In Silico and In Vitro Analysis of lncRNA XIST Reveals a Panel of Possible Lung Cancer Regulators and a Five-Gene Diagnostic Signature
Abstract
Simple Summary
Abstract
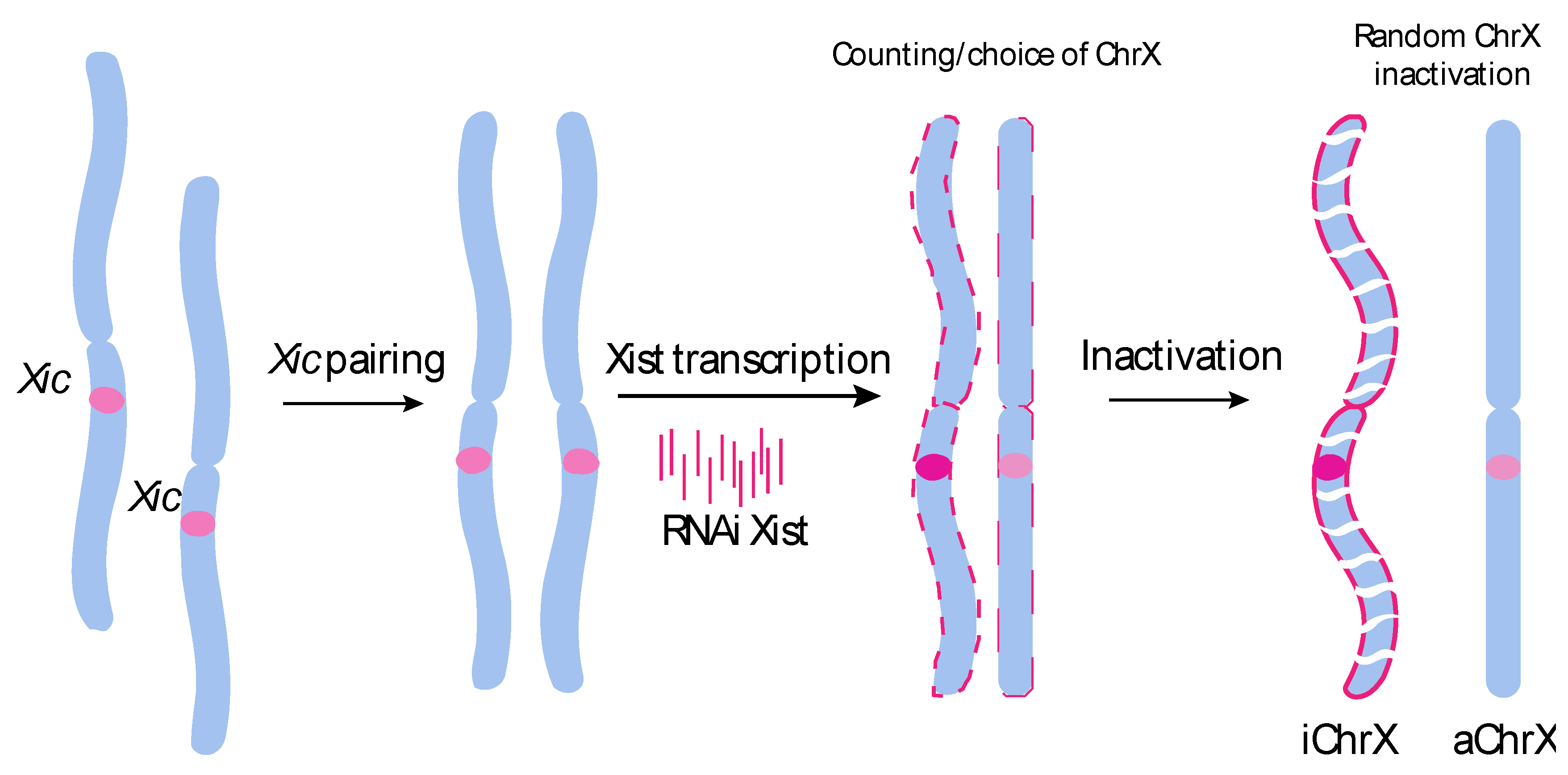
1. Introduction
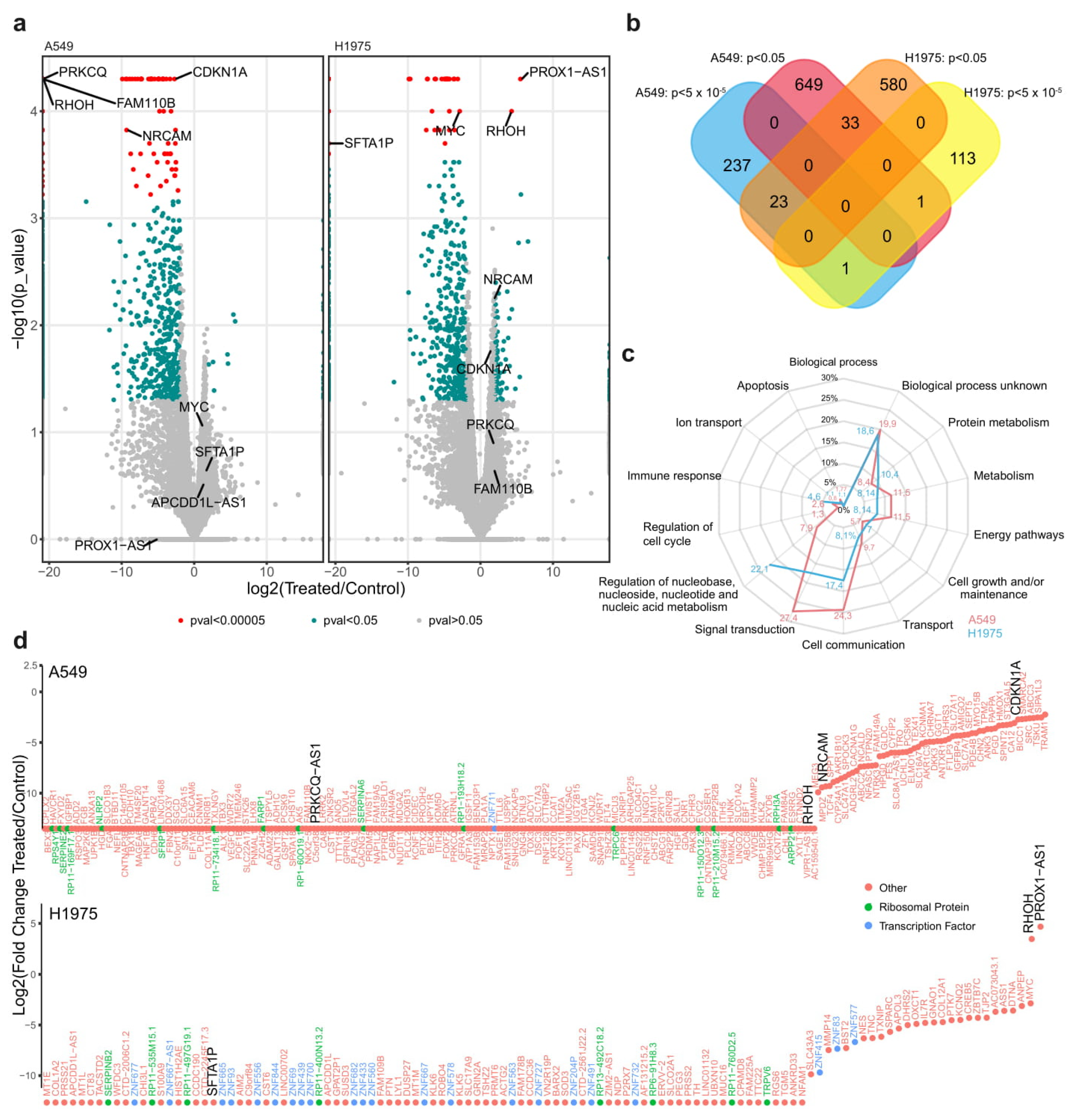
2. Results
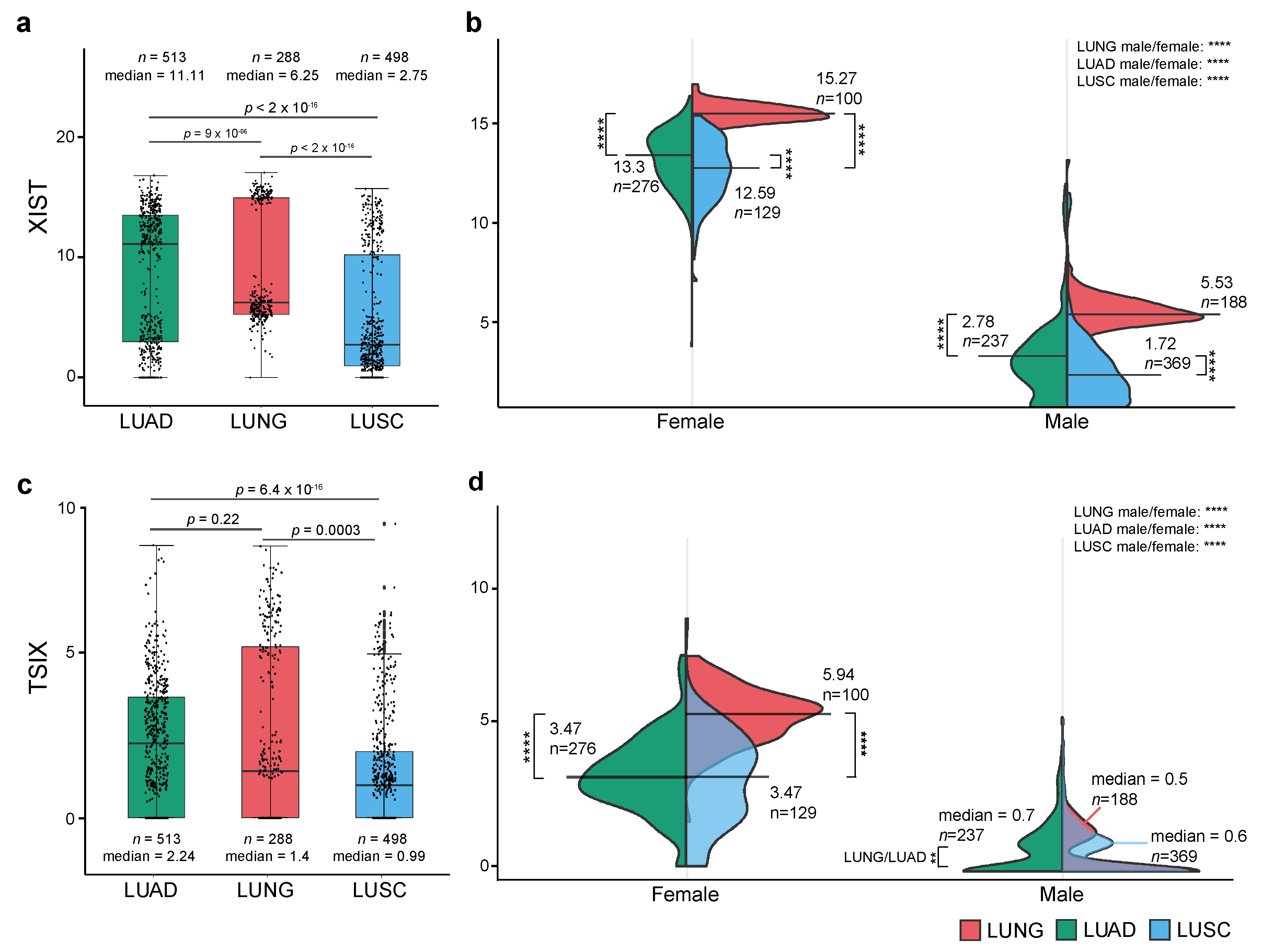
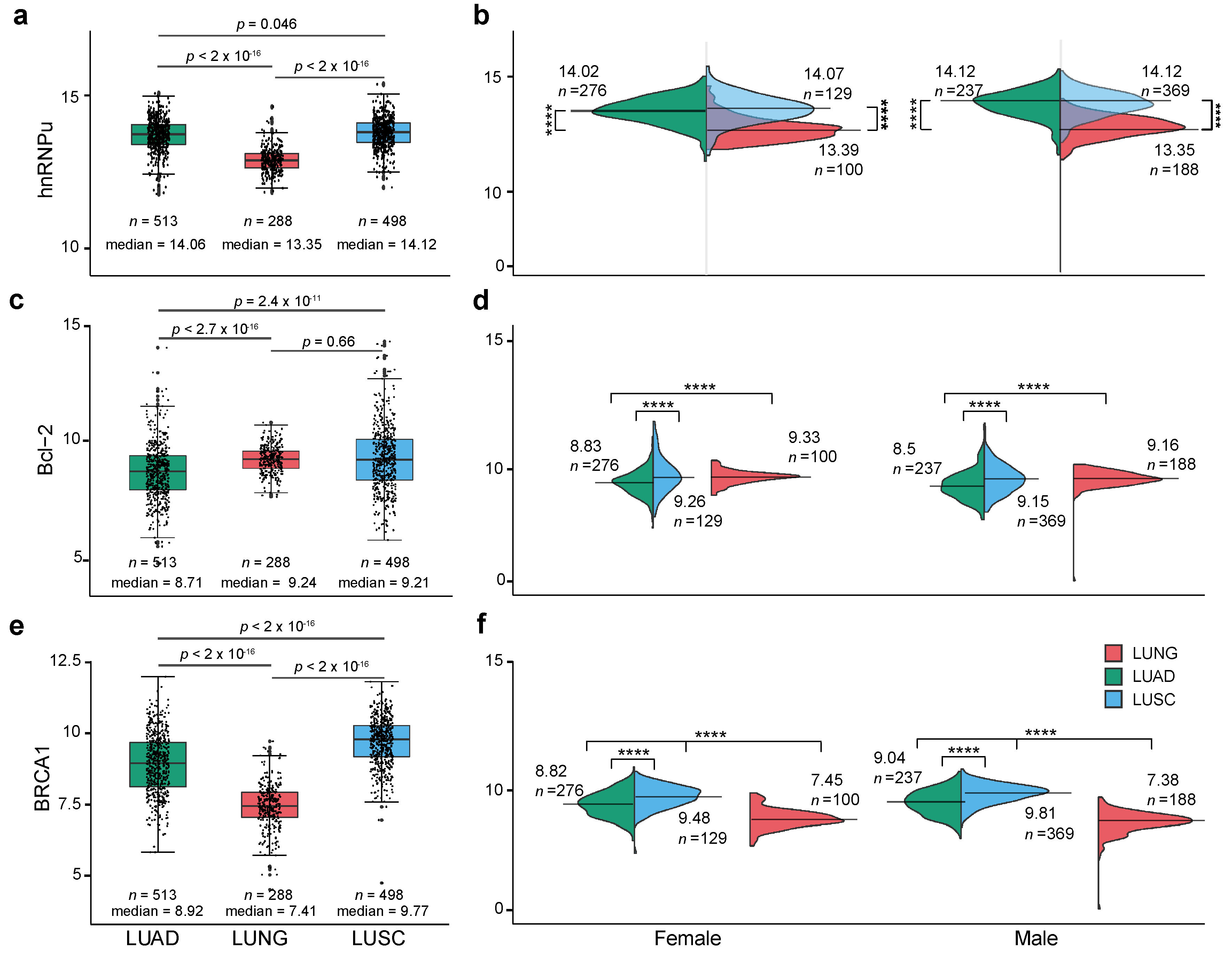
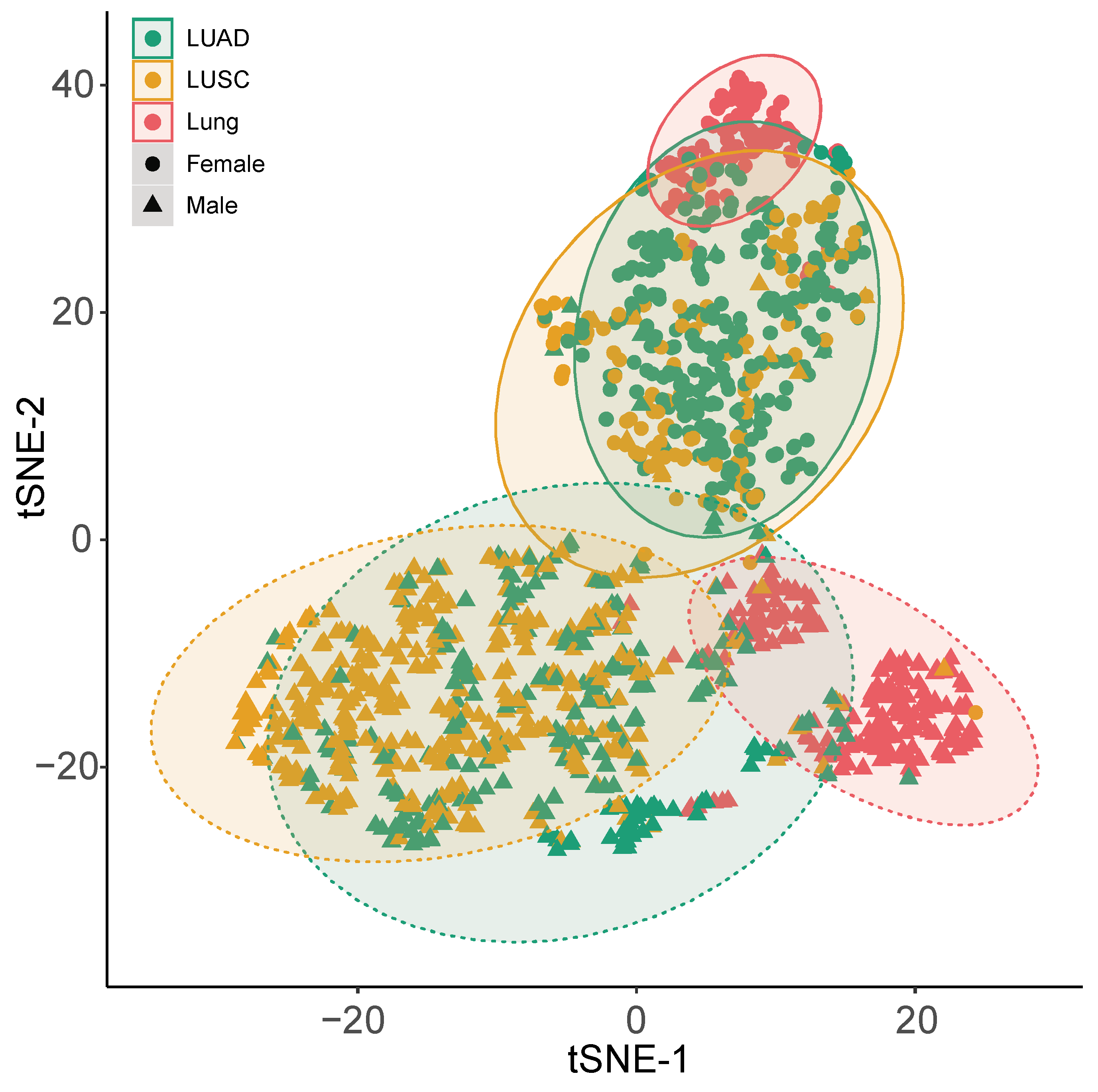
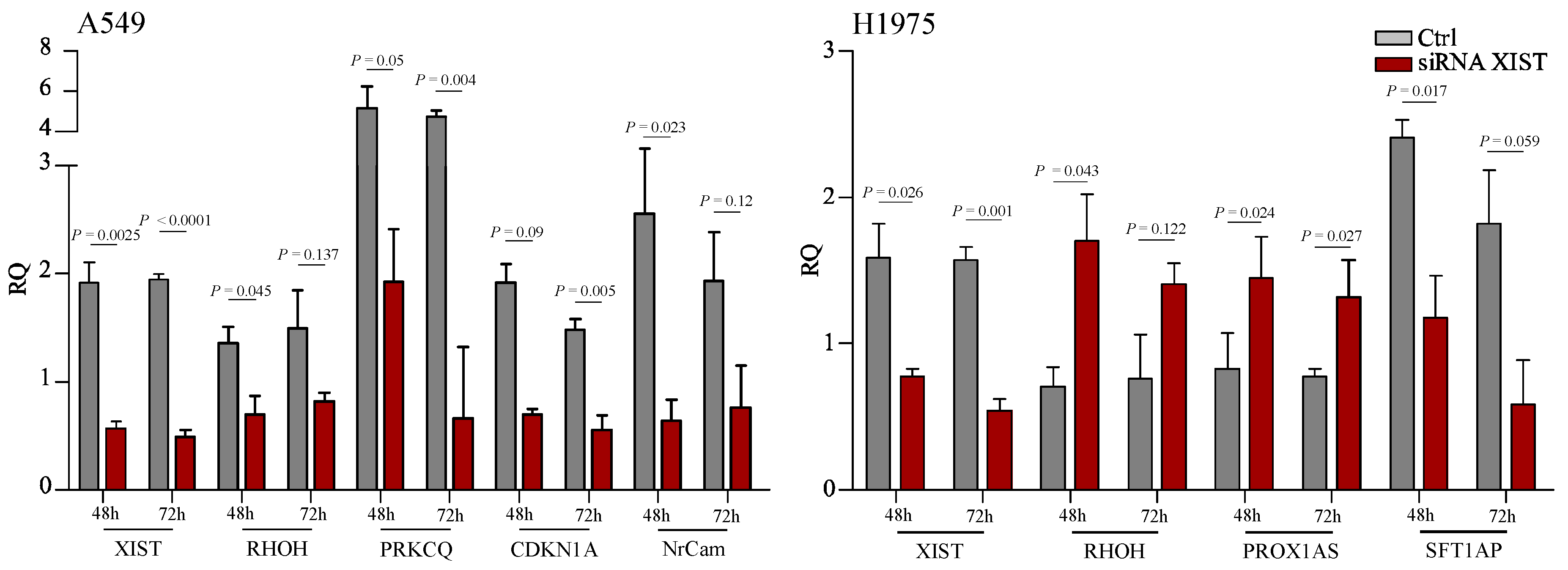
2.1. Expression Level of XIST, TSIX, hnRNPu, Bcl-2, and BRCA1 in NSCLC
2.2. Functional Analysis
3. Discussion
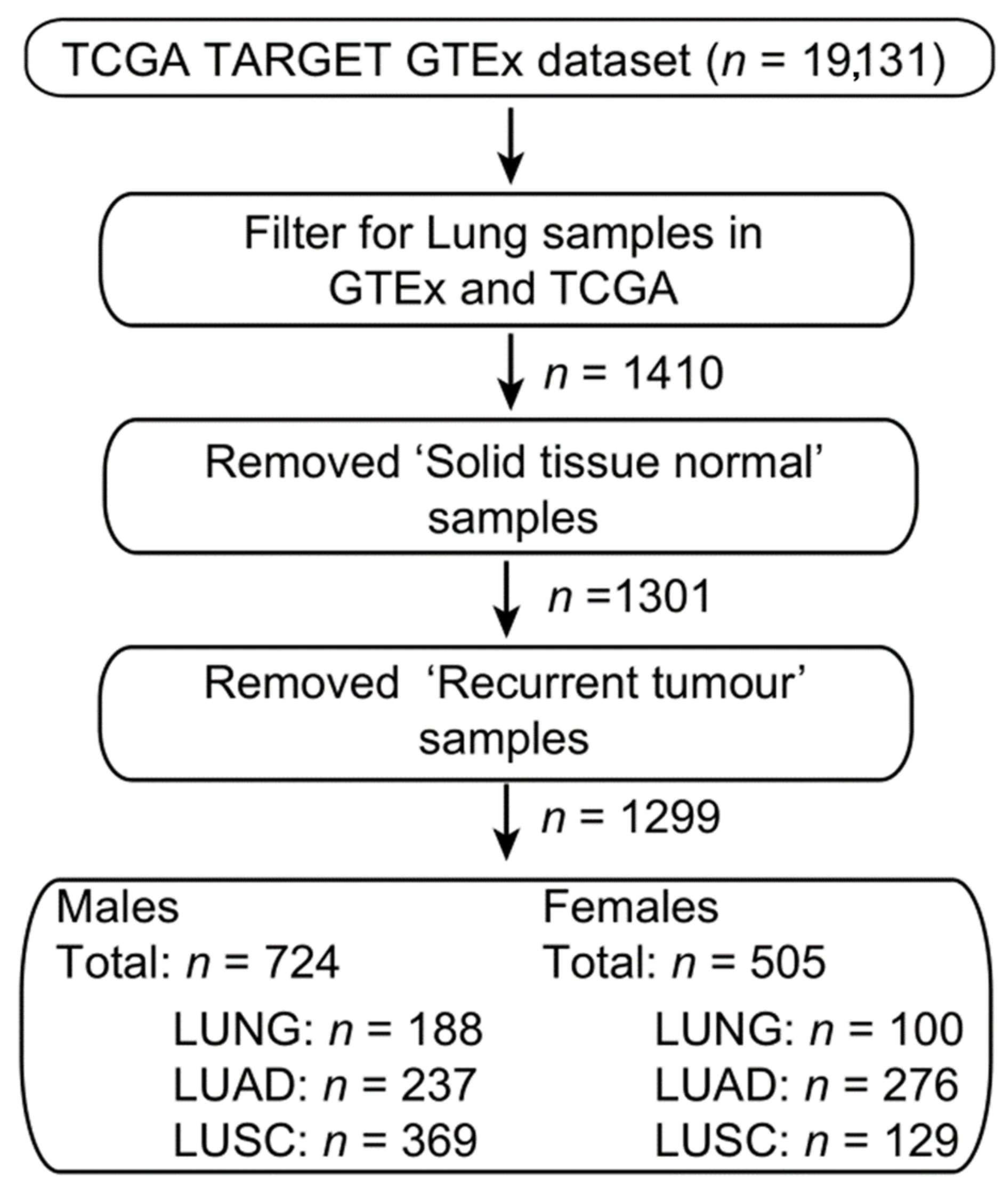
4. Materials and Methods
4.1. Cell Lines
4.2. siRNA for XIST
4.3. RNA Isolation, cDNA Synthesis, and qPCR
4.4. RT-qPCR
4.5. RNAseq
4.6. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Fraser, P. No-nonsense functions for long noncoding RNAs. Cell 2011, 145, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Delás, M.J.; Hannon, G.J. lncRNAs in development and disease: From functions to mechanisms. Open Biol. 2017, 7, 170121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Li, X.-B.; Hou, Y.-X.; Fang, N.-Z.; You, J.-C.; Zhou, Q.-H. The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancerThis article has been corrected since Advanced Online Publication, and an erratum is also printed in this issue. Acta Pharmacol. Sin. 2017, 38, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, Q.; Zhang, X.; Yang, C.; Cui, S.; Sun, Y.; Wang, L.; Fan, X.; Xu, S. The Long Non-Coding RNA XIST Controls Non-Small Cell Lung Cancer Proliferation and Invasion by Modulating miR-186-5p. Cell. Physiol. Biochem. 2017, 41, 2221–2229. [Google Scholar] [CrossRef]
- Inamura, K. Major Tumor Suppressor and Oncogenic Non-Coding RNAs: Clinical Relevance in Lung Cancer. Cells 2017, 6, 12. [Google Scholar] [CrossRef]
- Do, H.; Kim, W. Roles of Oncogenic Long Non-coding RNAs in Cancer Development. Genom. Inform. 2018, 16, e18. [Google Scholar] [CrossRef]
- Du, Z.; Sun, T.; Hacisuleyman, E.; Fei, T.; Wang, X.; Brown, M.; Rinn, J.L.; Lee, M.G.-S.; Chen, Y.; Kantoff, P.W.; et al. Integrative analyses reveal a long noncoding RNA-mediated sponge regulatory network in prostate cancer. Nat. Commun. 2016, 7, 10982. [Google Scholar] [CrossRef]
