Causal Inference between Rheumatoid Arthritis and Breast Cancer in East Asian and European Population: A Two-Sample Mendelian Randomization
Simple Summary
Abstract
1. Introduction
2. Results
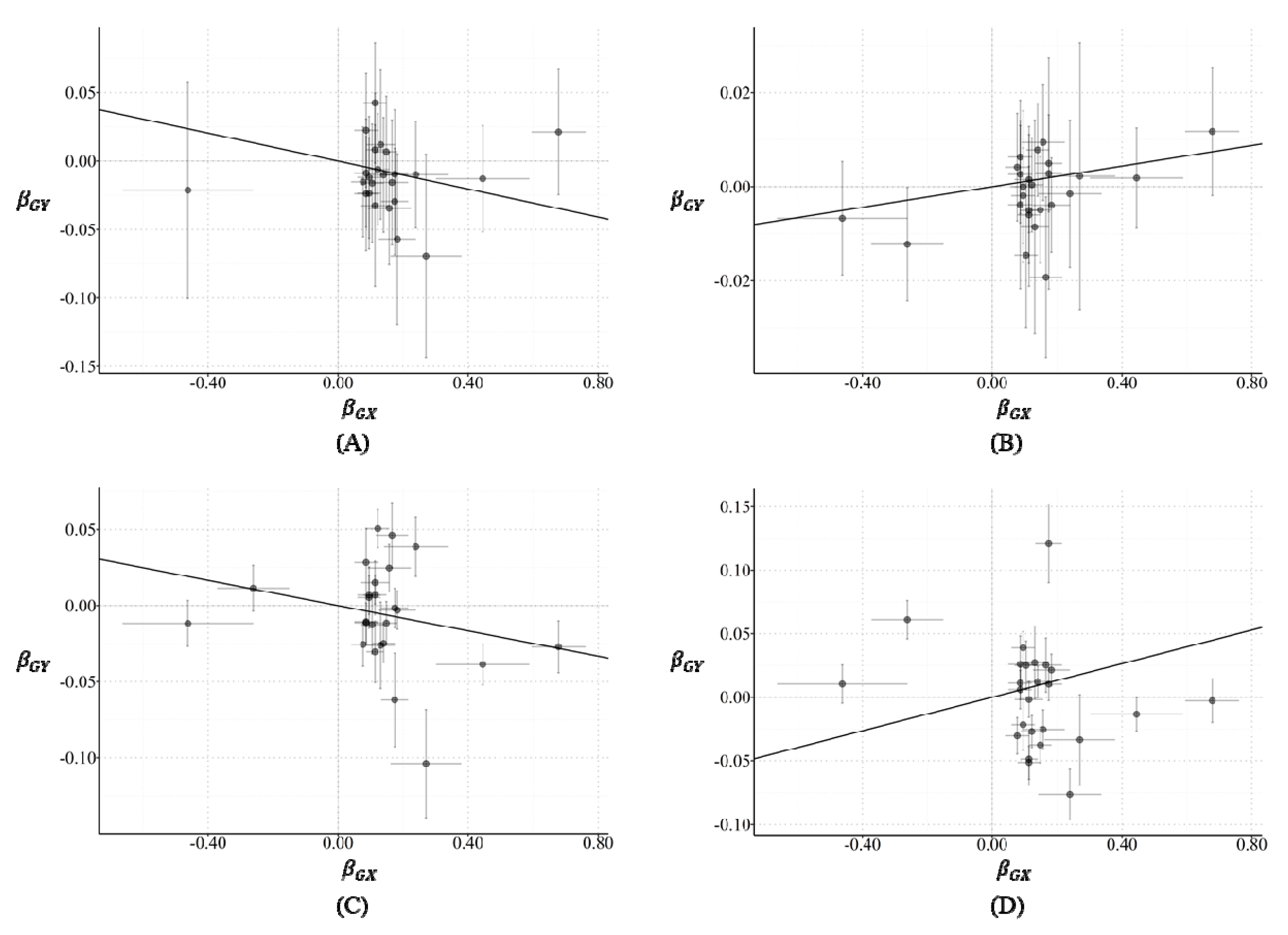
2.1. Two-Sample MR Analysis
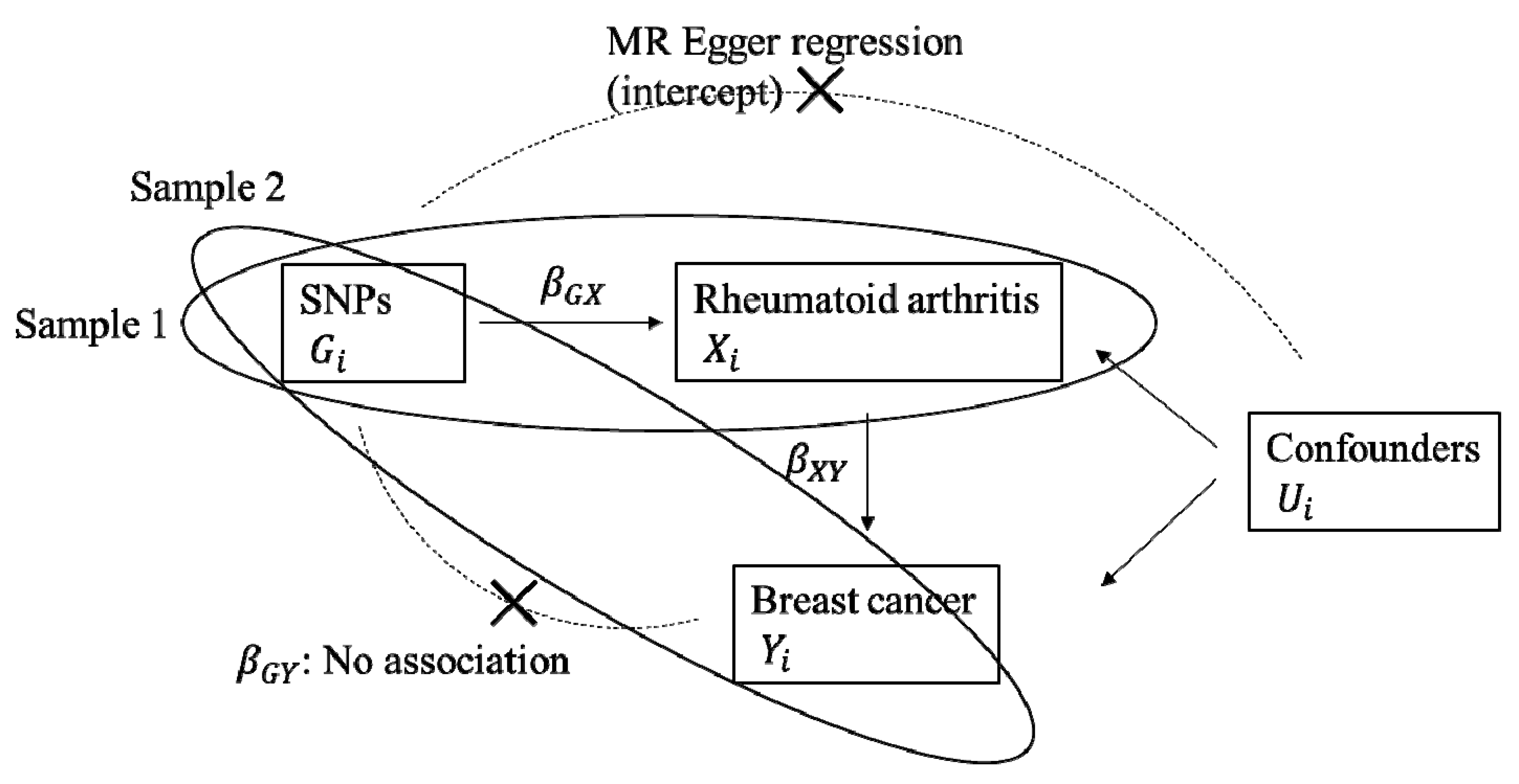
2.2. Horizontal Pleiotropy
2.3. Funnel Plot
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Exposure and Outcome
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raaschou, P.; Simard, J.F.; Hagelberg, C.A.; Askling, J.; ARTIS Study Group. A Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: Cohort study based on nationwide prospectively recorded data from Sweden. BMJ 2016, 352, i262. [Google Scholar] [CrossRef]
- Askling, J.; Fored, C.M.; Baecklund, E.; Brandt, L.; Backlin, C.; Ekbom, A.; Sundström, C.; Bertilsson, L.; Cöster, L.; Geborek, P.; et al. Haematopoietic malignancies in rheumatoid arthritis: Lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. BMJ 2005, 64, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, K.; Lindström, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemaçon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Liang, J.N.; Wang, Z.Y.; Zhou, D. Breast cancer risk in rheumatoid arthritis: An update meta-analysis. Biomed. Res. Int. 2014, 2014, 453012. [Google Scholar] [CrossRef] [PubMed]
- Infante-Rivard, C.; Rivard, G.E.; Derome, F.; Cusson, A.; Winikoff, R.; Chartrand, R.; Guay, J.-P. A retrospective cohort study of cancer incidence among patients treated with radiosynoviorthesis. Haemophilia 2012, 18, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Parikh-Patel, A.; White, R.H.; Allen, M.; Cress, R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control 2009, 20, 1001–1010. [Google Scholar] [CrossRef]
- Abásolo, L.; Júdez, E.; Descalzo, M.Á.; González-Álvaro, I.; Jover, J.A.; Carmona, L.; Group, E.S. (Eds.) Cancer in Rheumatoid Arthritis: Occurrence, Mortality, and Associated Factors in a South European Population; Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Hemminki, K.; Li, X.; Sundquist, K.; Sundquist, J. Cancer risk in hospitalized rheumatoid arthritis patients. Rheumatology (Oxford) 2008, 47, 698–701. [Google Scholar] [CrossRef]
- Buchbinder, R.; Barber, M.; Heuzenroeder, L.; Wluka, A.E.; Giles, G.; Hall, S.; Harkness, A.; Lewis, D.; Littlejohn, G.; Miller, M.H.; et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Care Res. News Alerts 2008, 59, 794–799. [Google Scholar] [CrossRef]
