Application of an Artificial Intelligence Algorithm to Prognostically Stratify Grade II Gliomas
Abstract
:1. Introduction
2. Results
2.1. Patients Included in the Study
2.2. Molecular Classification, EOR and ΔVT2T1 Independently Predict Patient Prognosis.
2.2.1. Overall Survival
2.2.2. Progression-Free Survival
2.2.3. Malignant Progression-Free Survival
2.2.4. Survival Curves
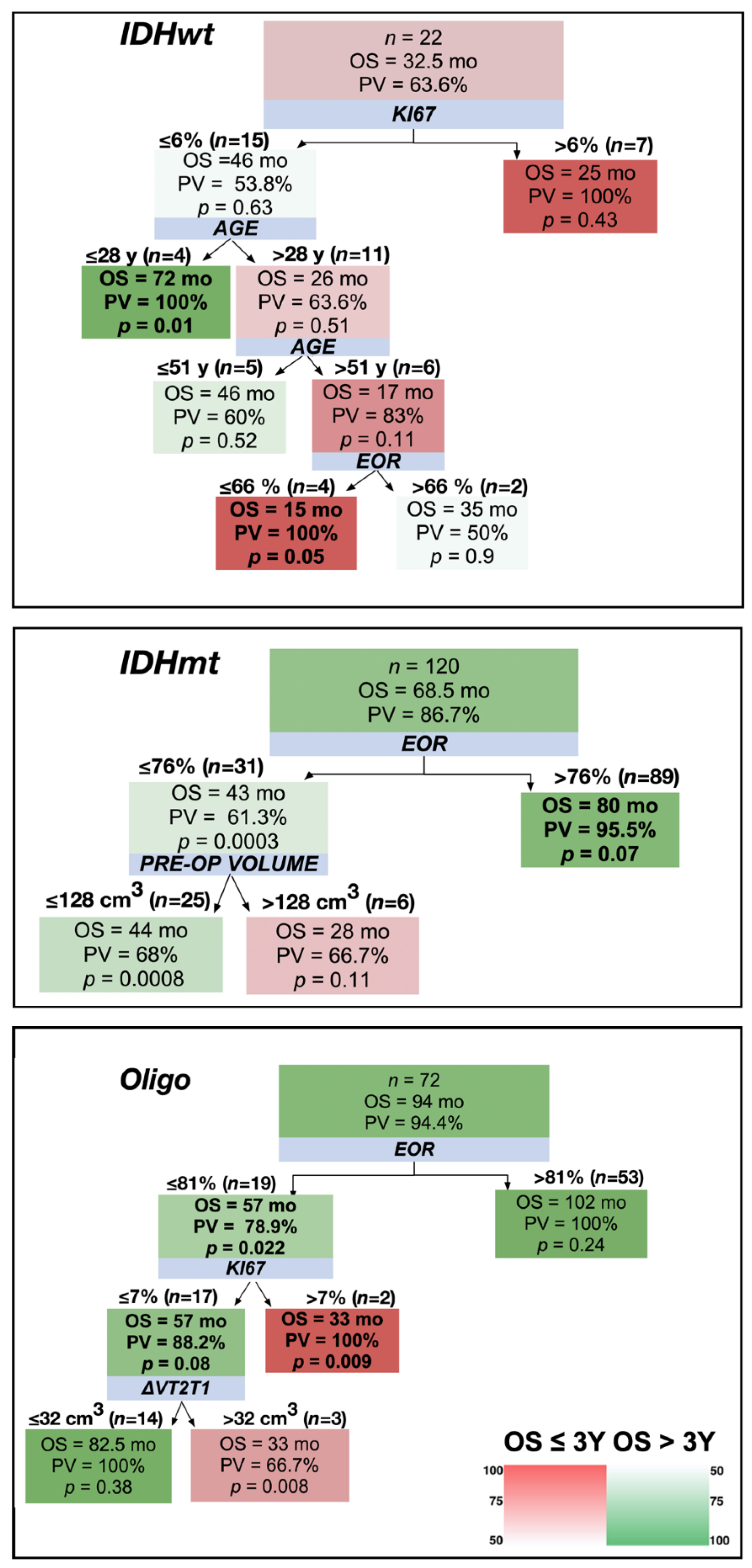
2.3. Adoption of a Decision Tree Approach to Stratify Patients
3. Discussion
4. Materials and Methods
4.1. Patients Included in the Study
4.2. Histological and Molecular Examination
4.3. Volumetric Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chammas, M.; Saadeh, F.; Maaliki, M.; Assi, H. Therapeutic Interventions in Adult Low-Grade Gliomas. J. Clin. Neurol. 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Soffietti, R.; Baumert, B.G.; Bello, L.; von Deimling, A.; Duffau, H.; Frenay, M.; Grisold, W.; Grant, R.; Graus, F.; Hoang-Xuan, K.; et al. Guidelines on management of low-grade gliomas: Report of an EFNS-EANO Task Force. Eur. J. Neurol. 2010, 17, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Chang, S.M. Grade II and III Oligodendroglioma and Astrocytoma. Neurol. Clin. 2018, 36, 467–484. [Google Scholar] [CrossRef] [PubMed]
- International Agence for Research on Cancer. WHO Classification of Tumours of the Central Nervous System (IARC WHO Classification of Tumours), 4th ed.; Louis, D.N., Ohgaki, H., Wiestler, O.D., Cavenee, W.K., Eds.; IARC Publications: Lyon, France, 2016; Volume 1. [Google Scholar]
- Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; Morozova, O.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef] [Green Version]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Baron, M.H.; Kujas, M.; et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: A series of 1097 cases: Clinical article. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Ius, T.; Isola, M.; Budai, R.; Pauletto, G.; Tomasino, B.; Fadiga, L.; Skrap, M. Low-grade glioma surgery in eloquent areas: Volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: Clinical article. J. Neurosurg. 2012, 117, 1039–1052. [Google Scholar] [CrossRef]
- Sanai, N.; Chang, S.; Berger, M.S. Low-grade gliomas in adults. J. Neurosurg. 2011, 115, 948–965. [Google Scholar] [CrossRef]