- Gendrel, A.V.; Heard, E. Fifty years of x-inactivation research. Development 2011, 138, 5049–5055. [Google Scholar] [CrossRef]
- Penny, G.D.; Kay, G.F.; Sheardown, S.A.; Rastan, S.; Brockdorff, N. Requirement for Xist in X chromosome inactivation. Nature 1996, 379, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, I.; Barakat, T.S.; Achame, E.M.; Monkhorst, K.; Kenter, A.; Rentmeester, E.; Grosveld, F.; Grootegoed, J.A.; Gribnau, J. RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell 2009, 139, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, X.; Jiang, X.; Zhao, H. X-inactive-specific transcript: A long noncoding RNA with complex roles in human cancers. Gene 2018, 679, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pintacuda, G.; Young, A.N.; Cerase, A. Function by Structure: Spotlights on Xist Long Non-coding RNA. Front. Mol. Biosci. 2017, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cerase, A.; Pintacuda, G.; Tattermusch, A.; Avner, P. Xist localization and function: New insights from multiple levels. Genome Biol. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Salvador, M.A.; Wicinski, J.; Cabaud, O.; Toiron, Y.; Finetti, P.; Josselin, E.; Lelièvre, H.; Kraus-Berthier, L.; Depil, S.; Bertucci, F.; et al. The histone deacetylase inhibitor abexinostat induces cancer stem cells differentiation in breast cancer with low Xist expression. Clin. Cancer Res. 2013, 19, 6520–6531. [Google Scholar] [CrossRef]
- Kobayashi, R.; Miyagawa, R.; Yamashita, H.; Morikawa, T.; Okuma, K.; Fukayama, M.; Ohtomo, K.; Nakagawa, K. Increased expression of long non-coding RNA XIST predicts favorable prognosis of cervical squamous cell carcinoma subsequent to definitive chemoradiation therapy. Oncol. Lett. 2016, 12, 3066–3074. [Google Scholar] [CrossRef]
- Chen, D.-L.; Chen, L.-Z.; Lu, Y.-X.; Zhang, D.-S.; Zeng, Z.-L.; Pan, Z.-Z.; Huang, P.; Wang, F.-H.; Li, Y.-H.; Ju, H.-Q.; et al. Long noncoding RNA XIST expedites metastasis and modulates epithelial-mesenchymal transition in colorectal cancer. Cell Death Dis. 2017, 8, e3011. [Google Scholar] [CrossRef]
