Fertility Preservation in Childhood Cancer: Endocrine Activity in Prepubertal Human Testis Xenografts Exposed to a Pubertal Hormone Environment
Simple Summary
Abstract
1. Introduction
2. Results
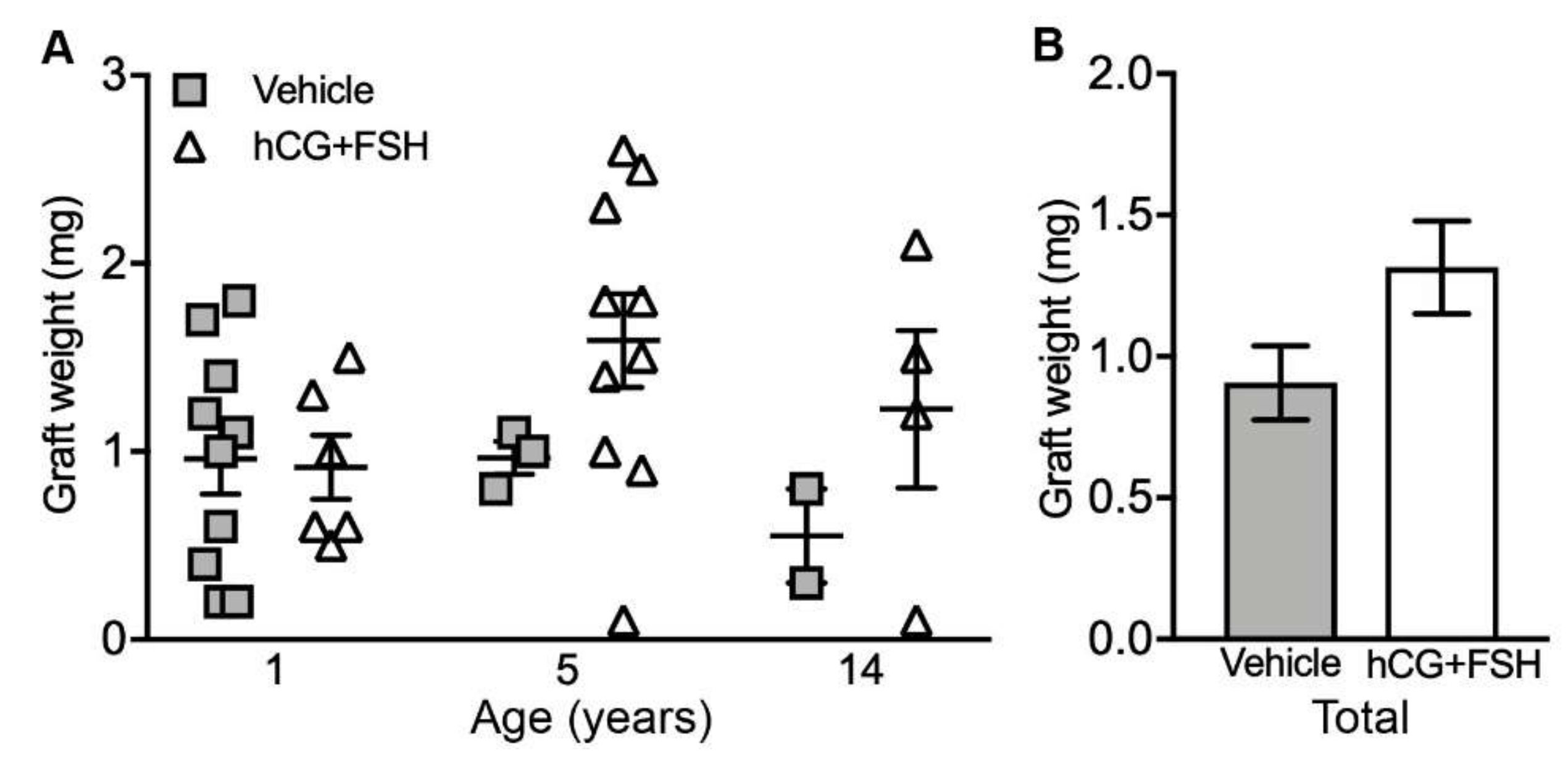
2.1. Graft Recovery Rate and Graft Weight
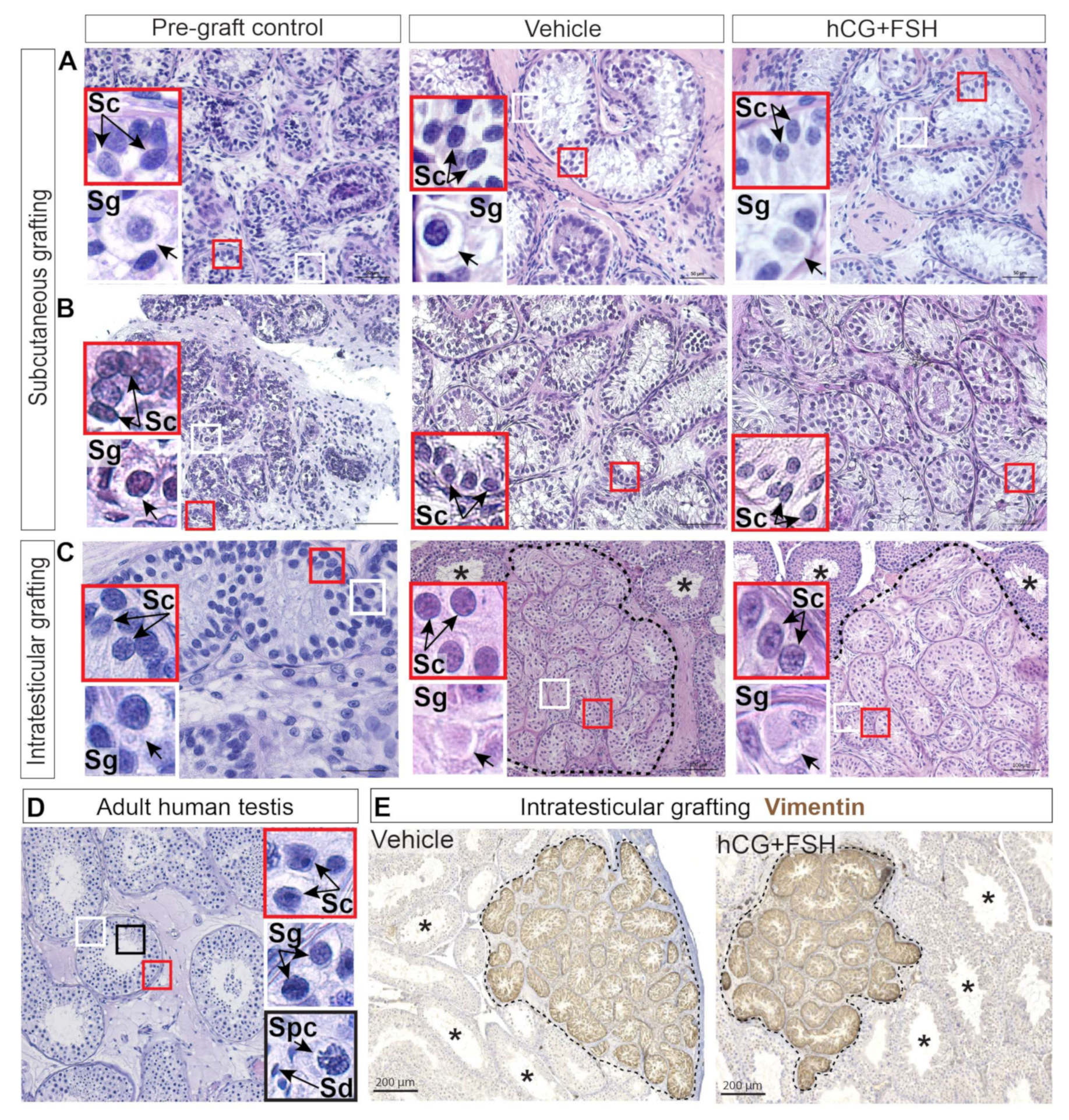
2.2. Histological Evaluation and Localisation of Prepubertal Human Testis Grafts
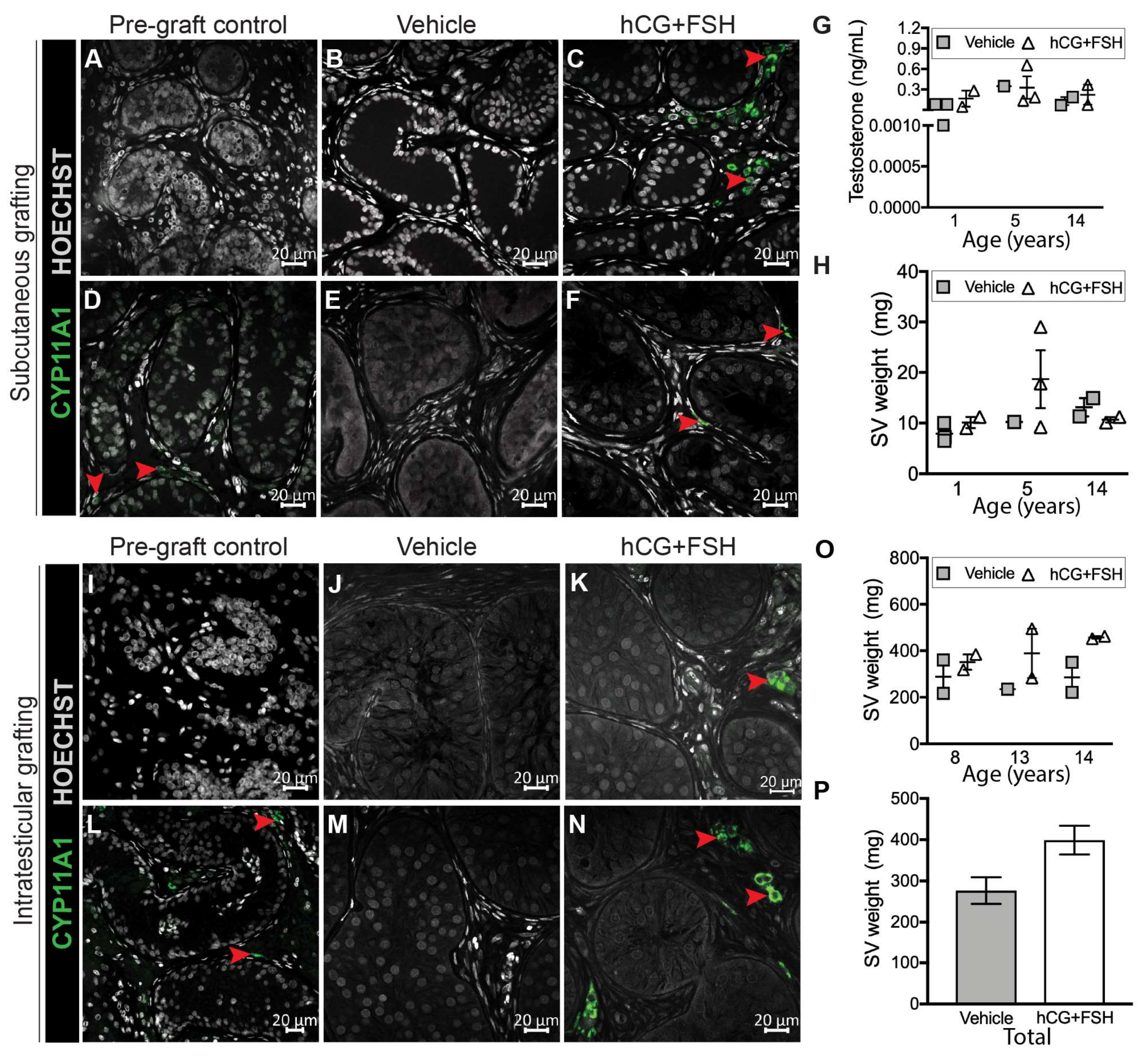
2.3. Effect of Exogenous Gonadotrophins on Steroidogenesis in Prepubertal Human Testis Grafts
2.4. Effect of Exogenous Gonadotrophins on Sertoli Cell Maturation
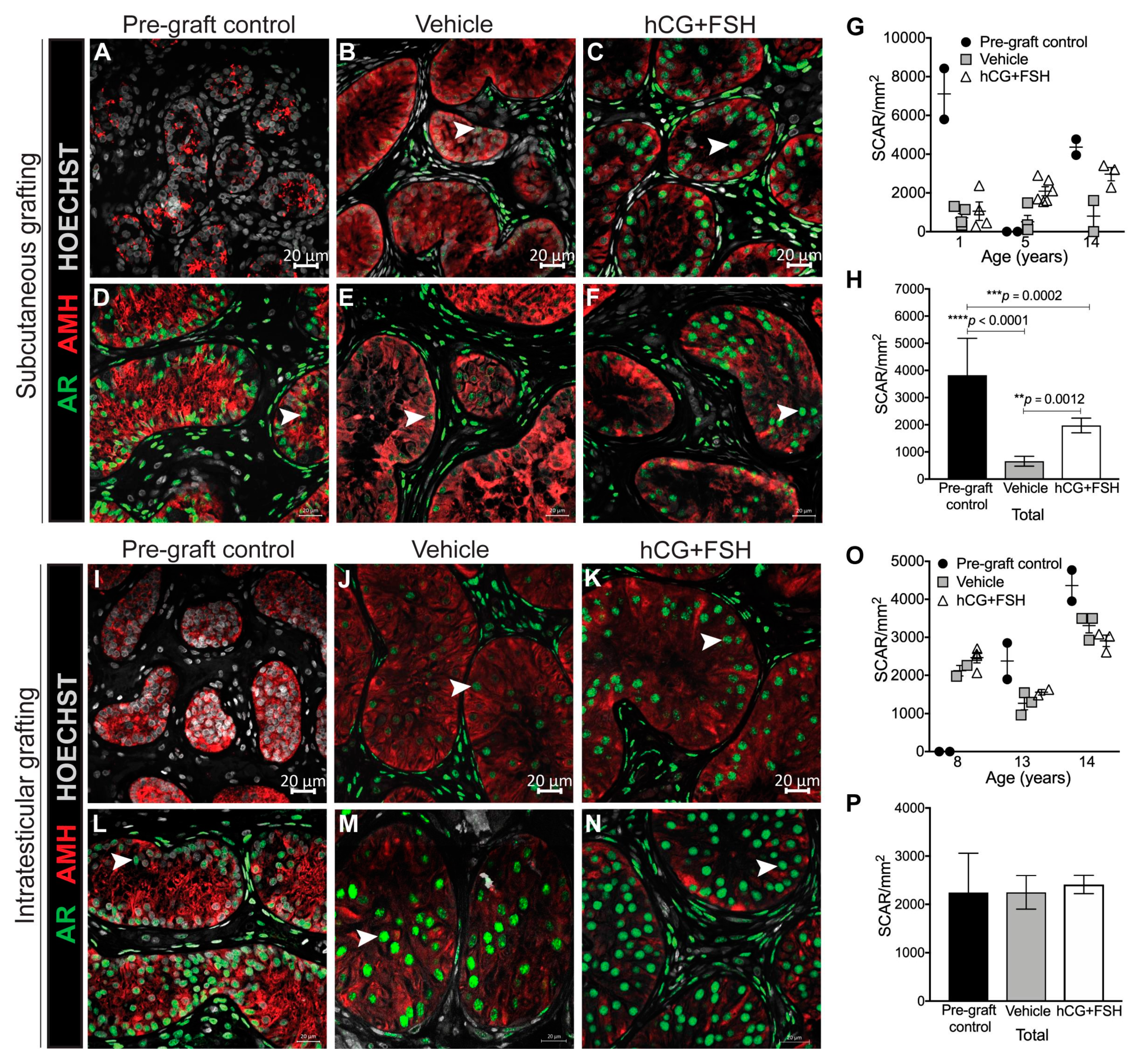
2.4.1. Expression of AR and AMH in Prepubertal Human Testis Grafts
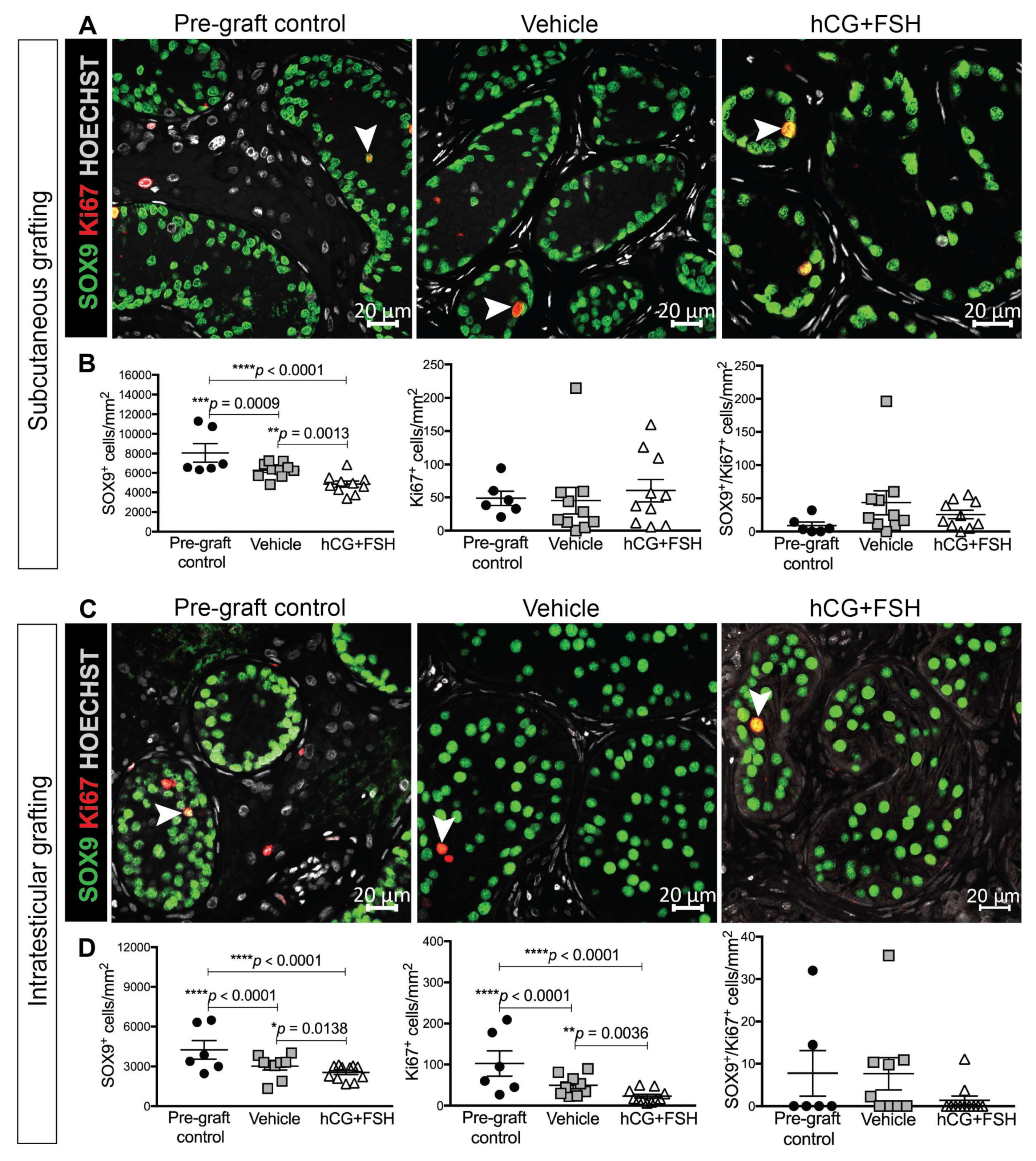
2.4.2. Expression of SOX9 and Ki67 in Prepubertal Human Testis Grafts
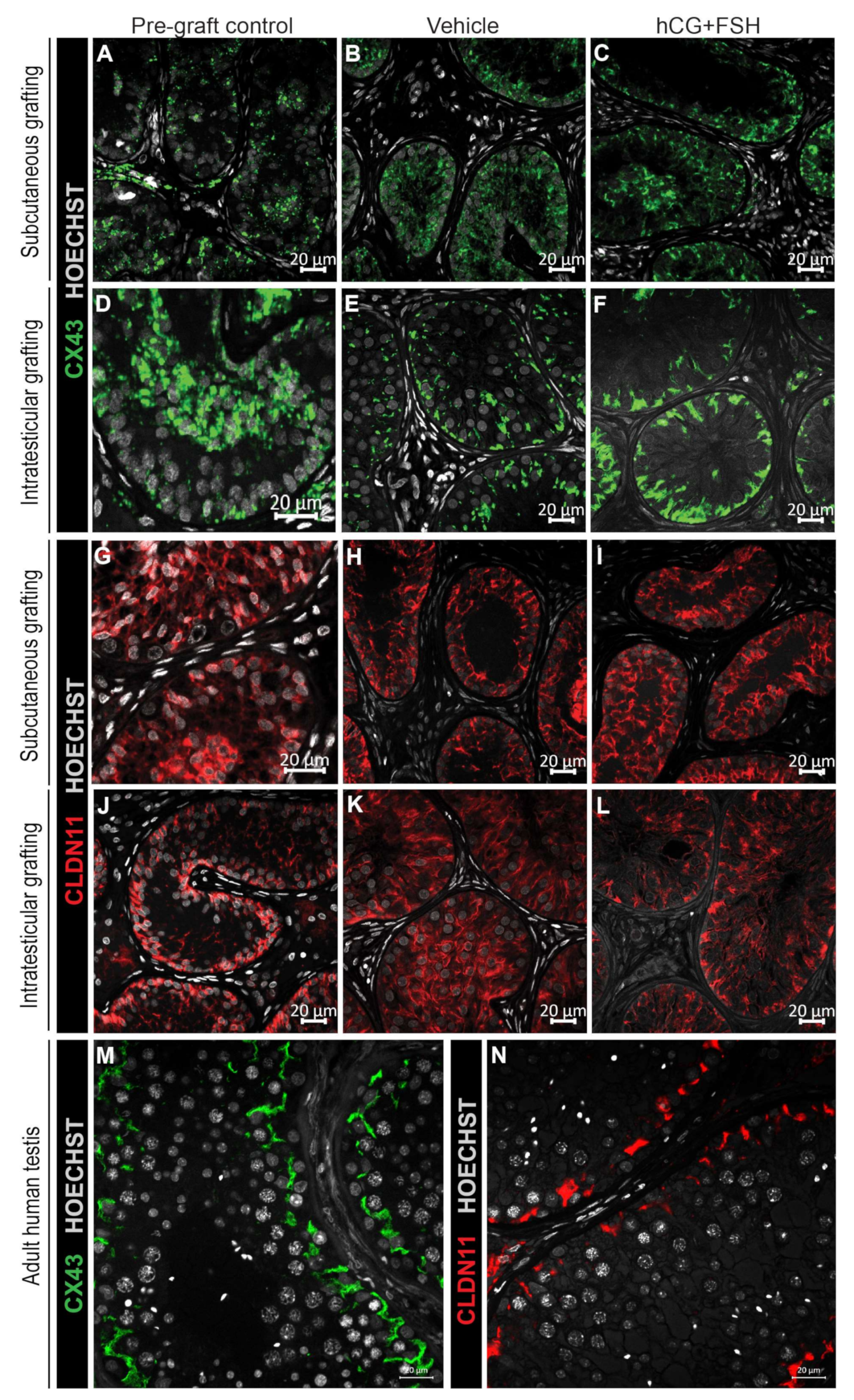
2.4.3. Expression of CX43 and CLDN11 in Prepubertal Human Testis Grafts
2.5. Effect of Exogenous Gonadotrophins on Germ Cell Survival in Prepubertal Human Testis Grafts
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Subcutaneous Grafting Procedure
4.4. Intratesticular Grafting Procedure
4.5. Treatment of Host Mice
4.6. Retrieval of Xenografts and Seminal Vesicles
4.7. Measurement of Plasma Testosterone Levels
4.8. Histology and Immunostaining
4.9. Image Acquisition and Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robison, L.L.; Hudson, M.M. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat. Rev. Cancer 2014, 14, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.T.; Saunders, P.T.; Sharpe, R.M.; Kelnar, C.J.; Wallace, W.H. Male fertility and strategies for fertility preservation following childhood cancer treatment. Endocr. Dev. 2009, 15, 101–134. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.T.; Phillips, B.T.; Orwig, K.E. Fruitful progress to fertility: Male fertility in the test tube. Nat. Med. 2011, 17, 1564–1565. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H.B. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567. [Google Scholar] [CrossRef]
- Stukenborg, J.B.; Jahnukainen, K.; Hutka, M.; Mitchell, R.T. Cancer treatment in childhood and testicular function: The importance of the somatic environment. Endocr. Connect. 2018, 7, R69–R87. [Google Scholar] [CrossRef] [PubMed]
