Integrin α6β4 in Colorectal Cancer: Expression, Regulation, Functional Alterations and Use as a Biomarker
Abstract
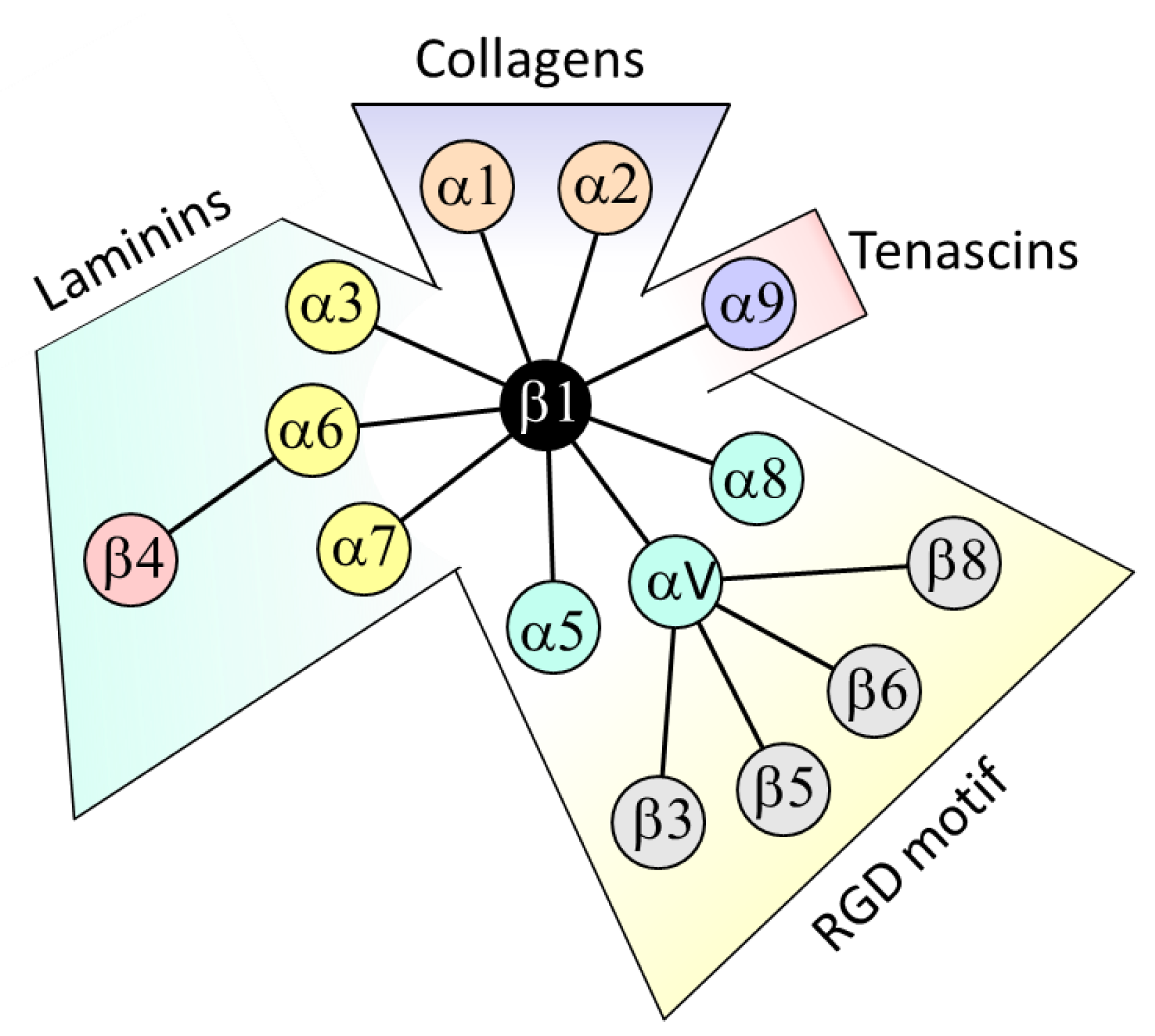
1. Introduction
2. β4 Integrin Subunit (ITGB4) in CRC
2.1. Expression
2.2. Regulation of Expression
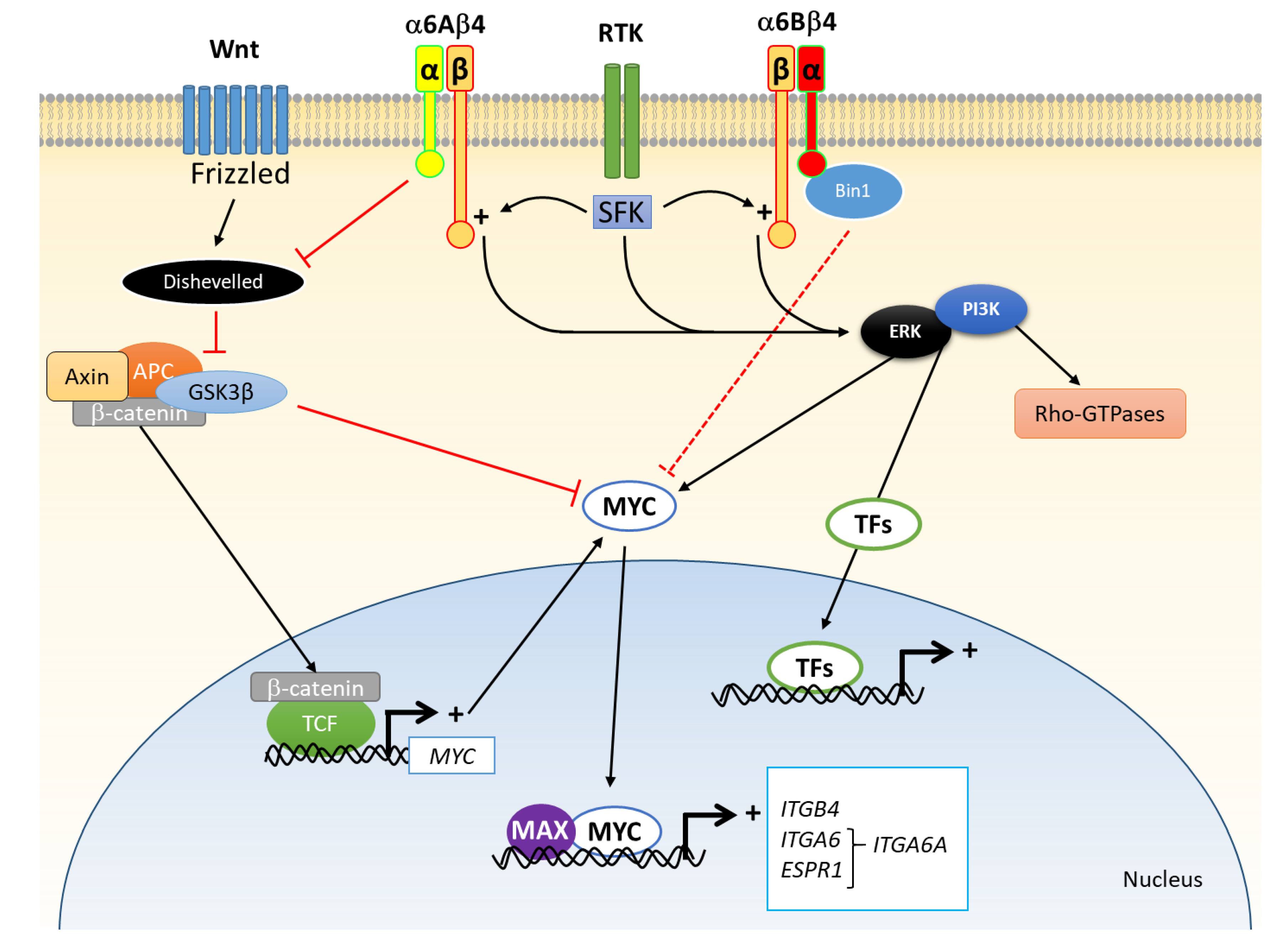
2.3. Change in Functionality
3. α6 Integrin Subunit (ITGA6) in CRC
3.1. Expression
3.2. Regulation of Expression
3.3. Change in Functionality
4. The Integrin α6β4 in CRC
5. Use of ITGA6 and ITGB4 as Biomarkers for CRC
5.1. Prognostic Factors
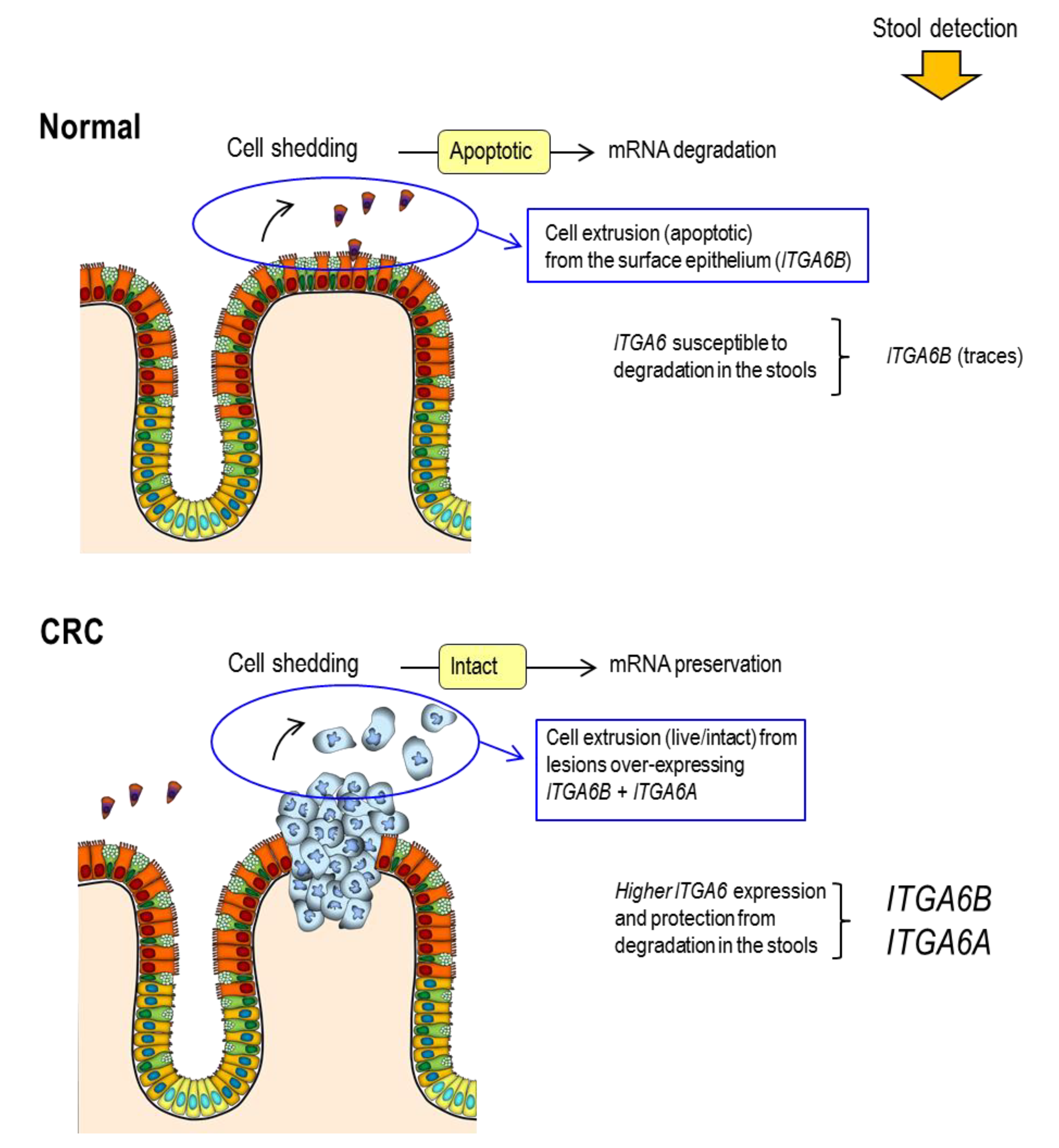
5.2. Screening Factors