- Chen, D.; Ren, J.; Wang, Y.; Li, B.; Gu, Y. Intraoperative monitoring of blood perfusion in port wine stains by laser Doppler imaging during vascular targeted photodynamic therapy: A preliminary study. Photodiagn. Photodyn. Ther. 2016, 14, 142–151. [Google Scholar] [CrossRef]
- Du, P.; Zhao, H.; Peng, R.; Liu, Q.; Yuan, J.; Peng, G.; Liao, Y. LncRNA-XIST interacts with miR-29c to modulate the chemoresistance of glioma cell to TMZ through DNA mismatch repair pathway. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Mo, Y.; Lu, Y.; Wang, P.; Huang, S.; He, L.; Li, D.; Li, F.; Huang, J.; Lin, X.; Li, X.; et al. Long non-coding RNA XIST promotes cell growth by regulating miR-139-5p/PDK1/AKT axis in hepatocellular carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ye, L.-F.; Zhang, C.; Peng, T.; Zhou, X.-H. Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene 2016, 592, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Sun, C.-C.; Gong, C. Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem. Biophys. Res. Commun. 2016, 478, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, Y.; Lu, Y.; Yang, B.; Tang, L. LncRNA XIST Promotes Pancreatic Cancer Proliferation Through miR-133a/EGFR. J. Cell. Biochem. 2017, 118, 3349–3358. [Google Scholar] [CrossRef]
- Li, G.-L.; Wu, Y.-X.; Li, Y.-M.; Li, J. High expression of long non-coding RNA XIST in osteosarcoma is associated with cell proliferation and poor prognosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2829–2834. [Google Scholar] [PubMed]
- Huang, K.-C.; Rao, P.H.; Lau, C.C.; Heard, E.; Ng, S.-K.; Brown, C.; Mok, S.C.; Berkowitz, R.S.; Ng, S.-W. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol. Cancer Ther. 2002, 1, 769–776. [Google Scholar]
- Yao, Y.; Ma, J.; Xue, Y.; Wang, P.; Li, Z.Z.; Liu, J.; Chen, L.; Xi, Z.; Teng, H.; Wang, Z.; et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015, 359, 75–86. [Google Scholar] [CrossRef]
