Circulating Interleukin-4 Is Associated with a Systemic T Cell Response against Tumor-Associated Antigens in Treatment-Naïve Patients with Resectable Non-Small-Cell Lung Cancer
Abstract
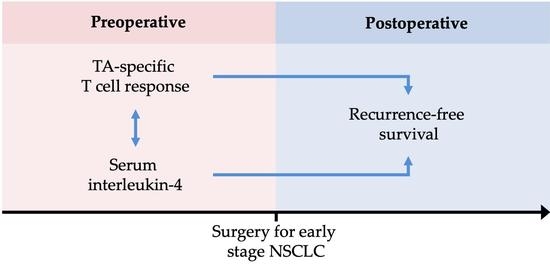
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Association of the TA-Specific Response with Clinical Parameters
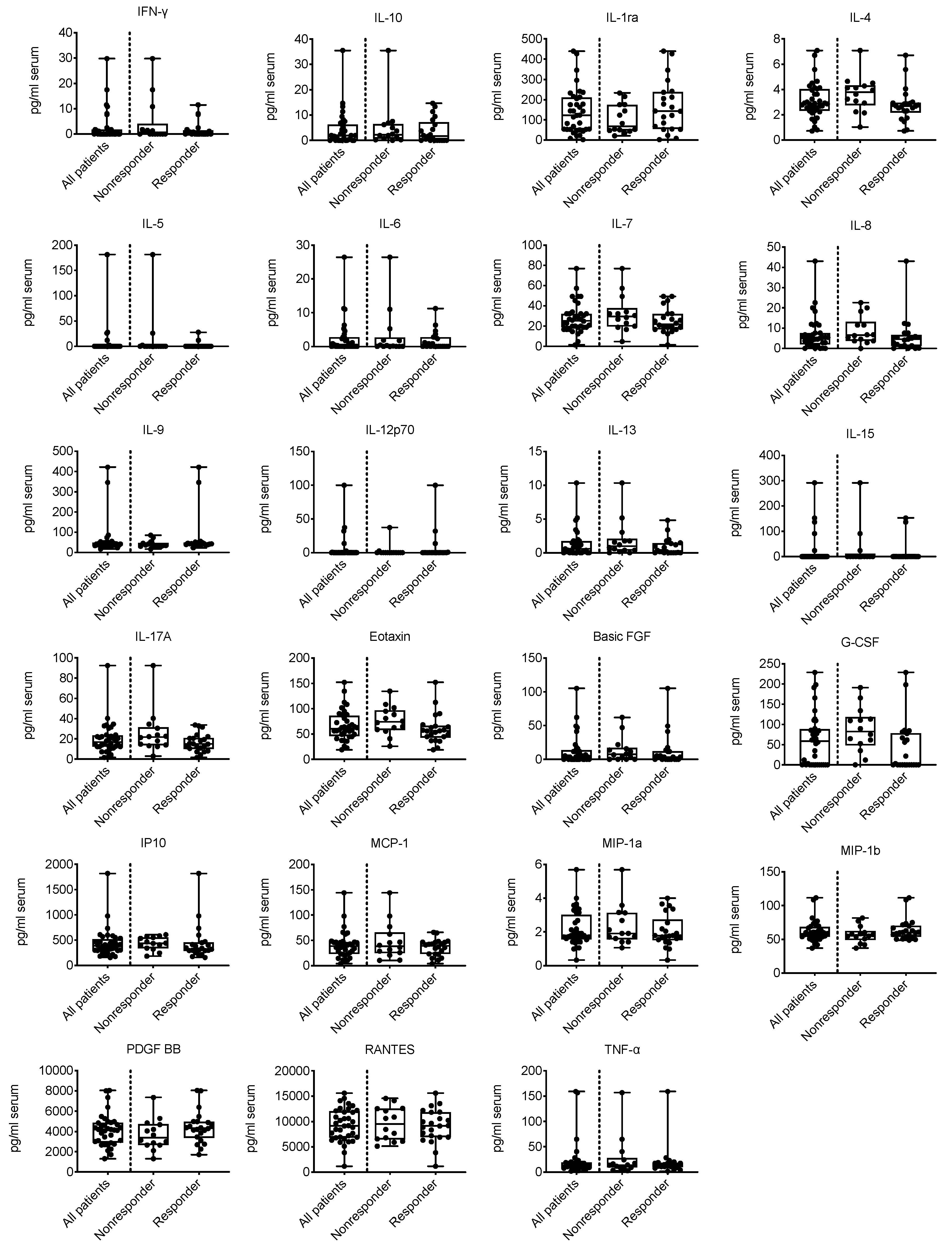
2.2. Association of the TA-Specific Response with Intratumor and Serum Cytokine Levels
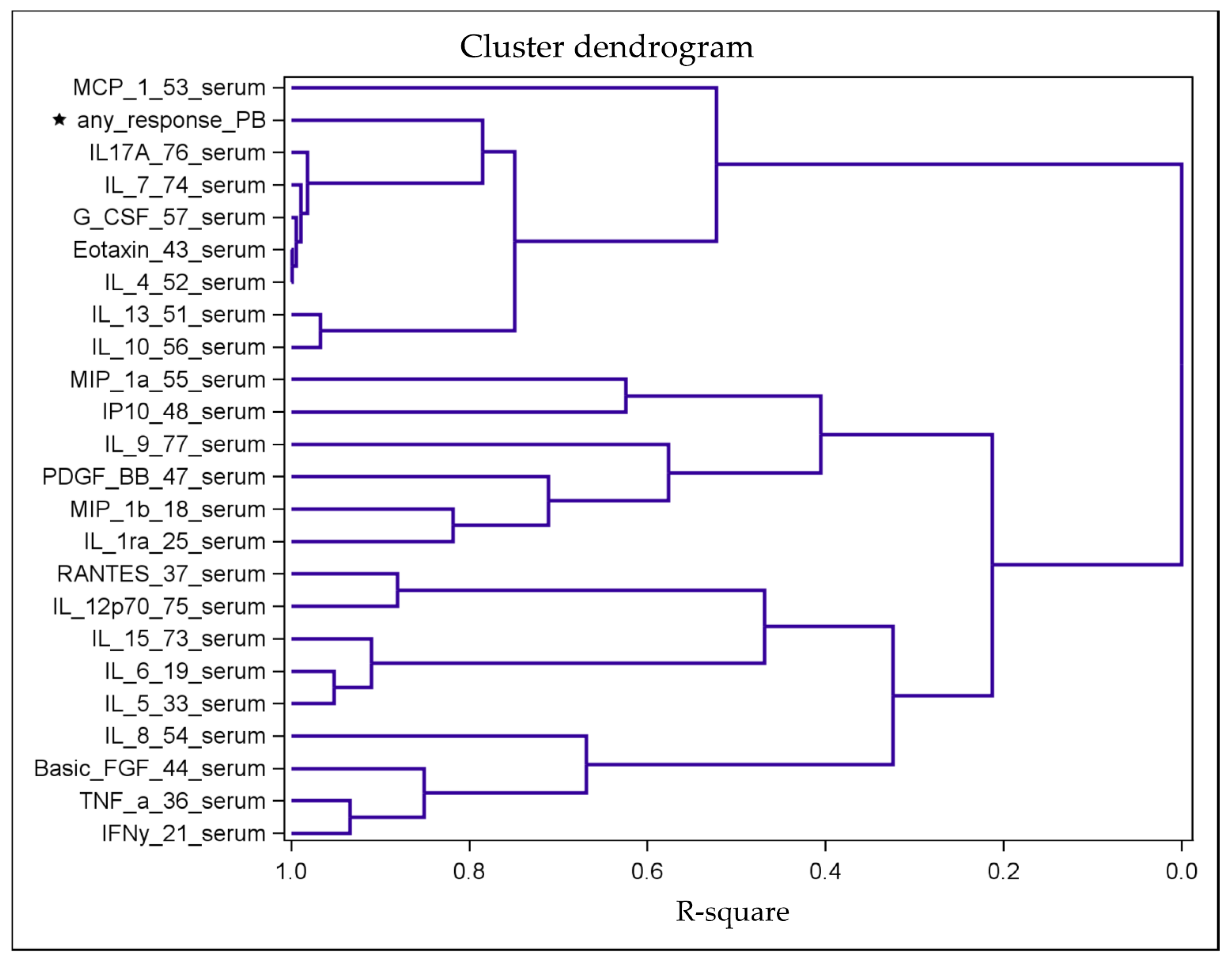
2.3. Identification of Distinct Clusters
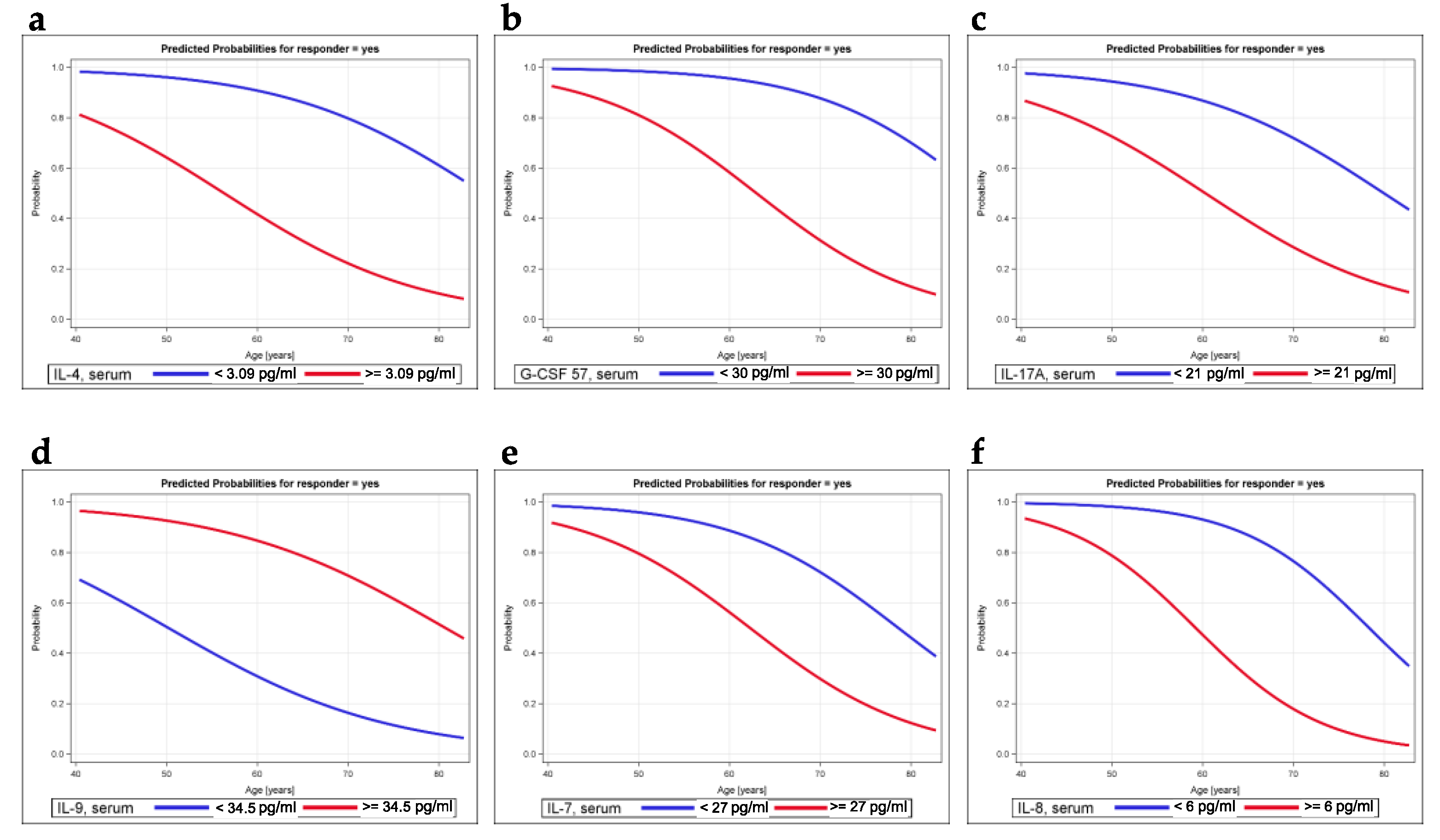
2.4. Modeling the Probability of Being a Responder
2.5. Multivariable Model for Being a Responder
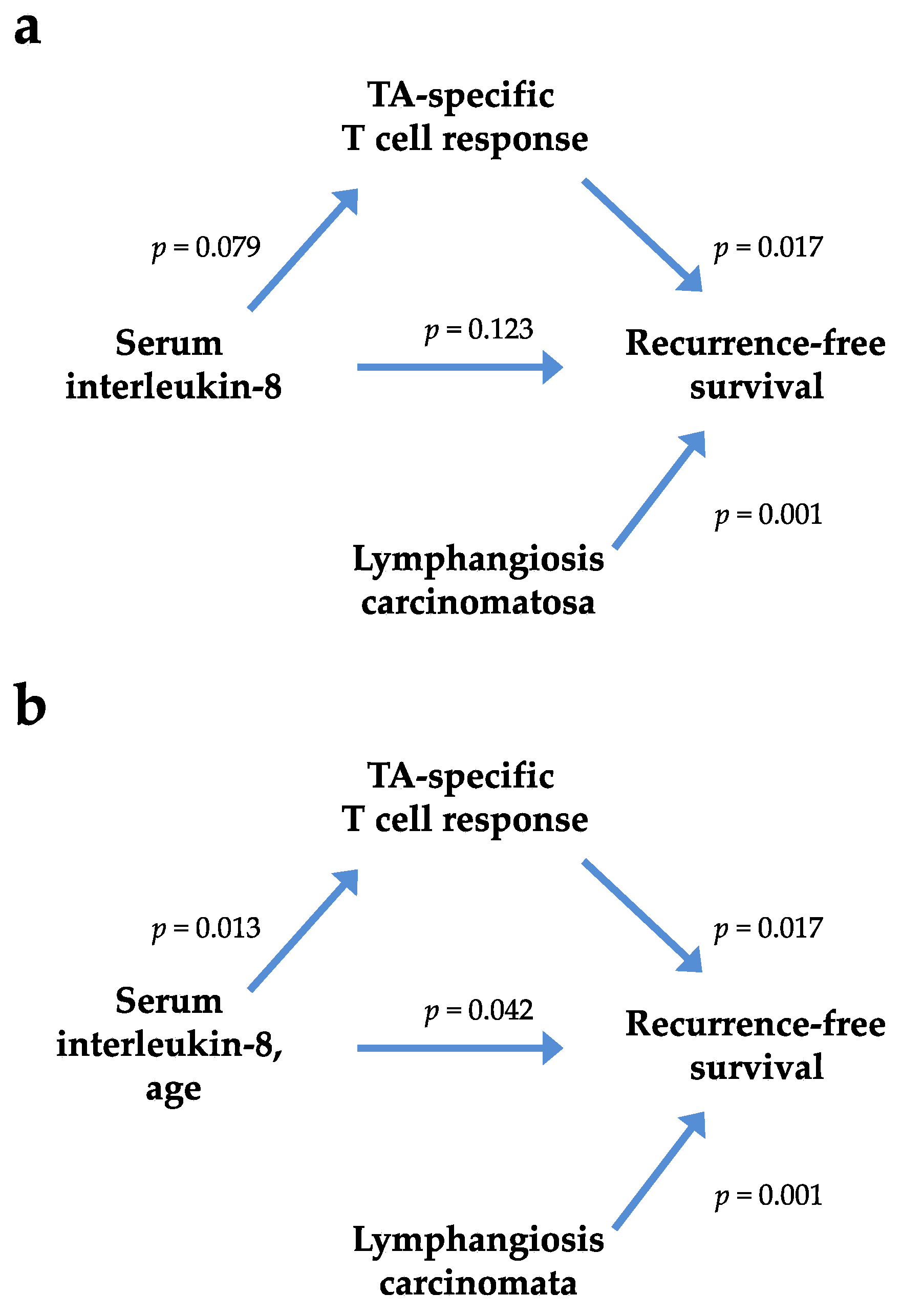
2.6. Associations between Cytokine Levels and Risk Factors for Tumor Recurrence
2.7. Association of Cytokine Levels with RFS
3. Discussion
4. Materials and Methods
4.1. Study Approval and Patients
4.2. Patient Samples
4.3. Preparation of Serum and Tumor Samples
4.4. Surgery
4.5. Postoperative Follow-Up
4.6. Cell Purification and Culture
4.7. Antigens
4.8. IFN-γ ELISPOT Assay
4.9. Cytokine Quantification
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ettinger, D.S.; Akerley, W.; Borghaei, H.; Chang, A.C.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Govindan, R.; Grannis, F.W.; et al. Non-small cell lung cancer, version 2.2013. J. Natl. Compr. Canc. Netw. 2013, 11, 645–653. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Goya, T.; Koshiishi, Y.; Sohara, Y.; Eguchi, K.; Mori, M.; Nakanishi, Y.; Tsuchiya, R.; Shimokata, K.; Inoue, H.; et al. A Japanese lung cancer registry study: Prognosis of 13,010 resected lung cancers. J. Thorac. Oncol. 2008, 3, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Muley, T.; Safi, S.; Rieken, S.; Bischoff, H.; Kappes, J.; Warth, A.; Herth, F.J.; Dienemann, H.; Hoffmann, H. The dynamic pattern of recurrence in curatively resected non-small cell lung cancer patients: Experiences at a single institution. Lung Cancer 2015, 90, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Remark, R.; Becker, C.; Gomez, J.E.; Damotte, D.; Dieu-Nosjean, M.C.; Sautes-Fridman, C.; Fridman, W.H.; Powell, C.A.; Altorki, N.K.; Merad, M.; et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am. J. Respir. Crit. Care Med. 2015, 191, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.; Yamauchi, Y.; Rathinasamy, A.; Stamova, S.; Eichhorn, M.; Warth, A.; Rauch, G.; Dienemann, H.; Hoffmann, H.; Beckhove, P. Functional T cells targeting tumor-associated antigens are predictive for recurrence-free survival of patients with radically operated non-small cell lung cancer. Oncoimmunology 2017, 6, e1360458. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Pine, S.R.; Mechanic, L.E.; Enewold, L.; Chaturvedi, A.K.; Katki, H.A.; Zheng, Y.L.; Bowman, E.D.; Engels, E.A.; Caporaso, N.E.; Harris, C.C. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J. Natl. Cancer Inst. 2011, 103, 1112–1122. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Onate, C.; Perez, G.; Alfaro, C.; Martin-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Morishima, C.; Velcheti, V.; Miller, J.S.; Lee, S.M.; Silk, A.W.; Holtan, S.G.; Lacroix, A.M.; Fling, S.P.; Kaiser, J.C.; et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin. Cancer Res. 2018, 24, 5552–5561. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.K.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Mariano, V.S.; Pastrez, P.R.A.; Pinto, M.C.; Castro, A.G.; Syrjanen, K.J.; Longatto-Filho, A. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS ONE 2017, 12, e0181125. [Google Scholar] [CrossRef] [PubMed]
- Barrera, L.; Montes-Servin, E.; Barrera, A.; Ramirez-Tirado, L.A.; Salinas-Parra, F.; Banales-Mendez, J.L.; Sandoval-Rios, M.; Arrieta, O. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Ann. Oncol. 2015, 26, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Imanishi, R.; Adachi, M.; Ikejima, S.; Nakata, J.; Morimoto, S.; Fujiki, F.; Nishida, S.; Tsuboi, A.; Hosen, N.; et al. Reader-free ELISPOT assay for immuno-monitoring in peptide-based cancer vaccine immunotherapy. Biomed. Rep. 2020, 12, 244–250. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Johnstone, J.; Millar, J.; Dorrington, M.G.; Habibagahi, M.; Lelic, A.; Loeb, M.; Bramson, J.L.; Bowdish, D.M. Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 2013, 93, 633–637. [Google Scholar] [CrossRef]
