The Interplay between Cancer Biology and the Endocannabinoid System—Significance for Cancer Risk, Prognosis and Response to Treatment
Simple Summary
Abstract
1. Introduction
2. The Interplay between Cancer Biology and the Endocannabinoid System
2.1. Breast Cancer
2.1.1. Cannabinoids and Hormone-Sensitive Breast Cancer
2.1.2. Cannabinoids and HER2-Positive Breast Cancer
2.1.3. Cannabinoids and Triple-Negative Breast Cancer
2.2. Gastrointestinal Malignancies
2.3. Gynecological Malignancies
2.3.1. Endometrial Cancer (EMC)
2.3.2. Ovarian Cancer (OC)
2.3.3. Cervical Cancer (CC)
2.4. Prostate Cancer (PC)
2.5. Thoracic Tumours
2.6. Thyroid Cancer (TC)
2.7. Central Nervous System Malignancies
2.8. Melanoma
3. Legal and Ethical Aspects of ECS Exploitation in Oncology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wu, J. Cannabis, cannabinoid receptors, and endocannabinoid system: Yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019, 40, 297–299. [Google Scholar] [CrossRef] [PubMed]
- HUGO(a), G.N.C. CNR1. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/2159 (accessed on 15 July 2020).
- HUGO(b), G.N.C. CNR2. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:2160 (accessed on 15 July 2020).
- HUGO(c), G.N.C. GPR18. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:4472 (accessed on 15 July 2020).
- HUGO(d), G.N.C. GPR55. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:4511 (accessed on 15 July 2020).
- HUGO(e), G.N.C. GPR119. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:19060 (accessed on 15 July 2020).
- HUGO(f), G.N.C. TRPV1. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:12716 (accessed on 21 July 2020).
- HUGO(g), G.N.C. TRPV2. Available online: https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:18082 (accessed on 21 July 2020).
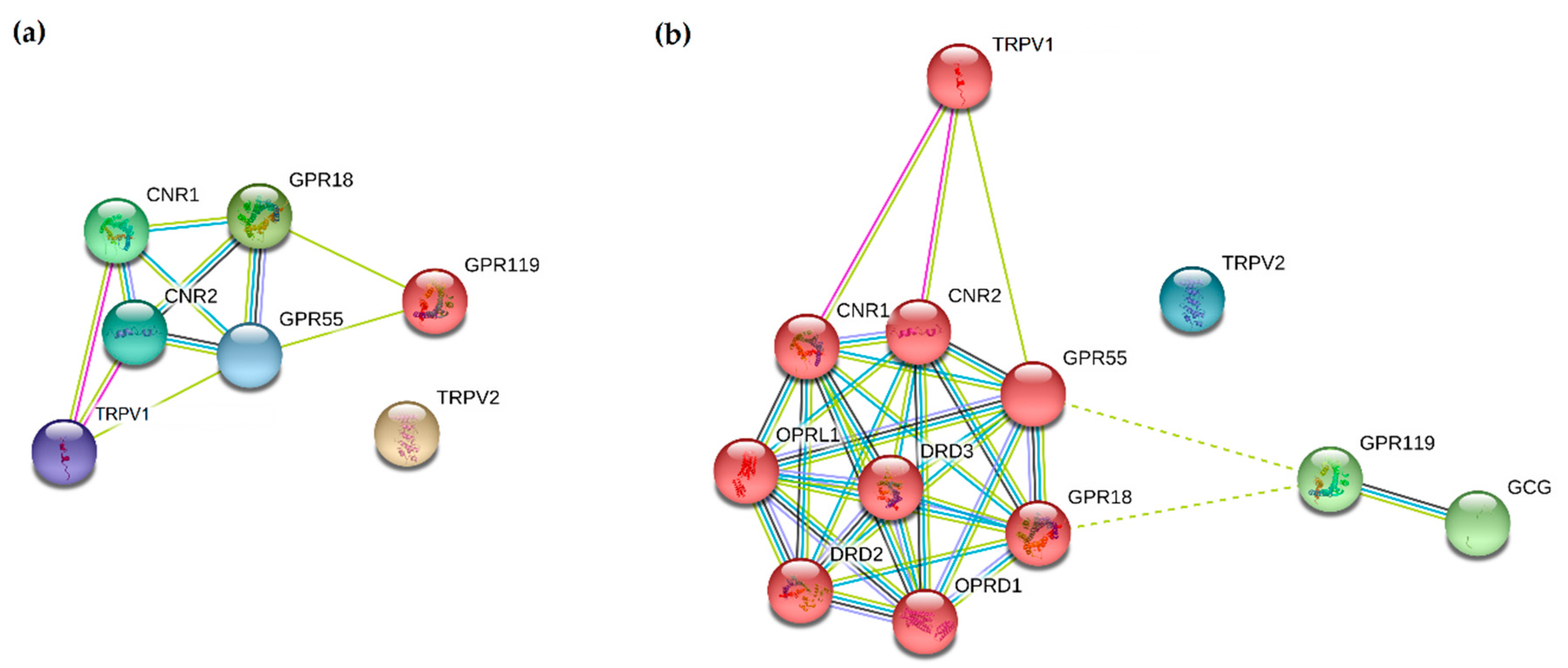
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Čavić, M.; Lluís, C.; Moreno, E.; Bakešová, J.; Canela, E.I.; Navarro, G. Production of functional recombinant G-protein coupled receptors for heteromerization studies. J. Neurosci. Methods 2011, 199, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Casadó, V.; Barrondo, S.; Spasic, M.; Callado, L.F.; Mallol, J.; Canela, E.; Lluís, C.; Meana, J.; Cortés, A.; Sallés, J.; et al. Gi protein coupling to adenosine A1-A2A receptor heteromers in human brain caudate nucleus. J. Neurochem. 2010, 114, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Andradas, C.; Medrano, M.; Caffarel, M.M.; Pérez-Gómez, E.; Blasco-Benito, S.; Gómez-Cañas, M.; Pazos, M.R.; Irving, A.J.; Lluís, C.; et al. Targeting CB2-GPR55 receptor heteromers modulates cancer cell signaling. J. Biol. Chem. 2014, 289, 21960–21972. [Google Scholar] [CrossRef] [PubMed]
- Human Protein Atlas Database. Available online: http://www.proteinatlas.org/pathology (accessed on 21 July 2020).