- Silver, D.P.; Dimitrov, S.D.; Feunteun, J.; Gelman, R.; Drapkin, R.; Lu, S.D.; Shestakova, E.; Velmurugan, S.; Denunzio, N.; Dragomir, S.; et al. Further evidence for BRCA1 communication with the inactive X chromosome. Cell 2007, 128, 991–1002. [Google Scholar] [CrossRef]
- Wang, P.; Chen, D.; Ma, H.; Li, Y. Long non-coding RNA MEG3 regulates proliferation and apoptosis in non-small cell lung cancer: Via the miR-205-5p/LRP1 pathway. RSC Adv. 2017, 7, 49710–49719. [Google Scholar] [CrossRef]
- Shen, L.; Li, C.; Liu, M.; Wei, D.; Chang, Q.; Cui, J. Prognostic and clinicopathological roles of long non-coding RNA XIST in human cancers: A meta-analysis. Transl. Cancer Res. 2018, 7, 1624–1633. [Google Scholar] [CrossRef]
- Sun, W.; Zu, Y.; Fu, X.; Deng, Y. Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol. Rep. 2017, 38, 3347–3354. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, G.; Cheng, Z.; Dai, L.; Jia, L.; Jing, X.; Wang, H.; Zhang, R.; Liu, M.; Jiang, T.; et al. Knockdown of LncRNA-XIST Suppresses Proliferation and TGF-β1-Induced EMT in NSCLC Through the Notch-1 Pathway by Regulation of miR-137. Genet. Test. Mol. Biomark. 2018, 22, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shi, G.; Li, Q.; Li, W.; Zhou, H. Long noncoding RNA XIST participates in bladder cancer by downregulating p53 via binding to TET1. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hu, W.; Zhu, W.; Zhang, F.; Lin-lin, L.; Liu, C.; Songyang, Y. Clinica Chimica Acta Long non coding RNA XIST as a prognostic cancer marker—A meta-analysis. Clin. Chim. Acta 2018, 482, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, F.; Jang, K.; Yoon, H.; Hew, K.E.; Kim, M.; Azzam, D.J.; Sun, J.; Zhao, D.; Ince, T.A.; Liu, W.; et al. Dual Src and MEK Inhibition Decreases Ovarian Cancer Growth and Targets Tumor Initiating Stem-Like Cells. Clin. Cancer Res. 2018, 24, 4874–4886. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, J.; Wang, J. Long noncoding RNA XIST promotes proliferation and invasion by targeting miR-141 in papillary thyroid carcinoma. OncoTargets Ther. 2018, 11, 5035–5043. [Google Scholar] [CrossRef] [PubMed]