- Rosenkranz, D.; Weyer, S.; Tolosa, E.; Gaenslen, A.; Berg, D.; Leyhe, T.; Gasser, T.; Stoltze, L. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J. Neuroimmunol. 2007, 188, 117–127. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, D.K.; Tscharke, D.C.; Jackson, R.J.; Ranasinghe, C. Reduced interleukin-4 receptor alpha expression on CD8+ T cells correlates with higher quality anti-viral immunity. PLoS ONE 2013, 8, e55788. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, N.; Olver, S.; Buttigieg, K.; Groves, P.; Janas, M.L.; Baz, A.; Kelso, A. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J. Immunol. 2005, 174, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, J.; Kowalska, M.; Kotowicz, B.; Fuksiewicz, M.; Glogowski, M.; Wojcik, E.; Chechlinska, M.; Steffen, J. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF—An independent prognostic factor. Oncology 2006, 70, 115–125. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, H.S.; Youn, J.C.; Shin, E.C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine levels in the serum of healthy subjects. Mediat. Inflamm. 2013, 2013, 434010. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Lee, P.; Sharma, S.; Mao, J.T.; Meissner, H.; Uyemura, K.; Modlin, R.; Wollman, J.; Dubinett, S.M. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995, 55, 3847–3853. [Google Scholar]
- Yamauchi, Y.; Safi, S.; Blattner, C.; Rathinasamy, A.; Umansky, L.; Juenger, S.; Warth, A.; Eichhorn, M.; Muley, T.; Herth, F.J.F.; et al. Circulating and tumor myeloid-derived suppressor cells in resectable non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 777–787. [Google Scholar] [CrossRef]