- Valli-Pulaski, H.; Peters, K.A.; Gassei, K.; Steimer, S.R.; Sukhwani, M.; Hermann, B.P.; Dwomor, L.; David, S.; Fayomi, A.P.; Munyoki, S.K.; et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum. Reprod. Oxf. Engl. 2019, 34, 966–977. [Google Scholar] [CrossRef]
- Stukenborg, J.B.; Wyns, C. Fertility sparing strategies for pre- and peripubertal male cancer patients. Ecancermedicalscience 2020, 14, 1016. [Google Scholar] [CrossRef]
- Goossens, E.; Jahnukainen, K.; Mitchell, R.; Van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.; et al. Fertility preservation in boys: Recent developments and new insights. Hum. Reprod. Open 2020, 2020. [Google Scholar] [CrossRef]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319. [Google Scholar] [CrossRef]
- Prince, F.P. Ultrastructure of immature Leydig cells in the human prepubertal testis. Anat. Rec. 1984, 209, 165–176. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Crépieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-stimulating Hormone (FSH) Action on Spermatogenesis: A Focus on Physiological and Therapeutic Roles. J. Clin. Med. 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M.; Zhang, F.P.; Huhtaniemi, I. Persistent expression of a truncated form of the luteinizing hormone receptor messenger ribonucleic acid in the rat testis after selective Leydig cell destruction by ethylene dimethane sulfonate. Endocrinology 1994, 135, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Stanton, P.; De Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2017. [Google Scholar]
- Hutka, M.; Smith, L.B.; Mitchell, R.T. Xenotransplantation as a model for human testicular development. Differentiation 2017, 97, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nozawa, S.; Yoshiike, M.; Arai, M.; Sasaki, C.; Iwamoto, T. Xenografting of testicular tissue from an infant human donor results in accelerated testicular maturation. Hum. Reprod. Oxf. Engl. 2010, 25, 1113–1122. [Google Scholar] [CrossRef]
- Wyns, C.; Van Langendonckt, A.; Wese, F.-X.; Donnez, J.; Curaba, M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum. Reprod. 2008, 23, 2402–2414. [Google Scholar] [CrossRef]
- Van Saen, D.; Goossens, E.; Haentjens, P.; Baert, Y.; Tournaye, H. Exogenous administration of recombinant human FSH does not improve germ cell survival in human prepubertal xenografts. Reprod. Biomed. Online 2013, 26, 286–298. [Google Scholar] [CrossRef]