- Tantai, J.; Hu, D.; Yang, Y.; Geng, J. Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 7887–7895. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, S.; Sheng, Q.; Guo, M.; Lehmann, B.; Pietenpol, J.; Samuels, D.C.; Shyr, Y. RNAseq by Total RNA Library Identifies Additional RNAs Compared to Poly(A) RNA Library. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Zheng, H.; Brennan, K.; Hernaez, M.; Gevaert, O. Benchmark of long non-coding RNA quantification for RNA sequencing of cancer samples. Gigascience 2019, 8. [Google Scholar] [CrossRef]
- Huang, R.; Jaritz, M.; Guenzl, P.; Vlatkovic, I.; Sommer, A.; Tamir, I.M.; Marks, H.; Klampfl, T.; Kralovics, R.; Stunnenberg, H.G.; et al. An RNA-Seq Strategy to Detect the Complete Coding and Non-Coding Transcriptome Including Full-Length Imprinted Macro ncRNAs. PLoS ONE 2011, 6, e27288. [Google Scholar] [CrossRef]
- Ilott, N.E.; Ponting, C.P. Predicting long non-coding RNAs using RNA sequencing. Methods 2013, 63, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Byerly, J.; Halstead-Nussloch, G.; Ito, K.; Katsyv, I.; Irie, H.Y. PRKCQ promotes oncogenic growth and anoikis resistance of a subset of triple-negative breast cancer cells. Breast Cancer Res. 2016, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Deeds, L.; Teodorescu, S.; Chu, M.; Yu, Q.; Chen, C.-Y. A p53-independent G1 cell cycle checkpoint induced by the suppression of protein kinase C alpha and theta isoforms. J. Biol. Chem. 2003, 278, 39782–39793. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, A.; Pasini, A.; Pirini, F.; Ravaioli, S.; Giordano, E.; Tesei, A.; Calistri, D.; Ulivi, P.; Fabbri, F.; Foca, F.; et al. CDKN1A upregulation and cisplatin-pemetrexed resistance in non-small cell lung cancer cells. Int. J. Oncol. 2020, 56, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Tanaka, F.; Takata, T.; Yanagihara, K.; Otake, Y.; Hanaoka, N.; Miyahara, R.; Nakagawa, T.; Kawano, Y.; Ishikawa, S.; et al. Clinical significance of p21 expression in non-small-cell lung cancer. J. Clin. Oncol. 2002, 20, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Groeger, A.M.; Caputi, M.; Esposito, V.; Baldi, A.; Rossiello, R.; Santini, D.; Mancini, A.; Kaiser, H.E.; Baldi, F. Expression of p21 in non small cell lung cancer relationship with PCNA. Anticancer Res. 2000, 20, 3301–3305. [Google Scholar]