- Aziz, N. Measurement of circulating cytokines and immune-activation markers by multiplex technology in the clinical setting: What are we really measuring? For. Immunopathol. Dis. Therap. 2015, 6, 19–22. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, J.; Wang, Z.; Zhang, J.; Xiao, M.; Wang, C.; Lu, Y.; Qin, Z. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008, 68, 8687–8694. [Google Scholar] [CrossRef]
- Guo, N.; Shen, G.; Zhang, Y.; Moustafa, A.A.; Ge, D.; You, Z. Interleukin-17 promotes migration and invasion of human cancer cells through upregulation of MTA1 expression. Front. Oncol. 2019, 9, 546. [Google Scholar] [CrossRef]
- Aliper, A.M.; Frieden-Korovkina, V.P.; Buzdin, A.; Roumiantsev, S.A.; Zhavoronkov, A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014, 3, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Levina, V.; Nolen, B.M.; Marrangoni, A.M.; Cheng, P.; Marks, J.R.; Szczepanski, M.J.; Szajnik, M.E.; Gorelik, E.; Lokshin, A.E. Role of eotaxin-1 signaling in ovarian cancer. Clin. Cancer Res. 2009, 15, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Mechanisms of immune suppression by myeloid-derived suppressor cells: The role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020, 10, 200111. [Google Scholar] [CrossRef] [PubMed]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef]
- Asselin-Paturel, C.; Echchakir, H.; Carayol, G.; Gay, F.; Opolon, P.; Grunenwald, D.; Chouaib, S.; Mami-Chouaib, F. Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int. J. Cancer 1998, 77, 7–12. [Google Scholar] [CrossRef]
- Ito, S.E.; Shirota, H.; Kasahara, Y.; Saijo, K.; Ishioka, C. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol. Immunother. 2017, 66, 1485–1496. [Google Scholar] [CrossRef]
- Volonte, A.; Di Tomaso, T.; Spinelli, M.; Todaro, M.; Sanvito, F.; Albarello, L.; Bissolati, M.; Ghirardelli, L.; Orsenigo, E.; Ferrone, S.; et al. Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. J. Immunol. 2014, 192, 523–532. [Google Scholar] [CrossRef]