- Yamada, T.; Nakajima, A.; Inoue, E.; Tanaka, E.; Taniguchi, A.; Momohara, S.; Yamanaka, H.J. Incidence of malignancy in Japanese patients with rheumatoid arthritis. Res. Artic. 2011, 31, 1487–1492. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, Y.-T.; Wang, C.-B.; Wu, C.-Y. The risk of cancer in patients with rheumatoid arthritis: A nationwide cohort study in Taiwan. Rheum. Arthritis 2011, 63, 352–358. [Google Scholar] [CrossRef]
- Moritomo, H.; Ueda, T.; Hiyama, T.; Hosono, N.; Mori, S.; Kornatsubara, Y. The risk of cancer in rheumatoid patients in Japan. Scand. J. Rheumatol. 1995, 24, 157–159. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Laragione, T.; Gulko, P.S. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol. Med. 2010, 16, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Hayer, S.; Niederreiter, B.; Sieghart, W.; Fuereder, T.; Zwerina, J.; Schett, G. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, Y.; Liu, L.; Yuan, Q. miR-125 regulates PI3K/Akt/mTOR signaling pathway in rheumatoid arthritis rats via PARP2. Biosci. Rep. 2019, 39, BSR20180890. [Google Scholar] [CrossRef]
- Vilquin, P.; Donini, C.F.; Villedieu, M.; Grisard, E.; Corbo, L.; Bachelot, T.; Vendrell, J.A.; Cohen, P.A. MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer. Breast Cancer Res. 2015, 17, 13. [Google Scholar] [CrossRef]
- He, H.; Xu, F.; Huang, W.; Luo, S.Y.; Lin, Y.T.; Zhang, G.H.; Du, Q.; Duan, R.H. miR-125a-5p expression is associated with the age of breast cancer patients. Genet. Mol. Res. 2015, 14, 17927–17933. [Google Scholar] [CrossRef]
- Young, M.R.; Wright, M.A.; Lozano, Y.; Matthews, J.P.; Benefield, J.; Prechel, M.M. Mechanisms of immune suppression in patients with head and neck cancer: Influence on the immune infiltrate of the cancer. Int. J. Cancer 1996, 67, 333–338. [Google Scholar] [CrossRef]
- Ben-Baruch, A. (Ed.) Inflammation-Associated Immune Suppression in Cancer: The Roles Played by Cytokines, Chemokines and Additional Mediators. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Moodycliffe, A.M.; Nghiem, D.; Clydesdale, G.; Ullrich, S.E. Immune suppression and skin cancer development: Regulation by NKT cells. Nat. Immunol. 2000, 1, 521–525. [Google Scholar] [CrossRef]