- Skrap, M.; Mondani, M.; Tomasino, B.; Weis, L.; Budai, R.; Pauletto, G.; Eleopra, R.; Fadiga, L.; Ius, T. Surgery of insular nonenhancing gliomas: Volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases. Neurosurgery 2012, 70, 1081–1093. [Google Scholar] [CrossRef] [Green Version]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claus, E.B.; Horlacher, A.; Hsu, L.; Schwartz, R.B.; Dello-Iacono, D.; Talos, F.; Jolesz, F.A.; Black, P.M. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer 2005, 103, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Nitta, M.; Muragaki, Y.; Maruyama, T.; Ikuta, S.; Komori, T.; Maebayashi, K.; Iseki, H.; Tamura, M.; Saito, T.; Okamoto, S.; et al. Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg. Focus 2015, 38, E7. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Cahill, D.P. Extent of Resection Versus Molecular Classification: What Matters When? Neurosurg. Clin. N Am. 2019, 30, 95–101. [Google Scholar] [CrossRef]
- Wijnenga, M.M.J.; Mattni, T.; French, P.J.; Rutten, G.J.; Leenstra, S.; Kloet, F.; Taphoorn, M.J.B.; van den Bent, M.J.; Dirven, C.M.F.; van Veelen, M.L.; et al. Does early resection of presumed low-grade glioma improve survival? A clinical perspective. J. Neurooncol. 2017, 133, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Richter, A.N.; Khoshgoftaar, T.M. A review of statistical and machine learning methods for modeling cancer risk using structured clinical data. Artif. Intell. Med. 2018, 90, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ius, T.; Cesselli, D.; Isola, M.; Pauletto, G.; Tomasino, B.; D’Auria, S.; Bagatto, D.; Pegolo, E.; Beltrami, A.P.; Loreto, C.D.; et al. Incidental Low-Grade Gliomas: Single-Institution Management Based on Clinical, Surgical, and Molecular Data. Neurosurgery 2019. [Google Scholar] [CrossRef]
- Ius, T.; Ciani, Y.; Ruaro, M.E.; Isola, M.; Sorrentino, M.; Bulfoni, M.; Candotti, V.; Correcig, C.; Bourkoula, E.; Manini, I.; et al. An NF-kappaB signature predicts low-grade glioma prognosis: A precision medicine approach based on patient-derived stem cells. Neuro. Oncol. 2018, 20, 776–787. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Reuss, D.E.; Mamatjan, Y.; Schrimpf, D.; Capper, D.; Hovestadt, V.; Kratz, A.; Sahm, F.; Koelsche, C.; Korshunov, A.; Olar, A.; et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: A grading problem for WHO. Acta Neuropathol. 2015, 129, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, D.T.; Duffau, H.; Cagnazzo, F.; Benedetto, N.; Morganti, R.; Perrini, P. IDH wild-type WHO grade II diffuse low-grade gliomas. A heterogeneous family with different outcomes. Systematic review and meta-analysis. Neurosurg. Rev. 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Lessons from brain mapping in surgery for low-grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol. 2005, 4, 476–486. [Google Scholar] [CrossRef]