- Ntemou, E.; Kadam, P.; Van Laere, S.; Van Saen, D.; Vicini, E.; Goossens, E. Effect of recombinant human vascular endothelial growth factor on testis tissue xenotransplants from prepubertal boys: A three-case study. Reprod. Biomed. Online 2019, 39, 119–133. [Google Scholar] [CrossRef]
- Baert, Y.; Van Saen, D.; Haentjens, P.; In’t Veld, P.; Tournaye, H.; Goossens, E. What is the best cryopreservation protocol for human testicular tissue banking? Hum. Reprod. 2013, 28, 1816–1826. [Google Scholar] [CrossRef]
- Izadyar, F.; Matthijs-Rijsenbilt, J.J.; Den Ouden, K.; Creemers, L.B.; Woelders, H.; De Rooij, D.G. Development of a cryopreservation protocol for type A spermatogonia. J. Androl. 2002, 23, 537–545. [Google Scholar]
- Paniagua, R.; Nistal, M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J. Anat. 1984, 139, 535–552. [Google Scholar] [PubMed]
- Wu, X.; Schmidt, J.A.; Avarbock, M.R.; Tobias, J.W.; Carlson, C.A.; Kolon, T.F.; Ginsberg, J.P.; Brinster, R.L. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. USA 2009, 106, 21672–21677. [Google Scholar] [CrossRef] [PubMed]
- Nistal, M.; Paniagua, R.; Gonzalez-Peramato, P.; Reyes-Mugica, M. Perspectives in pediatric pathology, chapter 3. Testicular development from birth to puberty: Systematic evaluation of the prepubertal testis. Pediatr. Dev. Pathol. 2015, 18, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Muciaccia, B.; Boitani, C.; Berloco, B.P.; Nudo, F.; Spadetta, G.; Stefanini, M.; De Rooij, D.G.; Vicini, E. Novel Stage Classification of Human Spermatogenesis Based on Acrosome Development1. Biol. Reprod. 2013, 89. [Google Scholar] [CrossRef]
- Van Saen, D.; Goossens, E.; Bourgain, C.; Ferster, A.; Tournaye, H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum. Reprod. Oxf. Engl. 2011, 26, 282–293. [Google Scholar] [CrossRef]
- Nistal, M.; Abaurrea, M.A.; Paniagua, R. Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. J. Anat. 1982, 134, 351–363. [Google Scholar]
- Hess, R.A.; França, L.R. Chapter 3—Structure of the Sertoli Cell. In Sertoli Cell Biology; Skinner, M.K., Griswold, M.D., Eds.; Academic Press: San Diego, CA, USA, 2005; pp. 19–40. [Google Scholar] [CrossRef]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef]