- Whiteside, T.L. (Ed.) Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Δ-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Coussens, L.M. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007, 9, 212. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rosenberg, N.A.; Huang, L.; Jewett, E.M.; Szpiech, Z.A.; Jankovic, I.; Boehnke, M. Genome-wide association studies in diverse populations. Nat. Rev. Genet. 2010, 11, 356–366. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ 2005, 330, 1076–1079. [Google Scholar] [CrossRef]
- Ishigaki, K.; Akiyama, M.; Kanai, M.; Takahashi, A.; Kawakami, E.; Sugishita, H.; Sakaue, S.; Matoba, N.; Low, S.-K.; Okada, Y.; et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat. Genet. 2020, 52, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Consortium, B.C.A. Commonly studied single-nucleotide polymorphisms and breast cancer: Results from the Breast Cancer Association Consortium. J. Natl. Cancer Inst. 2006, 98, 1382–1396. [Google Scholar]
- Chenevix-Trench, G.; Milne, R.L.; Antoniou, A.C.; Couch, F.J.; Easton, D.F.; Goldgar, D.E.; CIMBA. An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: The Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res. 2007, 9, 104. [Google Scholar] [CrossRef]
- Milne, R.L.; Kuchenbaecker, K.B.; Michailidou, K.; Beesley, J.; Kar, S.; Lindström, S.; Hui, S.; Lemaçon, A.; Soucy, P.; Dennis, J.; et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat. Genet. 2017, 49, 1767–1778. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Tilanus-Linthorst, M.M.A.; Lingsma, H.F.; Evans, D.G.; Thompson, D.; Kaas, R.; Manders, P.; van Asperen, C.J.; Adank, M.; Hooning, M.J.; Lim, G.E.K.; et al. Optimal age to start preventive measures in women with BRCA1/2 mutations or high familial breast cancer risk. Int. J. Cancer 2013, 133, 156–163. [Google Scholar] [CrossRef]
- Talhouet, S.D.; Peron, J.; Vuilleumier, A.; Friedlaender, A.; Viassolo, V.; Ayme, A.; Bodmer, A.; Treilleux, I.; Lang, N.; Tille, J.-C.; et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Wadström, H.; Pettersson, A.; Smedby, K.E.; Askling, J. Risk of breast cancer before and after rheumatoid arthritis, and the impact of hormonal factors. Ann. Rheum. Dis. 2020, 79, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, P.E.; van der Heijde, D.M.; St Clair, E.W.; Furst, D.E.; Breedveld, F.C.; Kalden, J.R.; Smolen, J.S.; Weisman, M.; Emery, P.; Feldmann, M.J.; et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N. Engl. J. Med. 2000, 343, 1594–1602. [Google Scholar] [CrossRef]