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- UALCAN Database. Available online: http://ualcan.path.uab.edu/analysis.html (accessed on 21 July 2020).
- National Cancer Institute and the National Human Genome Research Institute The Cancer Genome Atlas. Available online: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga/studied-cancers (accessed on 21 July 2020).
- Database, H.P.A. GPR119 in Pancreatic Cancer. Available online: https://www.proteinatlas.org/ENSG00000147262-GPR119/pathology/pancreatic+cancer (accessed on 21 July 2020).
- Database, H.P.A. TRPV2 in Renal Cancer. Available online: https://www.proteinatlas.org/ENSG00000187688-TRPV2/pathology/renal+cancer (accessed on 21 July 2020).
- Database, H.P.A. TRPV2 in Testicular Cancer. Available online: https://www.proteinatlas.org/ENSG00000187688-TRPV2/pathology/testis+cancer (accessed on 21 July 2020).
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- DeSantis, C.; Siegel, R.; Bandi, P.; Jemal, A. Breast cancer statistics, 2011. CA Cancer J. Clin. 2011, 61, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Baselga, J. Targeted therapies for breast cancer. J. Clin. Investig. 2011, 121, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Koehler, K.F.; Gustafsson, J.Å. Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discov. 2011, 10, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Srdic-Rajic, T.; Santibañez, J.F.; Kanjer, K.; Tisma-Miletic, N.; Cavic, M.; Galun, D.; Jevric, M.; Kardum, N.; Konic-Ristic, A.; Zoranovic, T. Iscador Qu inhibits doxorubicin-induced senescence of MCF7 cells. Sci. Rep. 2017, 7, 3763. [Google Scholar] [CrossRef]
- McAllister, S.D.; Soroceanu, L.; Desprez, P.-Y. The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. J. Neuroimmune Pharmacol. 2015, 10, 255–267. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Pérez-Gómez, E.; Guzmán, M.; Sánchez, C. Cannabinoids: A new hope for breast cancer therapy? Cancer Treat. Rev. 2012, 38, 911–918. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Melck, D.; Rueda, D.; Galve-Roperh, I.; De Petrocellis, L.; Guzmán, M.; Di Marzo, V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999, 463, 235–240. [Google Scholar] [CrossRef]
- Melck, D.; De Petrocellis, L.; Orlando, P.; Bisogno, T.; Laezza, C.; Bifulco, M.; Marzo, V.D.I. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 2000, 141, 118–126. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Sarrió, D.; Palacios, J.; Guzmán, M.; Sánchez, C. Δ9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res. 2006, 66, 6615–6621. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Moreno-Bueno, G.; Cerutti, C.; Palacios, J.; Guzman, M.; Mechta-Grigoriou, F.; Sanchez, C. JunD is involved in the antiproliferative effect of Δ9-tetrahydrocannabinol on human breast cancer cells. Oncogene 2008, 27, 5033–5044. [Google Scholar] [CrossRef]
- Zhang, H.; Berezov, A.; Wang, Q.; Zhang, G.; Drebin, J.; Murali, R.; Greene, M.I. ErbB receptors: From oncogenes to targeted cancer therapies. J. Clin. Investig. 2007, 117, 2051–2058. [Google Scholar] [CrossRef]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S.; et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; DeMorrow, S. Chapter 18 Changes in the Endocannabinoid System May Give Insight into new and Effective Treatments for Cancer. Vitam. Horm. 2009, 81, 469–485. [Google Scholar]
- Pisanti, S.; Bifulco, M. Endocannabinoid system modulation in cancer biology and therapy. Pharmacol. Res. 2009, 60, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and cancer: Therapeutic implication. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- Ursini-Siegel, J.; Schade, B.; Cardiff, R.D.; Muller, W.J. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat. Rev. Cancer 2007, 7, 389–397. [Google Scholar] [CrossRef]
- Nasser, M.W.; Qamri, Z.; Deol, Y.S.; Smith, D.; Shilo, K.; Zou, X.; Ganju, R.K. Crosstalk between chemokine receptor CXCR4 and cannabinoid receptor CB 2 in modulating breast cancer growth and invasion. PLoS ONE 2011, 6, e23901. [Google Scholar] [CrossRef]