- Brehm, R.; Rey, R.; Kliesch, S.; Steger, K.; Marks, A.; Bergmann, M. Mitotic activity of Sertoli cells in adult human testis: An immunohistochemical study to characterize Sertoli cells in testicular cords from patients showing testicular dysgenesis syndrome. Anat. Embryol. Berl. 2006, 211, 223–236. [Google Scholar] [CrossRef]
- Haverfield, J.T.; Stanton, P.G.; Meachem, S.J. 14-Adult Sertoli cell differentiation status in humans. In Sertoli Cell Biology, 2nd ed.; Griswold, M.D., Ed.; Academic Press: Oxford, UK, 2015; pp. 409–436. [Google Scholar] [CrossRef]
- Hutka, M.; Smith, L.B.; Goossens, E.; Wallace, W.H.B.; Stukenborg, J.B.; Mitchell, R.T. Exogenous Gonadotrophin Stimulation Induces Partial Maturation of Human Sertoli Cells in a Testicular Xenotransplantation Model for Fertility Preservation. J. Clin. Med. 2020, 9, 266. [Google Scholar] [CrossRef]
- Rajpert-De Meyts, E.; Jorgensen, N.; Graem, N.; Muller, J.; Cate, R.L.; Skakkebaek, N.E. Expression of anti-Mullerian hormone during normal and pathological gonadal development: Association with differentiation of Sertoli and granulosa cells. J. Clin. Endocrinol. Metab. 1999, 84, 3836–3844. [Google Scholar] [CrossRef]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The Regulation of Spermatogenesis by Androgens. Semin. Cell Dev. Biol. 2014, 2–13. [Google Scholar] [CrossRef] [PubMed]
- De Gendt, K.; Swinnen, J.V.; Saunders, P.T.; Schoonjans, L.; Dewerchin, M.; Devos, A.; Tan, K.; Atanassova, N.; Claessens, F.; Lecureuil, C.; et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. USA 2004, 101, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A.L.; De Gendt, K.; Atanassova, N.; Walker, M.; Sharpe, R.M.; Saunders, P.T.K.; Denolet, E.; Verhoeven, G. The Role of Androgens in Sertoli Cell Proliferation and Functional Maturation: Studies in Mice with Total or Sertoli Cell-Selective Ablation of the Androgen Receptor. Endocrinology 2005, 146, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- De Gendt, K.; Atanassova, N.; Tan, K.A.; De Franca, L.R.; Parreira, G.G.; McKinnell, C.; Sharpe, R.M.; Saunders, P.T.; Mason, J.I.; Hartung, S.; et al. Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology 2005, 146, 4117–4126. [Google Scholar] [CrossRef] [PubMed]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J. The human Leydig cell. In Male Hypogonadism: Basic, Clinical and Therapeutic Principles; Humana Press: Totowa, NJ, USA, 2017; pp. 25–47. [Google Scholar]