- Moreland, L.W.; Baumgartner, S.W.; Schiff, M.H.; Tindall, E.A.; Fleischmann, R.M.; Weaver, A.L.; Ettlinger, R.E.; Cohen, S.; Koopman, W.J.; Mohler, K.; et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)–Fc fusion protein. N. Engl. J. Med. 1997, 337, 141–147. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Koeller, M.; Weisman, M.H.; Emery, P. New therapies for treatment of rheumatoid arthritis. Lancet 2007, 370, 1861–1874. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007, 121, 1–14. [Google Scholar] [CrossRef]
- Prendergast, G.C. Immune escape as a fundamental trait of cancer: Focus on IDO. Oncogene 2008, 27, 3889. [Google Scholar] [CrossRef]
- Bidwell, B.N.; Slaney, C.Y.; Withana, N.P.; Forster, S.; Cao, Y.; Loi, S.; Andrews, D.; Mikeska, T.; Mangan, N.E.; Samarajiwa, S.A.; et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 2012, 18, 1224. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Leong, S.P.L.; Shen, Z.-Z.; Liu, T.-J.; Agarwal, G.; Tajima, T.; Paik, N.-S.; Sandelin, K.; Derossis, A.; Cody, H.; Foulkes, W.D. Is breast cancer the same disease in Asian and Western countries? World journal of surgery. World J. Surg. 2010, 34, 2308–2324. [Google Scholar] [CrossRef]
- Koike, T.; Inui, K. How can the treatment of rheumatoid arthritis be improved in Japan? Int. J. Clin. Rheumatol. 2015, 10, 235–244. [Google Scholar] [CrossRef]
- Freudenberg, J.; Lee, H.-S.; Han, B.-G.; Shin, H.D.; Kang, Y.M.; Sung, Y.-K.; Shim, S.-C.; Choi, C.-B.; Lee, A.T.; Gregersen, P.K.; et al. Genome-wide association study of rheumatoid arthritis in Koreans: Population-specific loci as well as overlap with European susceptibility loci. Arthritis Rheum. 2011, 63, 884–893. [Google Scholar] [CrossRef]
- Hu, H.J.; Jin, E.H.; Yim, S.H.; Yang, S.Y.; Jung, S.H.; Shin, S.H.; Kim, W.U.; Shim, S.C.; Kim, T.G.; Chung, Y.J. Common variants at the promoter region of the APOM confer a risk of rheumatoid arthritis. Exp. Mol. Med. 2011, 43, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Kochi, Y.; Okada, Y.; Suzuki, A.; Ikari, K.; Terao, C.; Takahashi, A.; Yamazaki, K.; Hosono, N.; Myouzen, K.; Tsunoda, T.; et al. A regulatory variant in CCR6 is associated with rheumatoid arthritis susceptibility. Nat. Genet. 2010, 42, 515–519. [Google Scholar] [CrossRef]