- Klein, M. Health-related quality of life aspects in patients with low-grade glioma. Adv. Tech. Stand. Neurosurg. 2010, 35, 213–235. [Google Scholar] [PubMed]
- Pekmezci, M.; Rice, T.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Hansen, H.; Sicotte, H.; Kollmeyer, T.M.; McCoy, L.S.; Sarkar, G.; et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017, 133, 1001–1016. [Google Scholar] [CrossRef]
- Patel, S.; Ngounou Wetie, A.G.; Darie, C.C.; Clarkson, B.D. Cancer secretomes and their place in supplementing other hallmarks of cancer. Adv. Exp. Med. Biol. 2014, 806, 409–442. [Google Scholar] [CrossRef]
- Xia, L.; Fang, C.; Chen, G.; Sun, C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: A systematic review and meta-analysis. BMC Cancer 2018, 18, 48. [Google Scholar] [CrossRef]
- Gousias, K.; Schramm, J.; Simon, M. Extent of resection and survival in supratentorial infiltrative low-grade gliomas: Analysis of and adjustment for treatment bias. Acta Neurochir (Wien.) 2014, 156, 327–337. [Google Scholar] [CrossRef]
- Still, M.E.H.; Roux, A.; Huberfeld, G.; Bauchet, L.; Baron, M.H.; Fontaine, D.; Blonski, M.; Mandonnet, E.; Guillevin, R.; Guyotat, J.; et al. Extent of Resection and Residual Tumor Thresholds for Postoperative Total Seizure Freedom in Epileptic Adult Patients Harboring a Supratentorial Diffuse Low-Grade Glioma. Neurosurgery 2019, 85, E332–E340. [Google Scholar] [CrossRef]
- Bourkoula, E.; Mangoni, D.; Ius, T.; Pucer, A.; Isola, M.; Musiello, D.; Marzinotto, S.; Toffoletto, B.; Sorrentino, M.; Palma, A.; et al. Glioma-associated stem cells: A novel class of tumor-supporting cells able to predict prognosis of human low-grade gliomas. Stem Cells 2014, 32, 1239–1253. [Google Scholar] [CrossRef]
- Galetsi, P.; Katsaliaki, K.; Kumar, S. Values, challenges and future directions of big data analytics in healthcare: A systematic review. Soc. Sci. Med. 2019, 241, 112533. [Google Scholar] [CrossRef]
- Blonski, M.; Pallud, J.; Goze, C.; Mandonnet, E.; Rigau, V.; Bauchet, L.; Fabbro, M.; Beauchesne, P.; Baron, M.H.; Fontaine, D.; et al. Neoadjuvant chemotherapy may optimize the extent of resection of World Health Organization grade II gliomas: A case series of 17 patients. J. Neurooncol. 2013, 113, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Blonski, M.; Taillandier, L.; Herbet, G.; Maldonado, I.L.; Beauchesne, P.; Fabbro, M.; Campello, C.; Goze, C.; Rigau, V.; Moritz-Gasser, S.; et al. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: A study of cognitive status and quality of life. J. Neurooncol. 2012, 106, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Dictus, C.; Hartmann, C.; Zurn, O.; Edler, L.; Hartmann, M.; Combs, S.; Herold-Mende, C.; Wirtz, C.R.; Unterberg, A. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien.) 2009, 151, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Hamisch, C.; Ruge, M.; Kellermann, S.; Kohl, A.C.; Duval, I.; Goldbrunner, R.; Grau, S.J. Impact of treatment on survival of patients with secondary glioblastoma. J. Neurooncol. 2017, 133, 309–313. [Google Scholar] [CrossRef]
- Ius, T.; Pauletto, G.; Cesselli, D.; Isola, M.; Turella, L.; Budai, R.; DeMaglio, G.; Eleopra, R.; Fadiga, L.; Lettieri, C.; et al. Second Surgery in Insular Low-Grade Gliomas. Biomed. Res. Int. 2015, 2015, 497610. [Google Scholar] [CrossRef] [Green Version]
- Jaeckle, K.A.; Decker, P.A.; Ballman, K.V.; Flynn, P.J.; Giannini, C.; Scheithauer, B.W.; Jenkins, R.B.; Buckner, J.C. Transformation of low grade glioma and correlation with outcome: An NCCTG database analysis. J. Neurooncol. 2011, 104, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Juratli, T.A.; Kirsch, M.; Robel, K.; Soucek, S.; Geiger, K.; von Kummer, R.; Schackert, G.; Krex, D. IDH mutations as an early and consistent marker in low-grade astrocytomas WHO grade II and their consecutive secondary high-grade gliomas. J. Neurooncol. 2012, 108, 403–410. [Google Scholar] [CrossRef]
- Martino, J.; Taillandier, L.; Moritz-Gasser, S.; Gatignol, P.; Duffau, H. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien.) 2009, 151, 427–436. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Hebb, A.; Barber, J.; Rostomily, R.; Silbergeld, D. Outcomes in Reoperated Low-Grade Gliomas. Neurosurgery 2015, 77, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.H.; Berger, M.S.; Lamborn, K.R.; Aldape, K.; McDermott, M.W.; Prados, M.D.; Chang, S.M. Repeated operations for infiltrative low-grade gliomas without intervening therapy. J. Neurosurg. 2003, 98, 1165–1169. [Google Scholar] [CrossRef] [Green Version]
- Thon, N.; Eigenbrod, S.; Kreth, S.; Lutz, J.; Tonn, J.C.; Kretzschmar, H.; Peraud, A.; Kreth, F.W. IDH1 mutations in grade II astrocytomas are associated with unfavorable progression-free survival and prolonged postrecurrence survival. Cancer 2012, 118, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Wang, Z.F.; Pan, Z.Y.; Peus, D.; Delgado-Fernandez, J.; Pallud, J.; Li, Z.Q. A Meta-Analysis of Survival Outcomes Following Reoperation in Recurrent Glioblastoma: Time to Consider the Timing of Reoperation. Front. Neurol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiman, L. Classification and Regression Trees; Routledge: New York, NY, USA, 1984; Available online: https://doi.org/10.1201/9781315139470 (accessed on 18 November 2019).
- Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]




| Clinical Feature | All Cases n = 241 (100%) | Astrocytoma, IDH Wildtype n = 27 (11.2%) | Astrocytoma, IDH Mutant n = 137 (56.8%) | Oligodendroglioma n = 77 (32.0%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Median (range) | N (%) by Class | % by Feature | Median (range) | N (%) by Class | % by Feature | Median (range) | N (%) by Class | % by Feature | Median (range) | p | |
| Sex | 0.766 | |||||||||||
| Male | 144 (59.8) | 18 (66.7) | 81 (59.1) | 45 (58.4) | ||||||||
| Female | 97 (40.2) | 9 (33.3) | 56 (40.9) | 32 (41.6) | ||||||||
| Age at Surgery (years) | 39 (19–75) | 51 (23–75) | 36 (19–74) * | 41 (22–66) ** | 0.0001 | |||||||
| Karnofsky Performance Status | 100 (80–100) | 100 (80–100) | 100 (80–100) | 100 (80–100) | 0.866 | |||||||
| Tumor side | 0.935 | |||||||||||
| Left | 129 (53.5) | 15 (55.6) | 11.6 | 74 (54.0) | 57.4 | 40 (51.9) | 31.0 | |||||
| Right | 112 (46.5) | 12 (44.4) | 10.7 | 63 (46.0) | 56.3 | 37 (48.1) | 33.0 | |||||
| Tumor location | 0.006 | |||||||||||
| Frontal lobe | 97 (40.2) | 5 (18.5) | 5.2 | 58 (42.3) | 59.8 | 34 (42.2) | 35.0 | |||||
| Insular lobe | 72 (29.2) | 9 (33.3) | 12.5 | 48 (35.0) | 66.7 | 15 (19.5) | 20.8 | |||||
| Parietal lobe | 33 (13.7) | 4 (14.8) | 12.1 | 18 (13.1) | 54.5 | 11 (14.3) | 33.3 | |||||
| Temporal lobe | 39 (16.2) | 9 (33.3) | 23.1 | 13 (9.5) | 33.3 | 17 (22.1) | 43.6 | |||||
| Pre-operative volume (cm3) | 44 (6–260) | 44 (6–133) | 45 (6–260) | 40 (8–159) | 0.1936 | |||||||
| ΔVT2T1 index (cm3) | 13 (0–95) | 18 (0–68) | 14 (0–95) | 11 (0–66) | 0.1841 | |||||||
| Post-operative volume (cm3) | 7 (0–125) | 11 (0–57) | 7 (0–125) | 6 (0–45) *, ** | 0.027 | |||||||
| <10 cm3 | 143 (59.3) | 11 (4.7) | 7.7 | 81 (59.1) | 56.6 | 51 (66.2) | 35.7 | |||||
| 10–19 cm3 | 51 (21.2) | 8 (29.6) | 15.7 | 25 (18.2) | 49.0 | 18 (23.4) | 35.3 | |||||
| 20–29 cm3 | 22 (9.4) | 1 (3.7) | 4.5 | 14 (10.2) | 63.6 | 7 (9.1) | 31.8 | |||||
| >30 cm3 | 25 (10.4) | 7 (25.9) | 28.0 | 17 (12.4) | 68.0 | 1 (1.3) | 4.0 | |||||
| Extent of Resection (%) | 86 (28–100) | 69 (34–100) | 86 (28–100) | 87 (50–100)* | 0.008 | |||||||
| ≥90% | 99 (41.1) | 9 (33.3) | 9.1 | 54 (39.4) | 54.5 | 36 (46.8) | 36.4 | |||||
| 70–89% | 97 (40.2) | 4 (14.8) | 4.1 | 60 (43.8) | 61.9 | 33 (42.9) | 34.0 | |||||
| <70% | 45 (18.7) | 14 (51.9) | 31.1 | 23 (16.8) | 51.1 | 8 (10.4) | 17.8 | |||||