- Coke, C.J.; Scarlett, K.A.; Chetram, M.A.; Jones, K.J.; Sandifer, B.J.; Davis, A.S.; Marcus, A.I.; Hinton, C.V. Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J. Biol. Chem. 2016, 291, 9991–10005. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; García-Taboada, E.; Villa-Morales, M.; Moreno, E.; Hamann, S.; Martín-Villar, E.; Flores, J.M.; et al. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Moreno, E.; Seijo-Vila, M.; Tundidor, I.; Andradas, C.; Caffarel, M.M.; Caro-Villalobos, M.; Urigüen, L.; Diez-Alarcia, R.; Moreno-Bueno, G.; et al. Therapeutic targeting of HER2–CB2R heteromers in HER2-positive breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3863–3872. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The endocannabinoid system as a target in cancer diseases: Are we there yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef]
- Laezza, C.; Pisanti, S.; Crescenzi, E.; Bifulco, M. Anandamide inhibits Cdk2 and activates Chk1 leading to cell cycle arrest in human breast cancer cells. FEBS Lett. 2006, 580, 6076–6082. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Δ-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [CrossRef]
- Gardner, B.; Zhu, L.X.; Sharma, S.; Tashkin, D.P.; Dubinett, S.M. Methanandamide increases COX-2 expression and tumor growth in murine lung cancer. FASEB J. 2003, 17, 2157–2159. [Google Scholar] [CrossRef]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Δ-9-Tetrahydrocannabinol Enhances Breast Cancer Growth and Metastasis by Suppression of the Antitumor Immune Response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 9, 2358. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Martín-Ruiz, A.; Martín, P.; Calvo, V.; Provencio, M.; García, J.M. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3β signaling pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef]
- Tutino, V.; Caruso, M.G.; De Nunzio, V.; Lorusso, D.; Veronese, N.; Gigante, I.; Notarnicola, M.; Giannelli, G. Down-Regulation of Cannabinoid Type 1 (CB1) Receptor and its Downstream Signaling Pathways in Metastatic Colorectal Cancer. Cancers (Basel) 2019, 11, 708. [Google Scholar] [CrossRef]
- Pagano, E.; Borrelli, F. Targeting cannabinoid receptors in gastrointestinal cancers for therapeutic uses: Current status and future perspectives. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 871–873. [Google Scholar] [CrossRef]
- Ortega, A.; García-Hernández, V.M.; Ruiz-García, E.; Meneses-García, A.; Herrera-Gómez, A.; Aguilar-Ponce, J.L.; Montes-Servín, E.; Prospero-García, O.; Del Angel, S.A. Comparing the effects of endogenous and synthetic cannabinoid receptor agonists on survival of gastric cancer cells. Life Sci. 2016, 165, 56–62. [Google Scholar] [CrossRef]
- Kogan, N.M.; Schlesinger, M.; Priel, E.; Rabinowitz, R.; Berenshtein, E.; Chevion, M.; Mechoulam, R. HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. 2007, 6, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Raup-Konsavage, W.M.; Johnson, M.; Legare, C.A.; Yochum, G.S.; Morgan, D.J.; Vrana, K.E. Synthetic Cannabinoid Activity Against Colorectal Cancer Cells. Cannabis Cannabinoid Res. 2018, 3, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, T.L.; Mabin, T.; Engelbrecht, A.-M. Cannabinoids: The lows and the highs of chemotherapy-induced nausea and vomiting. Future Oncol. 2019, 15, 1035–1049. [Google Scholar] [CrossRef]
- Vara, D.; Morell, C.; Rodríguez-Henche, N.; Diaz-Laviada, I. Involvement of PPARg in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Diseas 2013, 4, e618. [Google Scholar] [CrossRef]
- Wu, L.; Guo, C.; Wu, J. Therapeutic potential of PPARγ natural agonists in liver diseases. J. Cell. Mol. Med. 2020, 24, 2736–2748. [Google Scholar] [CrossRef]
- Pagano, E.; Borrelli, F.; Orlando, P.; Romano, B.; Monti, M.; Morbidelli, L.; Aviello, G.; Imperatore, R.; Capasso, R.; Piscitelli, F.; et al. Pharmacological inhibition of MAGL attenuates experimental colon carcinogenesis. Pharmacol. Res. 2017, 119, 227–236. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ferlay, J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 207–225. [Google Scholar] [CrossRef]