- Myouzen, K.; Kochi, Y.; Okada, Y.; Terao, C.; Suzuki, A.; Ikari, K.; Tsunoda, T.; Takahashi, A.; Kubo, M.; Taniguchi, A.; et al. Functional variants in NFKBIE and RTKN2 involved in activation of the NF-kappaB pathway are associated with rheumatoid arthritis in Japanese. PLoS Genet. 2012, 8, e1002949. [Google Scholar] [CrossRef]
- Okada, Y.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Kawaguchi, T.; Stahl, E.A.; Kurreeman, F.A.S.; Nishida, N.; et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat. Genet. 2012, 44, 511–516. [Google Scholar] [CrossRef]
- Terao, C.; Yamada, R.; Ohmura, K.; Takahashi, M.; Kawaguchi, T.; Kochi, Y.; Human Disease Genomics Working Group; RA Clinical and Genetic Study Consortium; Okada, Y.; Nakamura, Y.; et al. The human AIRE gene at chromosome 21q22 is a genetic determinant for the predisposition to rheumatoid arthritis in Japanese population. Hum. Mol. Genet. 2011, 20, 2680–2685. [Google Scholar] [CrossRef]
- Hirata, M.; Kamatani, Y.; Nagai, A.; Kiyohara, Y.; Ninomiya, T.; Tamakoshi, A.; Yamagata, Z.; Kubo, M.; Muto, K.; Mushiroda, T.; et al. Cross-sectional analysis of BioBank Japan clinical data: A large cohort of 200,000 patients with 47 common diseases. J. Epidemiol. 2017, 27 (Suppl. III), S9–S21. [Google Scholar] [CrossRef]
- Nagai, A.; Hirata, M.; Kamatani, Y.; Muto, K.; Matsuda, K.; Kiyohara, Y.; Ninomiya, T.; Tamakoshi, A.; Yamagata, Z.; Mushiroda, T.; et al. Overview of the BioBank Japan Project: Study design and profile. J. Epidemiol. 2017, 27 (Suppl. III), S2–S8. [Google Scholar] [CrossRef]
- Okada, Y.; Momozawa, Y.; Sakaue, S.; Kanai, M.; Ishigaki, K.; Akiyama, M.; Kishikawa, T.; Arai, Y.; Sasaki, T.; Kosaki, K.; et al. Deep whole-genome sequencing reveals recent selection signatures linked to evolution and disease risk of Japanese. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amos, C.I.; Dennis, J.; Wang, Z.; Byun, J.; Schumacher, F.R.; Gayther, S.A.; Casey, G.; Hunter, D.J.; Sellers, T.A.; Gruber, S.B.; et al. The OncoArray Consortium: A network for understanding the genetic architecture of common cancers. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 126–135. [Google Scholar] [CrossRef]
- Burgess, S.; Scott, R.A.; Timpson, N.J.; Smith, G.D.; Thompson, S.G.; EPIC-InterAct Consortium. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015, 30, 543–552. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Lawlor, D.A. Commentary: Two-sample Mendelian randomization: Opportunities and challenges. Int. J. Epidemiol. 2016, 45, 908. [Google Scholar] [CrossRef]