- Waldmann, T.A. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028472. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Velcheti, V.; Patel, M.R.; Garrett-Mayer, E.; Hill, E.G.; Ravenel, J.G.; Miller, J.S.; Farhad, M.; Anderton, K.; Lindsey, K.; et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: A non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018, 19, 694–704. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology 2016, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Faruki, H.; Mayhew, G.M.; Serody, J.S.; Hayes, D.N.; Perou, C.M.; Lai-Goldman, M. Lung adenocarcinoma and squamous cell carcinoma gene expression subtypes demonstrate significant differences in tumor immune landscape. J. Thorac. Oncol. 2017, 12, 943–953. [Google Scholar] [CrossRef]
- Althouse, A.D. Adjust for Multiple Comparisons? It’s Not That Simple. Ann. Thorac. Surg. 2016, 101, 1644–1645. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Palucka, A.K.; Coussens, L.M. The basis of oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef]
- Feuerer, M.; Beckhove, P.; Bai, L.; Solomayer, E.F.; Bastert, G.; Diel, I.J.; Pedain, C.; Oberniedermayr, M.; Schirrmacher, V.; Umansky, V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat. Med. 2001, 7, 452–458. [Google Scholar] [CrossRef]
- Dienemann, H.; Hoffmann, H.; Koebe, H.G. Technique and rationale of lymph node dissection in bronchial carcinoma. Chirurg 1998, 69, 412–417. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Lerut, T.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley: Oxford, UK, 2017; pp. 22–29. [Google Scholar]
- Reissfelder, C.; Timke, C.; Schmitz-Winnenthal, H.; Rahbari, N.N.; Koch, M.; Klug, F.; Roeder, F.; Edler, L.; Debus, J.; Buchler, M.W.; et al. A randomized controlled trial to investigate the influence of low dose radiotherapy on immune stimulatory effects in liver metastases of colorectal cancer. BMC Cancer 2011, 11, 419. [Google Scholar] [CrossRef]
- Kraemer, H.C. Encyclopaedia of statistical sciences. In Biserial Correlation; Kotz, S., Johnson, N.L., Eds.; Wiley: New York, NY, USA, 1982; Volume 1, pp. 276–279. [Google Scholar]

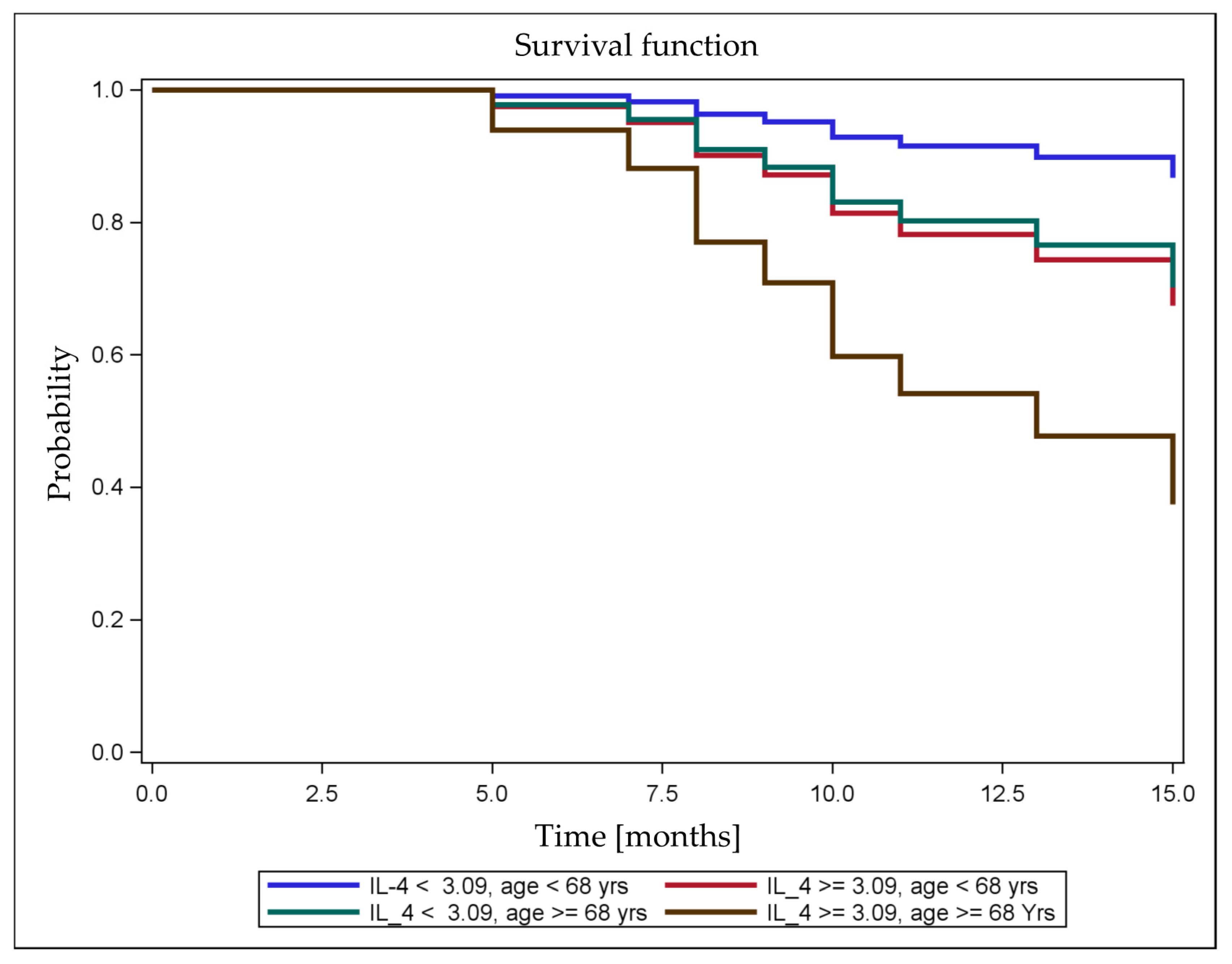