- Ledford, L.R.C.; Lockwood, S. Scope and Epidemiology of Gynecologic Cancers: An Overview. Semin. Oncol. Nurs. 2019, 35, 147–150. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and Function of the Endocannabinoid System in the Human Ovary. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010, 93, 1989–1996. [Google Scholar] [CrossRef]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The Levels of the Endocannabinoid Receptor CB2 and Its Ligand 2-Arachidonoylglycerol Are Elevated in Endometrial Carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef]
- Schmid, P.C.; Wold, L.E.; Krebsbach, R.J.; Berdyshev, E.V.; Schmid, H.H.O. Anandamide and other N-acylethanolamines in human tumors. Lipids 2002, 37, 907–912. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Maccarrone, M.; Konje, J.C. Identification of Novel Predictive Biomarkers for Endometrial Malignancies: N-Acylethanolamines. Front. Oncol. 2019, 9, 430. [Google Scholar] [CrossRef]
- Nomura, D.K.; Long, J.Z.; Niessen, S.; Hoover, H.S.; Ng, S.W.; Cravatt, B.F. Monoacylglycerol Lipase Regulates a Fatty Acid Network that Promotes Cancer Pathogenesis. Cell 2010, 140, 49–61. [Google Scholar] [CrossRef]
- Pĩeiro, R.; Maffucci, T.; Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 2011, 30, 142–152. [Google Scholar] [CrossRef]
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 211, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef]
- Skorić, M.; Gligorijević, N.; Čavić, M.; Todorović, S.; Janković, R.; Ristić, M.; Mišić, D.; Radulović, S. Cytotoxic activity of Nepeta rtanjensis Diklić & Milojević essential oil and its mode of action. Ind. Crops Prod. 2017, 100, 163–170. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zheng, C.-J.; Peng, C.; Zhang, H.; Jiang, Y.-P.; Han, T.; Qin, L.-P. Plants and cervical cancer: An overview. Expert Opin. Investig. Drugs 2013, 22, 1133–1156. [Google Scholar] [CrossRef]
- Contassot, E.; Tenan, M.; Schnüriger, V.; Pelte, M.F.; Dietrich, P.Y. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol. Oncol. 2004, 93, 182–188. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl. Cancer Inst. 2008, 100, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Webber, M.M.; Bello, D.; Quader, S. Immortalized and tumorigenic adult human prostatic epithelial cell lines: Characteristics and applications part 2. Tumorigenic cell lines. Prostate 1997, 30, 58–64. [Google Scholar] [CrossRef]
- Díaz-Laviada, I. The endocannabinoid system in prostate cancer. Nat. Rev. Urol. 2011, 8, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Serradell, O.; Poblete, C.E.; Sanchez, C.; Castellón, E.A.; Gallegos, I.; Huidobro, C.; Llanos, M.N.; Contreras, H.R. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol. Rep. 2015, 33, 1599–1608. [Google Scholar] [CrossRef]
- Endsley, M.P.; Aggarwal, N.; Isbell, M.A.; Wheelock, C.E.; Hammock, B.D.; Falck, J.R.; Campbell, W.B.; Nithipatikom, K. Diverse roles of 2-arachidonoylglycerol in invasion of prostate carcinoma cells: Location, hydrolysis and 12-lipoxygenase metabolism. Int. J. Cancer 2007, 121, 984–991. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Torres-Suárez, A.I. Phyto-, endo- and synthetic cannabinoids: Promising chemotherapeutic agents in the treatment of breast and prostate carcinomas. Expert Opin. Investig. Drugs 2016, 25, 1311–1323. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Nithipatikom, K.; Endsley, M.P.; Isbell, M.A.; Falck, J.R.; Iwamoto, Y.; Hillard, C.J.; Campbell, W.B. 2-Arachidonoylglycerol: A novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res. 2004, 64, 8826–8830. [Google Scholar] [CrossRef] [PubMed]
- Nomura, D.K.; Lombardi, D.P.; Chang, J.W.; Niessen, S.; Ward, A.M.; Long, J.Z.; Hoover, H.H.; Cravatt, B.F. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem. Biol. 2011, 18, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, M.; Gouveia-Figueira, S.; Persson, E.; Nording, M.; Fowler, C.J. The influence of monoacylglycerol lipase inhibition upon the expression of epidermal growth factor receptor in human PC-3 prostate cancer cells. BMC Res. Notes 2014, 7. [Google Scholar] [CrossRef]
- Mimeault, M.; Pommery, N.; Wattez, N.; Bailly, C.; Hénichart, J.P. Anti-proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: Implication of epidermal growth factor receptor down-regulation and ceramide production. Prostate 2003, 56, 1–12. [Google Scholar] [CrossRef]