- Rees, J.M.B.; Wood, A.M.; Dudbridge, F.; Burgess, S. Robust methods in Mendelian randomization via penalization of heterogeneous causal estimates. PLoS ONE 2019, 14, e0222362. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Hemani, G.; Smith, G.D. Invited Commentary: Detecting Individual and Global Horizontal Pleiotropy in Mendelian Randomization—A Job for the Humble Heterogeneity Statistic? Am. J. Epidemiol. 2018, 187, 2681–2685. [Google Scholar] [CrossRef] [PubMed]
- OYJS. MendelianRandomization: Mendelian Randomization Package. R package version 0.4.1. 2019. Available online: https://CRAN.R-project.org/package=MendelianRandomization (accessed on 23 August 2020).
| Data of Summary Statistics | SNPs, n | Beta (SE) | OR (95% CI) |
|---|---|---|---|
| BBJ | 24 | −0.051 (0.021) | 0.95 (0.91–0.99) |
| BCAC | 25 | 0.014 (0.005) | 1.01 (1.00–1.03) |
| BRCA1 carriers from CIMBA | 25 | −0.042 (0.007) | 0.96 (0.95–0.97) |
| BRCA2 carriers from CIMBA | 25 | 0.066 (0.038) | 1.07 (0.99–1.15) |
| Data of Summary Statistics | SNPs, n | Beta (SE) | p-Value |
|---|---|---|---|
| BBJ | 24 | −0.016 (0.008) | 0.04 |
| BCAC | 25 | −0.002 (0.002) | 0.31 |
| BRCA1 carriers from CIMBA | 25 | 0.004 (0.007) | 0.59 |
| BRCA2 carriers from CIMBA | 25 | −0.031 (0.007) | ≤0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.; Lee, S.; Park, S.K. Causal Inference between Rheumatoid Arthritis and Breast Cancer in East Asian and European Population: A Two-Sample Mendelian Randomization. Cancers 2020, 12, 3272. https://doi.org/10.3390/cancers12113272
Ahn C, Lee S, Park SK. Causal Inference between Rheumatoid Arthritis and Breast Cancer in East Asian and European Population: A Two-Sample Mendelian Randomization. Cancers. 2020; 12(11):3272. https://doi.org/10.3390/cancers12113272
Chicago/Turabian StyleAhn, Choonghyun, Sangjun Lee, and Sue K. Park. 2020. "Causal Inference between Rheumatoid Arthritis and Breast Cancer in East Asian and European Population: A Two-Sample Mendelian Randomization" Cancers 12, no. 11: 3272. https://doi.org/10.3390/cancers12113272
APA StyleAhn, C., Lee, S., & Park, S. K. (2020). Causal Inference between Rheumatoid Arthritis and Breast Cancer in East Asian and European Population: A Two-Sample Mendelian Randomization. Cancers, 12(11), 3272. https://doi.org/10.3390/cancers12113272