| Cytokine | n | Correlation Coefficients | Nonresponder (n = 14) | Responder (n = 22) | Wilcoxon Rank-Sum Test | |
|---|---|---|---|---|---|---|
| Rang Biserial | Spearman | Median | Median | p-Value | ||
| IFN-γ, serum | 36 | −0.0584 | −0.0528 | 0.37 | 0.02 | 0.770 |
| IL-10, serum | 35 | −0.1735 | −0.1486 | 2.21 | 1.62 | 0.402 |
| IL-1ra, serum | 36 | 0.2890 | 0.2441 | 68.61 | 143.36 | 0.162 |
| IL-4, serum | 36 | −0.4546 | −0.3841 | 3.79 | 2.68 | 0.030 |
| IL-5, serum | 36 | −0.1234 | −0.1733 | 0.00 | 0.00 | 0.325 |
| IL-6, serum | 35 | −0.0476 | −0.0430 | 0.21 | 0.00 | 0.818 |
| IL-7, serum | 36 | −0.2760 | −0.2336 | 29.50 | 21.85 | 0.181 |
| IL-8, serum | 36 | −0.2727 | −0.2308 | 6.52 | 4.78 | 0.186 |
| IL-9, serum | 36 | 0.3507 | 0.2963 | 35.56 | 42.59 | 0.091 |
| IL-12p70, serum | 36 | 0.0390 | 0.0507 | 0.00 | 0.00 | 0.785 |
| IL-13, serum | 36 | −0.2435 | −0.2076 | 0.99 | 0.275 | 0.234 |
| IL-15, serum | 35 | −0.1329 | −0.1828 | 0.00 | 0.00 | 0.307 |
| IL-17A, serum | 36 | −0.3571 | −0.3019 | 21.79 | 14.96 | 0.086 |
| Eotaxin, serum | 36 | −0.3961 | −0.3346 | 74.13 | 56.15 | 0.058 |
| Basic FGF, serum | 36 | −0.1786 | −0.1549 | 7.38 | 3.39 | 0.374 |
| G-CSF, serum | 36 | −0.4870 | −0.4192 | 82.41 | 1.64 | 0.019 |
| IP10, serum | 36 | −0.2857 | −0.2413 | 439.00 | 328.28 | 0.167 |
| MCP-1, serum | 36 | −0.0910 | −0.0768 | 38.38 | 39.02 | 0.664 |
| MIP-1a, serum | 36 | −0.1071 | −0.0906 | 1.91 | 1.78 | 0.607 |
| MIP-1b, serum | 36 | 0.2727 | 0.2304 | 56.055 | 60.155 | 0.187 |
| PDGF BB, serum | 36 | 0.2987 | 0.2523 | 3386.99 | 4328.51 | 0.149 |
| RANTES, serum | 36 | −0.0455 | −0.0384 | 9543.66 | 9162.47 | 0.834 |
| TNF-α, serum | 36 | 0.0227 | 0.0192 | 13.805 | 14.15 | 0.923 |
| Variable | Cutoff (pg/mL) | Crude Logistic Regression Model | Model Adjusted for Age | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | aOR (95% CI) | p-Value | ||
| IL-4, serum | 3.09 | 11.3 (2.3–55.0) | 0.003 | 13.8 (2.3–82.1) | 0.004 |
| IL-17A, serum | 21 | 4.5 (1.06–19.4) | 0.042 | 6.4 (1.2–34.0) | 0.029 |
| Eotaxin, serum | 58 | 5.3 (1.14–24.5) | 0.033 | 5.9 (1.13–31.1) | 0.036 |