- Nithipatikom, K.; Isbell, M.A.; Endsley, M.P.; Woodliff, J.E.; Campbell, W.B. Anti-proliferative effect of a putative endocannabinoid, 2-arachidonylglyceryl ether in prostate carcinoma cells. Prostaglandins Other Lipid Mediat. 2011, 94, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Miguel, A.; Díaz-Laviada, I. Δ9-Tetrahydrocannabinol induces apoptosis in human prostate PC-3 cells via a receptor-independent mechanism. FEBS Lett. 1999, 458, 400–404. [Google Scholar] [CrossRef]
- Sandeep Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of apoptosis by cannabinoids in prostate and colon cancer cells is phosphatase dependendent. Anticancer Res. 2011, 31, 3799–3807. [Google Scholar]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G 1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef]
- Olea-Herrero, N.; Vara, D.; Malagarie-Cazenave, S.; Díaz-Laviada, I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R()-Methanandamide and JWH-015: Involvement of CB 2. Br. J. Cancer 2009, 101, 940–950. [Google Scholar] [CrossRef]
- Scarlett, K.A.; White, E.S.Z.; Coke, C.J.; Carter, J.R.; Bryant, L.K.; Hinton, C.V. Agonist-induced CXCR4 and CB2 heterodimerization inhibits Ga13/ RhoA-mediated migration. Mol. Cancer Res. 2018, 16, 728–739. [Google Scholar] [CrossRef]
- Jankovic, R.; Goncalves, H.J.; Cavic, M.; Clemente, C.; Lind, M.; Murillo Carrasco, A.; Nadifi, S.; Khyatti, M.; Adebambo, T.; Egamberdiev, D. LungCARD—Report on worldwide research and clinical practices related to lung cancer. J. BUON 2019, 24, 11–19. [Google Scholar]
- Chen, R.; Manochakian, R.; James, L.; Azzouqa, A.-G.; Shi, H.; Zhang, Y.; Zhao, Y.; Zhou, K.; Lou, Y. Emerging therapeutic agents for advanced non-small cell lung cancer. J. Hematol. Oncol. 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Saini, D.; Dubey, A.; Roy, S.; Bharati, S.J.; Singh, N.; Khanna, M.; Prasad, C.P.; Singh, M.; Kumar, S.; et al. Feasibility of lung cancer screening in developing countries: Challenges, opportunities and way forward. Transl. Lung Cancer Res. 2019, 8, S106–S121. [Google Scholar] [CrossRef] [PubMed]
- Cavic, M.; Spasic, J.; Krivokuca, A.; Boljevic, I.; Kuburovic, M.; Radosavljevic, D.; Jankovic, R. TP53 and DNA-repair gene polymorphisms genotyping as a low-cost lung adenocarcinoma screening tool. J. Clin. Pathol. 2019, 72, 75–80. [Google Scholar] [CrossRef]
- Simmons, C.P.L.; Macleod, N.; Laird, B.J.A. Clinical management of pain in advanced lung cancer. Clin. Med. Insights. Oncol. 2012, 6, 331–346. [Google Scholar] [CrossRef]
- Aldington, S.; Harwood, M.; Cox, B.; Weatherall, M.; Beckert, L.; Hansell, A.; Pritchard, A.; Robinson, G.; Beasley, R. Cannabis use and risk of lung cancer: A case–control study. Eur. Respir. J. 2008, 31, 280–286. [Google Scholar] [CrossRef]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008, 21, 494–502. [Google Scholar] [CrossRef]
- Macleod, J.; Robertson, R.; Copeland, L.; McKenzie, J.; Elton, R.; Reid, P. Cannabis, tobacco smoking, and lung function: A cross-sectional observational study in a general practice population. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2015, 65, e89–e95. [Google Scholar] [CrossRef]
- Yarlagadda, K.; Singh, P.; Shrimanker, I.; Hoffman, J.C.; Nookala, V.K. Pot smokers puffing away lung health. Hear. Lung 2019, 48, 462–464. [Google Scholar] [CrossRef]
- Zhang, L.R.; Morgenstern, H.; Greenland, S.; Chang, S.-C.; Lazarus, P.; Teare, M.D.; Woll, P.J.; Orlow, I.; Cox, B.; Brhane, Y.; et al. Cannabis smoking and lung cancer risk: Pooled analysis in the International Lung Cancer Consortium. Int. J. Cancer 2015, 136, 894–903. [Google Scholar] [CrossRef]
- Jett, J.; Stone, E.; Warren, G.; Cummings, K.M. Cannabis Use, Lung Cancer, and Related Issues. J. Thorac. Oncol. 2018, 13, 480–487. [Google Scholar] [CrossRef]
- Smith, L.A.; Azariah, F.; Lavender, V.T.C.; Stoner, N.S.; Bettiol, S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst. Rev. 2015, 2015, CD009464. [Google Scholar] [CrossRef]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; Martín de Llano, J.J.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Ravi, J.; Elbaz, M.; Wani, N.A.; Nasser, M.W.; Ganju, R.K. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol. Carcinog. 2016, 55, 2063–2076. [Google Scholar] [CrossRef]