| Pathological Feature | All Cases n = 241 (100%) | Astrocytoma, IDH Wildtype n = 27 (11.2%) | Astrocytoma, IDH Mutant n = 137 (56.8%) | Oligodendroglioma n = 77 (32.0%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Median (range) | N (%) by Class | % by Category | Median (range) | N (%) by Class | % by Category | Median (range) | N (%) by Class | % by Category | Median (range) | p | |
| WHO 2007 LGG type | 0.0001 | |||||||||||
| Astrocytoma | 151 (62.7) | 25 (92.6) | 16.6 | 115 (83.9) | 76.2 | 11 (14.3) | 7.3 | |||||
| Oligodendroglioma | 25 (10.4) | 0 | 0 | 0 | 0 | 25 (32.5) | 100 | |||||
| Oligoastrocytoma | 65 (27.0) | 2 (7.4) | 3.1 | 22 (16.1) | 33.8 | 41 (53.2) | 63.1 | |||||
| Ki67 expression (%) | 4 (1–22) | 3 (1–22) | 5 (1–15) | 4 (1–12) | 0.284 | |||||||
| Number of Mitoses/10 HPF | 1 (0–10) | 1 (0–6) | 1 (0–10) | 1 (0–8) | 0.529 | |||||||
| P53 expression (%) (n = 232) | 160 (69.0) | 12 (46.2) | 123 | 90.4 | 25 | 35.7 | <0.0001 | |||||
| ATRX down-regulation (n = 203) | 116 (57.1) | 3 (18.8) | 105 | 91.3 | 8 | 11.1 | <0.0001 | |||||
| IDH1 / IDH2 mutation | 213 (88.8) | 0 | 137 | 100 * | 76 | 100 * | <0.0001 | |||||
| 1p/19q co-deletion (n = 238) | 77 (32.3) | 1 (4) | 0 | 0 | 76 | 100 | <0.0001 | |||||
| MGMT promoter methylation (n = 231) | 202 (87.5) | 15 (60) | 115 | 86.5 | 72 | 98.6 | <0.0001 | |||||
| Average MGMT promoter Methylation (%) (n = 195) | 25.5 (2.5–80.75) | 14.3 (3.2–43.7) | 24.1 (3.2–64.7) * | 32.4 (2.5–80.7) *, ** | 0.0001 | |||||||
| Clinicopathological Feature | Reference Variable | OS | PFS | MPFS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
| Age* | 1.022 | 1.007–1.037 | 0.004 | 1.006 | 0.994–1.019 | 0.334 | 1.019 | 1.005–1.033 | 0.006 | |
| Sex | Female | 0.988 | 0.688–1.420 | 0.951 | 1.139 | 0.844–1.537 | 0.395 | 0.964 | 0.695–1.336 | 0.824 |
| KPS* | 0.953 | 0.924–0.982 | 0.002 | 0.979 | 0.952–1.007 | 0.144 | 0.955 | 0.928–0.982 | 0.001 | |
| Tumor site | Left hemisphere | 0.760 | 0.533–1.083 | 0.128 | 0.680 | 0.509–0.910 | 0.01 | 0.741 | 0.538–1.019 | 0.065 |
| Pre–operative volume *,# | 3.288 | 1.874–5.766 | <0.0001 | 2.217 | 1.394–3.524 | 0.001 | 3.872 | 2.305–6.503 | <0.0001 | |
| Infiltrative growth index (ΔVT2T1) * | 1.034 | 1.025–1.044 | <0.0001 | 1.035 | 1.025–1.045 | <0.0001 | 1.036 | 1.027–1.044 | <0.0001 | |
| Post-operative volume * | 1.015 | 1.009–1.021 | <0.0001 | 1.021 | 1.015–1.027 | <0.0001 | 1.015 | 1.010–1.021 | <0.0001 | |
| % EOR * | 0.954 | 0.943–0.964 | <0.0001 | 0.961 | 0.952–0.970 | <0.0001 | 0.956 | 0.946–0.966 | <0.0001 | |
| WHO 2007 LGG type | Astrocytoma | |||||||||
| Oligodendroglioma | 0.255 | 0.117–0.554 | 0.001 | 0.441 | 0.259–0.751 | 0.003 | 0.417 | 0.233–0.748 | 0.003 | |