| G_CSF, serum | 30 | 8.7 (1.6–48.5) | 0.014 | 15.8 (2.0–122.3) | 0.008 |
| IL-7, serum | 27 | 2.9 (0.71–11.4) | 0.138 | 6.1 (1.03–36.1) | 0.037 |
| IL-9, serum | 34.5 | 0.1 (0.012–0.6) | 0.012 | 0.08 (0.011–0.57) | 0.012 |
| IL-1ra, serum | 178 | 0.24 (0.04–1.35) | 0.105 | 0.27 (0.05–1.63) | 0.154 |
| IL-8, serum | 6 | 3.56 (0.86–14.63) | 0.079 | 15.0 (1.78–126.5) | 0.013 |
| IP10, serum | 400 | 4.38 (1.03–18.63) | 0.046 | 3.69 (0.82–16.6) | 0.089 |
| MIP-1b, serum | 58.5 | 0.23 (0.05–0.97) | 0.046 | 0.26 (0.06–1.16) | 0.077 |
| PDGF BB, serum | 4100 | 0.26 (0.06–1.07) | 0.061 | 0.36 (0.08–1.67) | 0.193 |
| Variable | Wald Chi-Square p-Value | Adjusted OR (95% CI) |
|---|---|---|
| Age [year] | 0.0335 | 1.16 (1.00–1.35) |
| IL-4, serum (cutoff = 3.09 pg/mL) | 0.0468 | 23.23 (1.70–318.1) |
| IL-8, serum (cutoff = 6 pg/mL) | 0.0185 | 22.50 (1.12–453.3) |
| MIP-1b, serum (cutoff = 58.5 pg/mL) | 0.0422 | 0.054 (0.003–0.89) |
| Variable | Log Rank | M1: Crude Cox Regression Model | M2: Age-Adjusted Cox Regression Model | M3: Cox Regression Model Including Cytokine Levels, the TA-Specific Response and Age | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Cytokine | Cytokine | TA-Specific Response | ||||||
| p | HR (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p | |
| IL-4 | 0.097 | 2.8 (0.78–10.0) | 0.114 | 2.8 (0.78–9.9) | 0.116 | 1.4 (0.32–6.5) | 0.634 | 0.30 (0.06–1.6) | 0.154 |
| IL-17A | 0.175 | 2.3 (0.66–8.1) | 0.190 | 2.2 (0.62–7.6) | 0.222 | 1.3 (0.33–5.0) | 0.724 | 0.27 (0.06–1.2) | 0.091 |
| Eotaxin | 0.860 | 1.1 (0.32–4.0) | 0.861 | 1.1 (0.30–3.8) | 0.920 | 0.65 (0.17–2.6) | 0.535 | 0.21 (0.05–0.93) | 0.040 |
| G CSF | 0.118 | 3.2 (0.68–15.1) | 0.142 | 3.3 (0.69–15.5) | 0.134 | 2.0 (0.38–11.0 | 0.408 | 0.33 (0.07–1.4) | 0.138 |
| IL-7 | 0.482 | 2.1 (0.60–7.5) | 0.243 | 2.6 (0.71–9.2) | 0.152 | 1.8 (0.45–7.2) | 0.407 | 0.30 (0.07–1.3) | 0.098 |
| IL-9 | 0.685 | 0.76 (0.20–2.9) | 0.688 | 0.89 (0.23–3.5) | 0.869 | 1.9 (0.44–8.1) | 0.398 | 0.19 (0.04–0.83) | 0.028 |