- Teerds, K.J.; De Rooij, D.G.; Rommerts, F.F.; Van den Hurk, R.; Wensing, C.J. Stimulation of the proliferation and differentiation of Leydig cell precursors after the destruction of existing Leydig cells with ethane dimethyl sulphonate (EDS) can take place in the absence of LH. J. Androl. 1989, 10, 472–477. [Google Scholar] [CrossRef]
- Tena-Sempere, M.; Rannikko, A.; Kero, J.; Zhang, F.P.; Huhtaniemi, I.T. Molecular mechanisms of reappearance of luteinizing hormone receptor expression and function in rat testis after selective Leydig cell destruction by ethylene dimethane sulfonate. Endocrinology 1997, 138, 3340–3348. [Google Scholar] [CrossRef]
- Siril Ariyaratne, H.B.; Chamindrani Mendis-Handagama, S.; Buchanan Hales, D.; Ian Mason, J. Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol. Reprod. 2000, 63, 165–171. [Google Scholar] [CrossRef]
- Abney, T.O.; Zhai, J. Gene expression of luteinizing hormone receptor and steroidogenic enzymes during Leydig cell development. J. Mol. Endocrinol. 1998, 20, 119–127. [Google Scholar] [CrossRef]
- Payne, A.H.; Hardy, M.P. The Leydig Cell in Health and Disease. Springer 2007. [Google Scholar] [CrossRef]
- Rivarola, M.A.; Belgorosky, A.; Berensztein, E.; De Dávila, M.T. Human prepubertal testicular cells in culture: Steroidogenic capacity, paracrine and hormone control. J. Steroid. Biochem. Mol. Biol 1995, 53, 119–125. [Google Scholar] [CrossRef]
- Chen, H.; Jin, S.; Huang, S.; Folmer, J.; Liu, J.; Ge, R.; Zirkin, B.R. Transplantation of alginate-encapsulated seminiferous tubules and interstitial tissue into adult rats: Leydig stem cell differentiation in vivo? Mol. Cell Endocrinol. 2016, 436, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Rebourcet, D.; O’Shaughnessy, P.J.; Pitetti, J.L.; Monteiro, A.; O’Hara, L.; Milne, L.; Tsai, Y.T.; Cruickshanks, L.; Riethmacher, D.; Guillou, F.; et al. Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development 2014, 141, 2139–2149. [Google Scholar] [CrossRef]