- Preet, A.; Qamri, Z.; Nasser, M.W.; Prasad, A.; Shilo, K.; Zou, X.; Groopman, J.E.; Ganju, R.K. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev. Res. 2011, 4, 65–75. [Google Scholar] [CrossRef]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef]
- Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. PPARγ as a Novel Therapeutic Target in Lung Cancer. PPAR Res. 2016, 2016, 8972570. [Google Scholar] [CrossRef] [PubMed]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef]
- Remick, S.C.; Nagaiah, G.; Hossain, A.; Mooney, C.J.; Parmentier, J. Anaplastic thyroid cancer: A review of epidemiology, pathogenesis, and treatment. J. Oncol. 2011, 2011. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Portella, G.; Vitale, M.; Orlando, P.; De Petrocellis, L.; Di Marzo, V. Control by the endogenous cannabinoid system of ras oncogene-dependent tumor growth. FASEB J. 2001, 15, 2745–2747. [Google Scholar] [CrossRef]
- Portella, G.; Laezza, C.; Laccetti, P.; De Petrocellis, L.; Di Marzo, V.; Bifulco, M. Inhibitory effects of cannabinoid CB1 receptor stimulation on tumor growth and metastatic spreading: Actions on signals involved in angiogenesis and metastasis. FASEB J. 2003, 17, 1771–1773. [Google Scholar] [CrossRef]
- Miyagi, E.; Katoh, R.; Li, X.; Lu, S.; Suzuki, K.; Maeda, S.; Shibuya, M.; Kawaoi, A. Thyroid stimulating hormone downregulates vascular endothelial growth factor expression in FRTL-5 cells. Thyroid 2001, 11, 539–543. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Valenti, M.; Ligresti, A.; Portella, G.; Marzo, V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004, 18, 1606–1608. [Google Scholar] [CrossRef]
- Shi, Y.; Zou, M.; Baitei, E.Y.; Alzahrani, A.S.; Parhar, R.S.; Al-Makhalafi, Z.; Al-Mohanna, F.A. Cannabinoid 2 receptor induction by IL-12 and its potential as a therapeutic target for the treatment of anaplastic thyroid carcinoma. Cancer Gene Ther. 2008, 15, 101–107. [Google Scholar] [CrossRef]
- Kleihues, P.; Louis, D.N.; Scheithauer, B.W.; Rorke, L.B.; Reifenberger, G.; Burger, P.C.; Cavenee, W.K. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Wen, P.Y. Therapeutic Advances in the Treatment of Glioblastoma: Rationale and Potential Role of Targeted Agents. Oncologist 2006, 11, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, S.; Tosoni, A.; Brandes, A.A. Adjuvant chemotherapy in the treatment of high grade gliomas. Cancer Treat. Rev. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Velasco, G.; Carracedo, A.; Blázquez, C.; Lorente, M.; Aguado, T.; Haro, A.; Sánchez, C.; Galve-Roperh, I.; Guzmán, M. Cannabinoids and gliomas. Mol. Neurobiol. 2007, 36, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- Del Pulgar, T.G.; Velasco, G.; Sánchez, C.; Haro, A.; Guzmán, M. De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem. J. 2002, 363, 183–188. [Google Scholar] [CrossRef]
- Blázquez, C.; González-Feria, L.; Álvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef]
- Sánchez, C.; de Ceballos, M.L.; Gomez del Pulgar, T.; Rueda, D.; Corbacho, C.; Velasco, G.; Galve-Roperh, I.; Huffman, J.W.; Ramón y Cajal, S.; Guzmán, M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001, 61, 5784–5798. [Google Scholar]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of endocannabinoid system in human gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef]
- Sredni, S.T.; Huang, C.C.; Suzuki, M.; Pundy, T.; Chou, P.; Tomita, T. Spontaneous involution of pediatric low-grade gliomas: High expression of cannabinoid receptor 1 (CNR1) at the time of diagnosis may indicate involvement of the endocannabinoid system. Child’s Nerv. Syst. 2016, 32, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Grajkowska, W.; Gabrusiewicz, K.; Kaminska, B.; Konarska, L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007, 1137, 161–169. [Google Scholar] [CrossRef]
- Schley, M.; Ständer, S.; Kerner, J.; Vajkoczy, P.; Schüpfer, G.; Dusch, M.; Schmelz, M.; Konrad, C. Predominant CB2 receptor expression in endothelial cells of glioblastoma in humans. Brain Res. Bull. 2009, 79, 333–337. [Google Scholar] [CrossRef]
- Maccarrone, M.; Attinà, M.; Cartoni, A.; Bari, M.; Finazzi-Agrò, A. Gas chromatography-mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture. J. Neurochem. 2001, 76, 594–601. [Google Scholar] [CrossRef]