| IL-1ra | 0.908 | 0.92 (0.24–3.6) | 0.908 | 0.97 (0.25–3.8) | 0.969 | 1.8 (0.41–8.2) | 0.432 | 0.20 (0.04–0.90) | 0.037 |
| IL-8 | 0.106 | 2.7 (0.76–9.6) | 0.123 | 3.9 (1.05–14.5) | 0.042 | 2.2 (0.50–10.1) | 0.289 | 0.37 (0.07–1.9) | 0.227 |
| IP-10 | 0.446 | 1.6 (0.46–5.8) | 0.454 | 1.2 (0.32–4.7) | 0.760 | 1.0 (0.27–3.9) | 0.971 | 0.25 (0.06-0.99) | 0.048 |
| MIP 1b | 0.223 | 0.44 (0.11–1.7) | 0.234 | 0.48 (0.12–1.9) | 0.294 | 0.67 (0.16–2.8) | 0.578 | 0.27 (0.07–1.1) | 0.072 |
| PDGF | 0.712 | 1.3 (0.36–4.5) | 0.715 | 1.7 (0.45–6.2) | 0.438 | 2.8 (0.70–11.0) | 0.115 | 0.18 (0.04–0.76) | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safi, S.; Yamauchi, Y.; Hoffmann, H.; Weichert, W.; Jost, P.J.; Winter, H.; Muley, T.; Beckhove, P. Circulating Interleukin-4 Is Associated with a Systemic T Cell Response against Tumor-Associated Antigens in Treatment-Naïve Patients with Resectable Non-Small-Cell Lung Cancer. Cancers 2020, 12, 3496. https://doi.org/10.3390/cancers12123496
Safi S, Yamauchi Y, Hoffmann H, Weichert W, Jost PJ, Winter H, Muley T, Beckhove P. Circulating Interleukin-4 Is Associated with a Systemic T Cell Response against Tumor-Associated Antigens in Treatment-Naïve Patients with Resectable Non-Small-Cell Lung Cancer. Cancers. 2020; 12(12):3496. https://doi.org/10.3390/cancers12123496
Chicago/Turabian StyleSafi, Seyer, Yoshikane Yamauchi, Hans Hoffmann, Wilko Weichert, Philipp J. Jost, Hauke Winter, Thomas Muley, and Philipp Beckhove. 2020. "Circulating Interleukin-4 Is Associated with a Systemic T Cell Response against Tumor-Associated Antigens in Treatment-Naïve Patients with Resectable Non-Small-Cell Lung Cancer" Cancers 12, no. 12: 3496. https://doi.org/10.3390/cancers12123496
APA StyleSafi, S., Yamauchi, Y., Hoffmann, H., Weichert, W., Jost, P. J., Winter, H., Muley, T., & Beckhove, P. (2020). Circulating Interleukin-4 Is Associated with a Systemic T Cell Response against Tumor-Associated Antigens in Treatment-Naïve Patients with Resectable Non-Small-Cell Lung Cancer. Cancers, 12(12), 3496. https://doi.org/10.3390/cancers12123496