- Racine, C.; Rey, R.; Forest, M.G.; Louis, F.; Ferre, A.; Huhtaniemi, I.; Josso, N.; Di Clemente, N. Receptors for anti-mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc. Natl. Acad. Sci. USA 1998, 95, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Rouiller-Fabre, V.; Carmona, S.; Merhi, R.A.; Cate, R.; Habert, R.; Vigier, B. Effect of Anti-Mullerian Hormone on Sertoli and Leydig Cell Functions in Fetal and Immature Rats. Endocrinology 1998, 139, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Salva, A.; Hardy, M.P.; Wu, X.F.; Sottas, C.M.; MacLaughlin, D.T.; Donahoe, P.K.; Lee, M.M. Müllerian-inhibiting substance inhibits rat Leydig cell regeneration after ethylene dimethanesulphonate ablation. Biol. Reprod. 2004, 70, 600–607. [Google Scholar] [CrossRef]
- Lee, M.M.; Seah, C.C.; Masiakos, P.T.; Sottas, C.M.; Preffer, F.I.; Donahoe, P.K.; Maclaughlin, D.T.; Hardy, M.P. Müllerian-inhibiting substance type II receptor expression and function in purified rat Leydig cells. Endocrinology 1999, 140, 2819–2827. [Google Scholar] [CrossRef]
- Rey, R.; Lordereau-Richard, I.; Carel, J.C.; Barbet, P.; Cate, R.L.; Roger, M.; Chaussain, J.L.; Josso, N. Anti-müllerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. J. Clin. Endocrinol. Metab. 1993, 77, 1220–1226. [Google Scholar] [CrossRef]
- Boukari, K.; Meduri, G.; Brailly-Tabard, S.; Guibourdenche, J.; Ciampi, M.-L.; Massin, N.; Martinerie, L.; Picard, J.-Y.; Rey, R.; Lombes, M.; et al. Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J. Clin. Endocrinol. Metab. 2009, 94, 1818–1825. [Google Scholar] [CrossRef]
- Rey, R.A.; Musse, M.; Venara, M.; Chemes, H.E. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: Its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc. Res. Tech. 2009, 72, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Kim, S.S.; Gosden, R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction 2002, 124, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sandhowe-Klaverkamp, R.; Schlatt, S. Differentiation of Testis Xenografts in the Prepubertal Marmoset Depends on the Sex and Status of the Mouse Host. Front. Endocrinol. Lausanne 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Rey, R.; Lukas-Croisier, C.; Lasala, C.; Bedecarrás, P. AMH/MIS: What we know already about the gene, the protein and its regulation. Mol. Cell Endocrinol. 2003, 211, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Hazra, R.; Corcoran, L.; Robson, M.; McTavish, K.J.; Upton, D.; Handelsman, D.J.; Allan, C.M. Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol. Endocrinol. 2013, 27, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Van Saen, D.; Goossens, E.; Aerts, J.L.; Haentjens, P.; Tournaye, H. Does early cell death cause germ cell loss after intratesticular tissue grafting? Fertil. Steril. 2013, 99, 1264.e1261–1272.e1261. [Google Scholar] [CrossRef]