- Petersen, G.; Moesgaard, B.; Schmid, P.C.; Schmid, H.H.O.; Broholm, H.; Kosteljanetz, M.; Hansen, H.S. Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non-tumour brain tissue. J. Neurochem. 2005, 93, 299–309. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R.; Eichele, K.; Weinzierl, U.; Brune, K. Up-regulation of cyclooxygenase-2 expression is involved in R(+)-methanandamide-induced apoptotic death of human neuroglioma cells. Mol. Pharmacol. 2004, 66, 1643–1651. [Google Scholar] [CrossRef]
- Bari, M.; Battista, N.; Fezza, F.; Finazzi-Agrò, A.; Maccarrone, M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells: Implications for anandamide-induced apoptosis. J. Biol. Chem. 2005, 280, 12212–12220. [Google Scholar] [CrossRef]
- Ma, C.; Wu, T.T.; Jiang, P.C.; Li, Z.Q.; Chen, X.J.; Fu, K.; Wang, W.; Gong, R. Anti-carcinogenic activity of anandamide on human glioma in vitro and in vivo. Mol. Med. Rep. 2016, 13, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Grabiec, U.; Ghadban, C.; Feese, K.; Dehghani, F. The influence of biomechanical properties and cannabinoids on tumor invasion. Cell Adhes. Migr. 2017, 11, 54–67. [Google Scholar] [CrossRef]
- Fowler, C.J.; Jonsson, K.O.; Andersson, A.; Juntunen, J.; Järvinen, T.; Vandevoorde, S.; Lambert, D.M.; Jerman, J.C.; Smart, D. Inhibition of C6 glioma cell proliferation by anandamide, 1-arachidonoylglycerol, and by a water soluble phosphate ester of anandamide: Variability in response and involvement of arachidonic acid. Biochem. Pharmacol. 2003, 66, 757–767. [Google Scholar] [CrossRef]
- Jacobsson, S.O.; Wallin, T.; Fowler, C.J. Inhibition of rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J. Pharmacol. Exp. Ther. 2001, 299, 951–959. [Google Scholar]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. J. Pharmacol. Exp. Ther. 2004, 308, 838–845. [Google Scholar] [CrossRef]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of Δ9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Grahovac, J.; Srdić-Rajić, T.; Francisco Santibañez, J.; Pavlović, M.; Čavić, M.; Radulović, S. Telmisartan induces melanoma cell apoptosis and synergizes with vemurafenib in vitro by altering cell bioenergetics. Cancer Biol. Med. 2019, 16, 247–263. [Google Scholar] [CrossRef]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a Potential New and Novel Treatment for Melanoma: A Pilot Study in a Murine Model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, J.; Zhao, H.; Fang, X.; Li, H. Cannabinoid receptor 2 is upregulated in melanoma. J. Cancer Res. Ther. 2012, 8, 549–554. [Google Scholar] [CrossRef]
- Glodde, N.; Jakobs, M.; Bald, T.; Tüting, T.; Gaffal, E. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015, 138, 35–40. [Google Scholar] [CrossRef]
- Adinolfi, B.; Romanini, A.; Vanni, A.; Martinotti, E.; Chicca, A.; Fogli, S.; Nieri, P. Anticancer activity of anandamide in human cutaneous melanoma cells. Eur. J. Pharmacol. 2013, 718, 154–159. [Google Scholar] [CrossRef]
- Hamtiaux, L.; Masquelier, J.; Muccioli, G.G.; Bouzin, C.; Feron, O.; Gallez, B.; Lambert, D.M. The association of N-palmitoylethanolamine with the FAAH inhibitor URB597 impairs melanoma growth through a supra-additive action. BMC Cancer 2012, 12, 92. [Google Scholar] [CrossRef]
- Carpi, S.; Fogli, S.; Polini, B.; Montagnani, V.; Podestà, A.; Breschi, M.C.; Romanini, A.; Stecca, B.; Nieri, P. Tumor-promoting effects of cannabinoid receptor type 1 in human melanoma cells. Toxicol. Vitr. 2017, 40, 272–279. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(ut)annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Ladin, D.A.; Soliman, E.; Griffin, L.; Van Dross, R. Preclinical and Clinical Assessment of Cannabinoids as Anti-Cancer Agents. Front. Pharmacol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Nardini, C. The ethics of clinical trials. Ecancermedicalscience 2014, 8. [Google Scholar] [CrossRef]
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef]
- Hayry, M. Prescribing cannabis: Freedom, autonomy, and values. J. Med. Ethics 2004, 30, 333–336. [Google Scholar] [CrossRef]
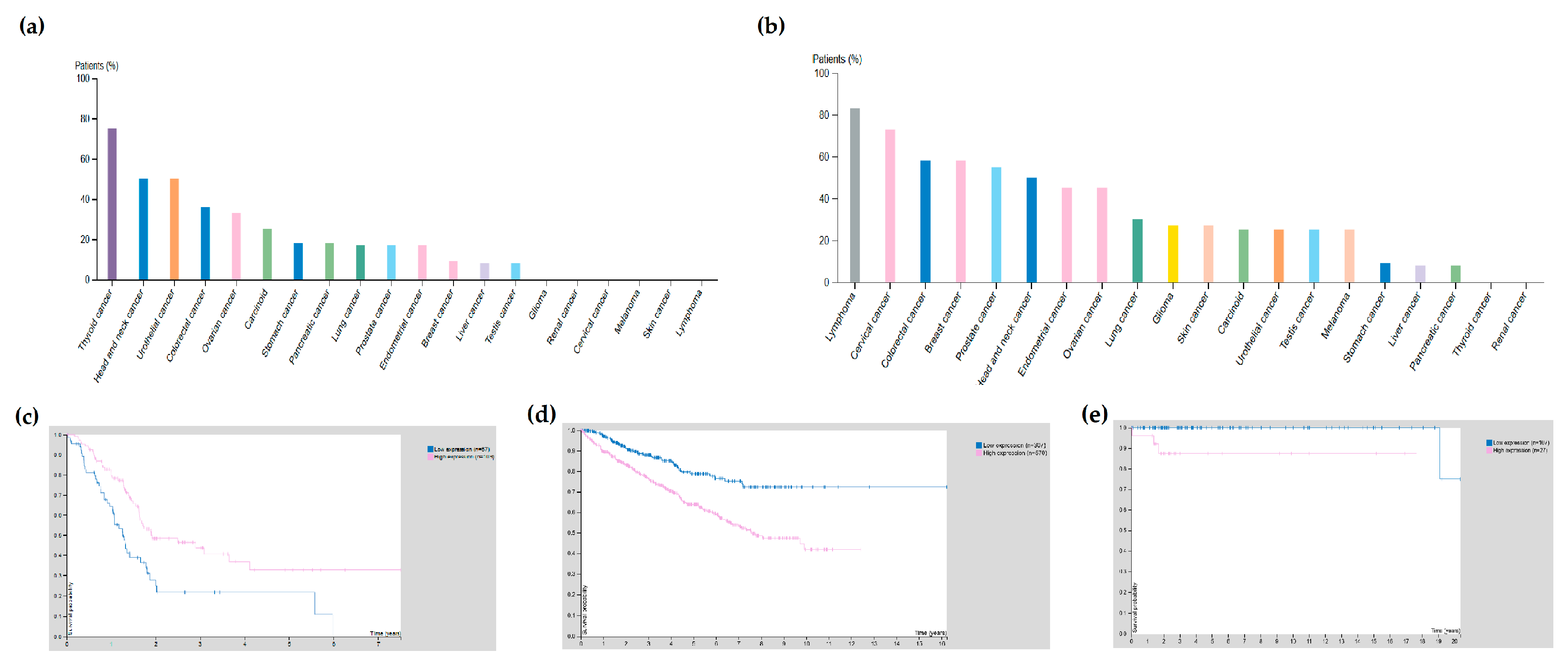
| Cancer Type | Receptor | Cancer Subtype | Expression in Tumour vs. Normal Tissue | p Value |
|---|---|---|---|---|