- Górka, B.; Skubis-Zegadło, J.; Mikula, M.; Bardadin, K.; Paliczka, E.; Czarnocka, B. NrCAM, a neuronal system cell-adhesion molecule, is induced in papillary thyroid carcinomas. Br. J. Cancer 2007, 97, 531–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Sui, F.; Ma, J.; Ren, X.; Guan, H.; Yang, Q.; Shi, J.; Ji, M.; Shi, B.; Sun, Y.; et al. Positive Feedback Loops Between NrCAM and Major Signaling Pathways Contribute to Thyroid Tumorigenesis. J. Clin. Endocrinol. Metab. 2017, 102, 613–624. [Google Scholar] [CrossRef]
- Rohrbeck, A.; Neukirchen, J.; Rosskopf, M.; Pardillos, G.G.; Geddert, H.; Schwalen, A.; Gabbert, H.E.; von Haeseler, A.; Pitschke, G.; Schott, M.; et al. Gene expression profiling for molecular distinction and characterization of laser captured primary lung cancers. J. Transl. Med. 2008, 6, 69. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; Kaplan, A.; Raveh, S.; Gavert, N.; Sakurai, T.; Ben-Ze’ev, A. The shed ectodomain of Nr-CAM stimulates cell proliferation and motility, and confers cell transformation. Cancer Res. 2005, 65, 11605–11612. [Google Scholar] [CrossRef]
- Haga, R.B.; Ridley, A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 2016, 7, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Tajadura-Ortega, V.; Garg, R.; Allen, R.; Owczarek, C.; Bright, M.D.; Kean, S.; Mohd-Noor, A.; Grigoriadis, A.; Elston, T.C.; Hahn, K.M.; et al. An RNAi screen of Rho signalling networks identifies RhoH as a regulator of Rac1 in prostate cancer cell migration. BMC Biol. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.F.; Campbell, S.E.; Diette, G.B.; Gabrielson, E.W.; Kim, S.; Shim, H.; Dang, C.V. c-Myc suppresses the tumorigenicity of lung cancer cells and down-regulates vascular endothelial growth factor expression. Cancer Res. 2000, 60, 143–149. [Google Scholar] [PubMed]
- Rapp, U.R.; Korn, C.; Ceteci, F.; Karreman, C.; Luetkenhaus, K.; Serafin, V.; Zanucco, E.; Castro, I.; Potapenko, T. MYC is a metastasis gene for non-small-cell lung cancer. PLoS ONE 2009, 4, e6029. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct. Target. Ther. 2018, 3, 5. [Google Scholar] [CrossRef]