- Honaramooz, A.; Snedaker, A.; Boiani, M.; Schöler, H.; Dobrinski, I.; Schlatt, S. Sperm from neonatal mammalian testes grafted in mice. Nature 2002, 418, 778. [Google Scholar] [CrossRef]
- Geens, M.; De Block, G.; Goossens, E.; Frederickx, V.; Van Steirteghem, A.; Tournaye, H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum. Reprod. 2006, 21, 390–396. [Google Scholar] [CrossRef]
- Schlatt, S.; Honaramooz, A.; Ehmcke, J.; Goebell, P.J.; Rübben, H.; Dhir, R.; Dobrinski, I.; Patrizio, P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum. Reprod. 2006, 21, 384–389. [Google Scholar] [CrossRef]
- Poels, J.; Van Langendonckt, A.; Many, M.-C.; Wese, F.-X.; Wyns, C. Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod. 2013, 28, 578–589. [Google Scholar] [CrossRef]
- Mitchell, R.T.; Saunders, P.T.; Childs, A.J.; Cassidy-Kojima, C.; Anderson, R.A.; Wallace, W.H.; Kelnar, C.J.; Sharpe, R.M. Xenografting of human fetal testis tissue: A new approach to study fetal testis development and germ cell differentiation. Hum. Reprod 2010, 25, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Van den Driesche, S.; Macdonald, J.; Anderson, R.A.; Johnston, Z.C.; Chetty, T.; Smith, L.B.; McKinnell, C.; Dean, A.; Homer, N.Z.; Jorgensen, A.; et al. Prolonged exposure to acetaminophen reduces testosterone production by the human fetal testis in a xenograft model. Sci. Transl. Med. 2015, 7, 288ra280. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Gonzalez, P.; Anderson, R.A.; Macdonald, J.; Van den Driesche, S.; Kilcoyne, K.; Jorgensen, A.; McKinnell, C.; Macpherson, S.; Sharpe, R.M.; Mitchell, R.T. Effects of Exposure to Acetaminophen and Ibuprofen on Fetal Germ Cell Development in Both Sexes in Rodent and Human Using Multiple Experimental Systems. Environ. Health Perspect 2018, 126, 047006. [Google Scholar] [CrossRef] [PubMed]

| Age (yrs) | Diagnosis | Pre-Biopsy Chemotherapy | Biopsy Condition | Most Advanced Germ Cell Type |
|---|---|---|---|---|
| 1 | Ependymoma | No | Fresh | Spermatogonia |
| 5 | Medulloblastoma | No | Fresh | Spermatogonia |
