.3 |
| DCIS | 4/15 | 26.7 |
| Invasive lobular carcinoma | 2/15 | 13.3 |
| Adenosquamous carcinoma | 1/15 | 6.7 |
| Benign | 20/35 | 57.1 |
| Sclerosing adenosis, Usual ductal hyperplasia, Apocrine metaplasia, Periductal inflammation, Dense stromal fibrosis with calcifications in benign epithelium | 9/20 | 45.0 |
| Fibroadenoma/fibroadenomatoid changes | 4/20 | 20.0 |
| Fat necrosis, Scar tissue | 5/20 | 25.0 |
| Atypical lobular hyperplasia | 2/20 | 10.0 |
| Probably Benign Imaging Findings | n | % |
|---|---|---|
| Focal NME | 16/23 | 69.6 |
| Regional NME | 3/23 | 13.0 |
| Focus | 2/23 | 8.7 |
| Circumscribed enhancing mass Bilateral segmental NME | 1/23 1/23 | 4.3 4.3 |
| Performance Metric | Low-Energy Images (95% CI) | CEM (Low-Energy + Iodine Images) (95% CI) | p-Value |
|---|---|---|---|
| Sensitivity | 27.8% (9.7–53.5) | 66.7% (40.9–86.7) | 0.01 |
| Specificity | 98.7% (97.7–99.2) | 95.8% (94.2–97.0) | <0.001 |
| PPV1 | 29.4% (10.3–55.8) | 24.0% (12.9–38.1) | 0.53 |
| NPV | 98.6% (97.5–99.2) | 99.3% (98.6–99.8) | 0.009 |
| Interval Cancer | Imaging Size, cm | Pathology Size, cm | Pathology Grade | Molecular Subtype of Invasive Cancers | How Interval Cancer was Detected |
|---|---|---|---|---|---|
| IDC | 1.2 | 1.3 | 3 | Triple-negative | Palpable |
| IDC | - | Multiple foci | 3 | Her2+ | Screening ultrasound at 6 months, 29 days |
| IDC | 0.5 | 0.5 | 3 | Luminal A | Screening MRI at 8 months, 14 days |
| IMC | 1.5 | 2.0 | 3 | Luminal A | Palpable |
| DCIS | 0.2 | 1.1 | 2 | Screening mammography at 11 months, 9 days | |
| Paget’s with DCIS | - | 1.0 | 2 | Areolar eczematous changes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gluskin, J.; Rossi Saccarelli, C.; Avendano, D.; Marino, M.A.; Bitencourt, A.G.V.; Pilewskie, M.; Sevilimedu, V.; Sung, J.S.; Pinker, K.; Jochelson, M.S. Contrast-Enhanced Mammography for Screening Women after Breast Conserving Surgery. Cancers 2020, 12, 3495. https://doi.org/10.3390/cancers12123495
Gluskin J, Rossi Saccarelli C, Avendano D, Marino MA, Bitencourt AGV, Pilewskie M, Sevilimedu V, Sung JS, Pinker K, Jochelson MS. Contrast-Enhanced Mammography for Screening Women after Breast Conserving Surgery. Cancers. 2020; 12(12):3495. https://doi.org/10.3390/cancers12123495
Chicago/Turabian StyleGluskin, Jill, Carolina Rossi Saccarelli, Daly Avendano, Maria Adele Marino, Almir G. V. Bitencourt, Melissa Pilewskie, Varadan Sevilimedu, Janice S. Sung, Katja Pinker, and Maxine S. Jochelson. 2020. "Contrast-Enhanced Mammography for Screening Women after Breast Conserving Surgery" Cancers 12, no. 12: 3495. https://doi.org/10.3390/cancers12123495
APA StyleGluskin, J., Rossi Saccarelli, C., Avendano, D., Marino, M. A., Bitencourt, A. G. V., Pilewskie, M., Sevilimedu, V., Sung, J. S., Pinker, K., & Jochelson, M. S. (2020). Contrast-Enhanced Mammography for Screening Women after Breast Conserving Surgery. Cancers, 12(12), 3495. https://doi.org/10.3390/cancers12123495