- Soucek, L.; Whitfield, J.R.; Sodir, N.M.; Massó-Vallés, D.; Serrano, E.; Karnezis, A.N.; Swigart, L.B.; Evan, G.I. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes Dev. 2013, 27, 504–513. [Google Scholar] [CrossRef]
- Jäger, N.; Schlesner, M.; Jones, D.T.W.; Raffel, S.; Mallm, J.P.; Junge, K.M.; Weichenhan, D.; Bauer, T.; Ishaque, N.; Kool, M.; et al. XHypermutation of the inactive X chromosome is a frequent event in cancer. Cell 2013, 155, 567. [Google Scholar] [CrossRef]
- Shiratsuchi, H.; Wang, Z.; Chen, G.; Ray, P.; Lin, J.; Zhang, Z.; Zhao, L.; Beer, D.; Ray, D.; Ramnath, N. Oncogenic Potential of CYP24A1 in Lung Adenocarcinoma. J. Thorac. Oncol. 2017, 12, 269–280. [Google Scholar] [CrossRef]
- Liu, W.; Song, J.; Du, X.; Zhou, Y.; Li, Y.; Li, R.; Lyu, L.; He, Y.; Hao, J.; Ben, J.; et al. AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater. 2019, 91, 195–208. [Google Scholar] [CrossRef]
- Li, C.-G.; Pu, M.-F.; Li, C.-Z.; Gao, M.; Liu, M.-X.; Yu, C.-Z.; Yan, H.; Peng, C.; Zhao, Y.; Li, Y.; et al. MicroRNA-1304 suppresses human non-small cell lung cancer cell growth in vitro by targeting heme oxygenase-1. Acta Pharmacol. Sin. 2017, 38, 110–119. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zheng, G.; Yang, Y.; Du, L.; Dong, Z.; Zhang, X.; Wang, C. Clinical evaluation and therapeutic monitoring value of serum tumor markers in lung cancer. Int. J. Biol. Markers 2016, 31, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; He, J.; Lv, B.; Xi, X.; He, G.; He, J. PTK7 expression is associated with lymph node metastasis, ALK and EGFR mutations in lung adenocarcinomas. Histol. Histopathol. 2020, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Wang, J. Expression of pleiotrophin in small cell lung cancer. J. Biol. Regul. Homeost. Agents 2015, 29, 175–179. [Google Scholar] [PubMed]
- Jen, J.; Wang, Y.-C. Zinc finger proteins in cancer progression. J. Biomed. Sci. 2016, 23, 53. [Google Scholar] [CrossRef]

| Primer | Sequence 5′–3′ |