| 8 | Aplastic Anaemia | No | Cryopreserved | Spermatogonia |
| 13 | Myelodysplastic Syndrome | No | Cryopreserved | Spermatogonia |
| 13 | Acute Lymphoblastic Leukaemia | Yes * | Cryopreserved | Spermatocytes |
| 14 | Anaplastic Large Cell Lymphoma | Yes ** | Fresh | Spermatogonia |
| Subcutaneous - Castrate | Subcutaneous - Intact | Intratesticular - Intact | ||||
|---|---|---|---|---|---|---|
| Treatment | Vehicle | hCG+FSH | Vehicle | hCG+FSH | Vehicle | hCG+FSH |
| Testis Tissue | n = 6 | n = 6 | n = 3 | n = 3 | n = 4 | n = 4 |
| Patient Age (Years) | 1, 5, 8, 13, 13, 14 | 1, 5, 8, 13, 13, 14 | 8, 13, 13 | 8, 13, 13 | 8, 13, 13, 14 | 8, 13, 13, 14 |
| Recipient Mouse | n = 10 | n = 11 | n = 6 | n = 6 | n = 8 | n = 8 |
| Graft Recovery Rate (%) | 15/43 (35) | 26/47 (55) | 4/18 (22) | 4/18 (22) | 9/16 (56) | 11/15 (73) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutka, M.; Kadam, P.; Van Saen, D.; Homer, N.Z.M.; Onofre, J.; Wallace, W.H.B.; Smith, L.B.; Stukenborg, J.-B.; Goossens, E.; Mitchell, R.T. Fertility Preservation in Childhood Cancer: Endocrine Activity in Prepubertal Human Testis Xenografts Exposed to a Pubertal Hormone Environment. Cancers 2020, 12, 2830. https://doi.org/10.3390/cancers12102830
Hutka M, Kadam P, Van Saen D, Homer NZM, Onofre J, Wallace WHB, Smith LB, Stukenborg J-B, Goossens E, Mitchell RT. Fertility Preservation in Childhood Cancer: Endocrine Activity in Prepubertal Human Testis Xenografts Exposed to a Pubertal Hormone Environment. Cancers. 2020; 12(10):2830. https://doi.org/10.3390/cancers12102830
Chicago/Turabian StyleHutka, Marsida, Prashant Kadam, Dorien Van Saen, Natalie Z. M. Homer, Jaime Onofre, W. Hamish B. Wallace, Lee B. Smith, Jan-Bernd Stukenborg, Ellen Goossens, and Rod T. Mitchell. 2020. "Fertility Preservation in Childhood Cancer: Endocrine Activity in Prepubertal Human Testis Xenografts Exposed to a Pubertal Hormone Environment" Cancers 12, no. 10: 2830. https://doi.org/10.3390/cancers12102830
APA StyleHutka, M., Kadam, P., Van Saen, D., Homer, N. Z. M., Onofre, J., Wallace, W. H. B., Smith, L. B., Stukenborg, J.-B., Goossens, E., & Mitchell, R. T. (2020). Fertility Preservation in Childhood Cancer: Endocrine Activity in Prepubertal Human Testis Xenografts Exposed to a Pubertal Hormone Environment. Cancers, 12(10), 2830. https://doi.org/10.3390/cancers12102830