| Oligoastrocytoma | 0.637 | 0.425–0.955 | 0.029 | 0.641 | 0.459–0.895 | 0.009 | 0.624 | 0.431–0.902 | 0.012 | |
| % Ki67* | 1.084 | 1.016–1.157 | 0.014 | 1.059 | 1.004–1.117 | 0.033 | 1.051 | 0.990–1.115 | 0.102 | |
| Number of Mitosis 10HPF * | 1.025 | 0.913–1.150 | 0.677 | 1.053 | 0.958–1.158 | 0.283 | 1.019 | 0.918–1.131 | 0.722 | |
| IDH1 or IDH2mutation | No | 0.189 | 0.111–0.322 | <0.0001 | 0.368 | 0.233–0.579 | <0.0001 | 0.259 | 0.158–0.422 | <0.0001 |
| Chromosome 1p/19qco–deletion | No | 0.463 | 0.308–0.696 | <0.0001 | 0.591 | 0.431–0.810 | 0.001 | 0.529 | 0.370–0.754 | <0.0001 |
| Molecular class | IDH wild type | |||||||||
| IDH mutant | 0.236 | 0.137–0.405 | <0.0001 | 0.434 | 0.272–0.691 | <0.0001 | 0.313 | 0.190–0.516 | <0.0001 | |
| IDH mutant and 1p/19q codeletion | 0.117 | 0.063–0.216 | <0.0001 | 0.269 | 0.163–0.448 | <0.0001 | 0.177 | 0.101–0.308 | <0.0001 | |
| P53 expression * | 1.094 | 0.735–1.629 | 0.658 | 1.281 | 0.924–1.775 | 0.137 | 1.048 | 0.736–1.492 | 0.796 | |
| ATRX downregulation | Yes | 1.039 | 0.694–1.557 | 0.850 | 0.939 | 0.679–1.296 | 0.701 | 1.089 | 0.766–1.548 | 0.634 |
| MGMT promoter methylation | No | 0.497 | 0.292–0.847 | 0.010 | 0.447 | 0.286–0.699 | <0.0001 | 0.571 | 0.351–0.927 | 0.023 |
| Radiotherapy | No | 5.596 | 2.459–12.732 | <0.0001 | 1.926 | 1.295–2.864 | 0.001 | 5.115 | 2.690–9.725 | <0.0001 |
| Chemotherapy | No | 1.876 | 1.107–3.179 | 0.019 | 1.916 | 1.288–2.852 | 0.001 | 2.365 | 1.458–3.837 | <0.0001 |
| Second surgery | No | 0.522 | 0.360–0.757 | 0.001 | 1.220 | 0.913–1.632 | 0.179 | 0.899 | 0.651–1.242 | 0.518 |
| OS | PFS | MPFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Variable | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| KPS* | 0.948 | 0.913–0.984 | 0.006 | 0.954 | 0.922–0.988 | 0.008 | ||||
| Pre–operative volume*# | 3.010 | 1.428–6.345 | 0.004 | |||||||
| Infiltrative growth index (ΔVT2T1)* | 1.024 | 1.006–1.041 | 0.008 | 1.019 | 1.002–1.036 | 0.024 | ||||
| Post–operative volume * | 0.980 | 0.962–0.998 | 0.028 | 0.980 | 0.964–0.996 | 0.016 | ||||
| % EOR * | 0.952 | 0.933–0.972 | <0.0001 | 0.971 | 0.956–0.986 | <0.0001 | 0.957 | 0.940–0.974 | <0.0001 | |
| % Ki67 * | 1.075 | 1.013–1.142 | 0.018 | |||||||
| Molecular class | IDH wild type | |||||||||
| IDH1/2 mutant | 0.374 | 0.187–0.749 | 0.005 | 0.409 | 0.237–0.706 | 0.001 | 0.316 | 0.169–0.593 | <0.0001 | |
| IDH mutant and 1p/19q codeletion | 0.179 | 0.083–0.388 | <0.0001 | 0.290 | 0.159–0.527 | <0.0001 | 0.175 | 0.088–0.346 | <0.0001 | |
| Second Surgery | No | 0.644 | 0.423–0.979 | 0.039 | ||||||