|---|---|
| 18S RNA-Forward | ATGGCCGTTCTGAGTTGGTG |
| 18S RNA-Reverse | CGCTGAGCCAGTCAGTGTAG |
| PRKCQ-Forward | CTTGTGGCAGCTTTGGATGT |
| PRKCQ-Reverse | CGTTTCTGACGCACATGTTT |
| NrCAM-Forward | TTGTGCAAAGAGGGAGCATG |
| NrCAM-Reverse | GGGCAGTTCCCTGTTGTCCT |
| CDKN1A-Forward | GCAGACCAGCATGACAGATTT |
| CDKN1A-Reverse | GGATTAGGGCTTCCTCTTGGA |
| RHOH Forward | GAGAAGTAACATTCTGCAAATCGC |
| RHOH Reverse | AGCACACGCCATTCAGCAAG |
| XIST-Forward | AGGTCAGGCAGAGGAAGTCA |
| XIST-Reverse | AGGTCAGGCAGAGGAAGTCA |
| ROX1-AS1 Forward | CTAGTTAGCAGGGGCAGCAC |
| PROX1-AS1 Reverse | AACAGAGAGGCGTGGAAGAA |
| MYC-Forward | TGGACATCCGCAAAGACCTGTAC |
| MYC-Reverse | TCAGGAGGAGCAATGATCTTGA |
| SFTA1P-Forward | CAGCATTCCAGGTGGGCTTT |
| SFTA1P-Reverse | CCTTGTTTGGCTTACTCGTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katopodis, P.; Dong, Q.; Halai, H.; Fratila, C.I.; Polychronis, A.; Anikin, V.; Sisu, C.; Karteris, E. In Silico and In Vitro Analysis of lncRNA XIST Reveals a Panel of Possible Lung Cancer Regulators and a Five-Gene Diagnostic Signature. Cancers 2020, 12, 3499. https://doi.org/10.3390/cancers12123499
Katopodis P, Dong Q, Halai H, Fratila CI, Polychronis A, Anikin V, Sisu C, Karteris E. In Silico and In Vitro Analysis of lncRNA XIST Reveals a Panel of Possible Lung Cancer Regulators and a Five-Gene Diagnostic Signature. Cancers. 2020; 12(12):3499. https://doi.org/10.3390/cancers12123499
Chicago/Turabian StyleKatopodis, Periklis, Qiduo Dong, Heerni Halai, Cristian I. Fratila, Andreas Polychronis, Vladimir Anikin, Cristina Sisu, and Emmanouil Karteris. 2020. "In Silico and In Vitro Analysis of lncRNA XIST Reveals a Panel of Possible Lung Cancer Regulators and a Five-Gene Diagnostic Signature" Cancers 12, no. 12: 3499. https://doi.org/10.3390/cancers12123499
APA StyleKatopodis, P., Dong, Q., Halai, H., Fratila, C. I., Polychronis, A., Anikin, V., Sisu, C., & Karteris, E. (2020). In Silico and In Vitro Analysis of lncRNA XIST Reveals a Panel of Possible Lung Cancer Regulators and a Five-Gene Diagnostic Signature. Cancers, 12(12), 3499. https://doi.org/10.3390/cancers12123499