| Breast cancer | CNR1 | Breast invasive carcinoma | Under-expressed | 7.09 × 10−11 |
| CNR2 | Breast invasive carcinoma | Under-expressed | 1.55 × 10−2 | |
| GPR18 | Breast invasive carcinoma | Over-expressed | 3.60 × 10−7 | |
| Gastrointestinal malignancies | CNR1 | Cholangiocarcinoma | Over-expressed | 3.16 × 10−2 |
| Colon adenocarcinoma | Under-expressed | 1.58 × 10−7 | ||
| Hepatocellular carcinoma | Over-expressed | 3.52 × 10−11 | ||
| Rectum adenocarcinoma | Under-expressed | 9.80 × 10−3 | ||
| CNR2 | Colon adenocarcinoma | Under-expressed | 6.57 × 10−4 | |
| Rectum adenocarcinoma | Under-expressed | 2.83 × 10−2 | ||
| GPR18 | Colon adenocarcinoma | Under-expressed | 3.30 × 10−6 | |
| GPR55 | Colon adenocarcinoma | Under-expressed | 2.16 × 10−4 | |
| GPR119 | Colon adenocarcinoma | Under-expressed | 1.55 × 10−5 | |
| Hepatocellular carcinoma | Under-expressed | 3.58 × 10−5 | ||
| Pancreatic adenocarcinoma | Over-expressed | 1.73 × 10−2 | ||
| Rectum adenocarcinoma | Under-expressed | 2.84 × 10−3 | ||
| TRPV1 | Hepatocellular carcinoma | Over-expressed | 4.75 × 10−6 | |
| Stomach adenocarcinoma | Over-expressed | 1.32 × 10−3 | ||
| TRPV2 | Cholangiocarcinoma | Over-expressed | 5.71 × 10−7 | |
| Hepatocellular carcinoma | Over-expressed | 4.27 × 10−9 | ||
| Stomach adenocarcinoma | Over-expressed | 1.22 × 10−8 | ||
| Gynaecological malignancies | CNR1 | Uterine corpus endometrial carcinoma | Under-expressed | 1.54 × 10−2 |
| GPR18 | Cervical squamous cell carcinoma | Over-expressed | 1.18 × 10−3 | |
| GPR55 | Cervical squamous cell carcinoma | Over-expressed | 1.64 × 10−9 | |
| Uterine corpus endometrial carcinoma | Under-expressed | 9.88 × 10−7 | ||
| Prostate cancer | CNR1 | Prostate adenocarcinoma | Under-expressed | 3.45 × 10−6 |
| TRPV1 | Prostate adenocarcinoma | Over-expressed | 1.05 × 10−4 | |
| TRPV2 | Prostate adenocarcinoma | Under-expressed | 3.56 × 10−2 | |
| Thoracic tumours | CNR1 | Lung adenocarcinoma | Under-expressed | 1.62 × 10−12 |
| Lung squamocellular carcinoma | Under-expressed | 4.06 × 10−7 | ||
| TRPV1 | Lung adenocarcinoma | Over-expressed | <1 × 10−12 | |
| Lung squamous cell carcinoma | Over-expressed | 6.11 × 10−10 | ||
| TRPV2 | Lung adenocarcinoma | Under-expressed | <1 × 10−12 | |
| Lung squamous cell carcinoma | Under-expressed | 1.62 × 10−12 | ||
| Thyroid cancer | CNR1 | Thyroid carcinoma | Under-expressed | 3.05 × 10−2 |
| CNR2 | Thyroid carcinoma | Under-expressed | 1.72 × 10−5 | |
| GPR18 | Thyroid carcinoma | Under-expressed | 3.94 × 10−3 | |
| GPR55 | Thyroid carcinoma | Over-expressed | 2.24 × 10−4 | |
| TRPV1 | Thyroid carcinoma | Under-expressed | 3.74 × 10−2 | |
| Central nervous system malignancies | GPR18 | Glioblastoma multiforme | Over-expressed | 1.60 × 10−6 |
| TRPV1 | Glioblastoma multiforme | Under-expressed | 4.78 × 10−2 | |
| Melanoma (primary vs. metastasis) | CNR2 | Skin cutaneous melanoma | Over-expressed | 1.22 × 10−6 |
| GPR18 | Skin cutaneous melanoma | Over-expressed | 1.61 × 10−9 | |
| GPR119 | Skin cutaneous melanoma | Under-expressed | 1.95 × 10−2 | |
| TRPV2 | Skin cutaneous melanoma | Under-expressed | 3.81 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, E.; Cavic, M.; Krivokuca, A.; Canela, E.I. The Interplay between Cancer Biology and the Endocannabinoid System—Significance for Cancer Risk, Prognosis and Response to Treatment. Cancers 2020, 12, 3275. https://doi.org/10.3390/cancers12113275
Moreno E, Cavic M, Krivokuca A, Canela EI. The Interplay between Cancer Biology and the Endocannabinoid System—Significance for Cancer Risk, Prognosis and Response to Treatment. Cancers. 2020; 12(11):3275. https://doi.org/10.3390/cancers12113275
Chicago/Turabian StyleMoreno, Estefanía, Milena Cavic, Ana Krivokuca, and Enric I. Canela. 2020. "The Interplay between Cancer Biology and the Endocannabinoid System—Significance for Cancer Risk, Prognosis and Response to Treatment" Cancers 12, no. 11: 3275. https://doi.org/10.3390/cancers12113275
APA StyleMoreno, E., Cavic, M., Krivokuca, A., & Canela, E. I. (2020). The Interplay between Cancer Biology and the Endocannabinoid System—Significance for Cancer Risk, Prognosis and Response to Treatment. Cancers, 12(11), 3275. https://doi.org/10.3390/cancers12113275