| All Patients | ||
| OS <3 Years | OS >5 Years | OS >10 Years |
| EOR ≤ 76% | EOR >74% PV = 83.8% | EOR >86% |
| IDHwt PV = 78.6% | Oligodendroglioma | |
| ΔVT2T1 ≤ 10 cm3 or 2nd surgery PV = 100% (if ΔVT2T1 ≤ 10 cm3); 71.4% (if 2nd surgery) | ||
| EOR ≤74% | EOR >86% | |
| Age ≤58 years | IDHmt | |
| 2nd surgery PV = 58.3% | Ki67 ≤ 4% PV = 64.7% | |
| IDHwt | ||
| OS <3 years | OS >5 years | OS >10 years |
| Ki67 >6% PV = 100% | Ki67 ≤2% | – |
| Age ≤58 years PV = 100% | ||
| IDHmt | ||
| OS < 3 years | OS > 5 years | OS > 10 years |
| EOR ≤76% | EOR >75% | EOR >86% |
| Pre-operative volume >128 cm3 PV = 66.7% | ΔVT2T1 ≤40 cm3 PV = 84.2% | Age ≤25 years |
| Ki67 ≤4% PV = 100% | ||
| EOR ≤75% | EOR >86% | |
| 2nd surgery PV = 75% | Age >25 years | |
| Ki67<1% PV = 100% | ||
| Oligodendroglioma | ||
| EOR ≤81% | EOR >81% PV = 95.8% | EOR >92% |
| Ki67 >7% or ΔVT2T1 >32 cm3 PV = 100% (if Ki67 >7%); 71.4% (if ΔVT2T1 > 32 cm3) | ΔVT2T1 ≤13 cm3 PV = 100% | |
| EOR ≤81% | ||
| ΔVT2T1 Ki67 ≤7% PV = 88.9% | ||
| Decision Tree Version | OS >3 years | OS >5 years | OS >10 years |
|---|---|---|---|
| Decision Tree | 0.799 | 0.777 | 0.716 |
| Random Forest | 0.841 | 0.782 | 0.777 |
| ADA Boost | 0.785 | 0.686 | 0.676 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesselli, D.; Ius, T.; Isola, M.; Del Ben, F.; Da Col, G.; Bulfoni, M.; Turetta, M.; Pegolo, E.; Marzinotto, S.; Scott, C.A.; et al. Application of an Artificial Intelligence Algorithm to Prognostically Stratify Grade II Gliomas. Cancers 2020, 12, 50. https://doi.org/10.3390/cancers12010050
Cesselli D, Ius T, Isola M, Del Ben F, Da Col G, Bulfoni M, Turetta M, Pegolo E, Marzinotto S, Scott CA, et al. Application of an Artificial Intelligence Algorithm to Prognostically Stratify Grade II Gliomas. Cancers. 2020; 12(1):50. https://doi.org/10.3390/cancers12010050
Chicago/Turabian StyleCesselli, Daniela, Tamara Ius, Miriam Isola, Fabio Del Ben, Giacomo Da Col, Michela Bulfoni, Matteo Turetta, Enrico Pegolo, Stefania Marzinotto, Cathryn Anne Scott, and et al. 2020. "Application of an Artificial Intelligence Algorithm to Prognostically Stratify Grade II Gliomas" Cancers 12, no. 1: 50. https://doi.org/10.3390/cancers12010050
APA StyleCesselli, D., Ius, T., Isola, M., Del Ben, F., Da Col, G., Bulfoni, M., Turetta, M., Pegolo, E., Marzinotto, S., Scott, C. A., Mariuzzi, L., Di Loreto, C., Beltrami, A. P., & Skrap, M. (2020). Application of an Artificial Intelligence Algorithm to Prognostically Stratify Grade II Gliomas. Cancers, 12(1), 50. https://doi.org/10.3390/cancers12010050