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cloutier, G.; Sallenbach-Morrissette, A.; Beaulieu, J.F. Non-integrin laminin receptors in epithelia. Tissue Cell 2019, 56, 71–78. [Google Scholar] [CrossRef]
- Beaulieu, J.F. Integrins and human intestinal cell functions. Front. Biosci. 1999, 4, 310–321. [Google Scholar] [CrossRef]
- Beaulieu, J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997, 31, 1–76. [Google Scholar] [CrossRef]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2019, 56, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Keane, T.J.; Badylak, S.F. The extracellular matrix of the gastrointestinal tract: A regenerative medicine platform. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Boudjadi, S.; Carrier, J.C.; Beaulieu, J.F. Integrin alpha1 subunit is up-regulated in colorectal cancer. Biomark. Res. 2013, 1, 16. [Google Scholar] [CrossRef]
- Boudjadi, S.; Carrier, J.C.; Groulx, J.F.; Beaulieu, J.F. Integrin alpha1beta1 expression is controlled by c-MYC in colorectal cancer cells. Oncogene 2016, 35, 1671–1678. [Google Scholar] [CrossRef]
- Boudjadi, S.; Bernatchez, G.; Senicourt, B.; Beausejour, M.; Vachon, P.H.; Carrier, J.C.; Beaulieu, J.F. Involvement of the Integrin alpha1beta1 in the Progression of Colorectal Cancer. Cancers 2017, 9, 96. [Google Scholar] [CrossRef]
- Basora, N.; Desloges, N.; Chang, Q.; Bouatrouss, Y.; Gosselin, J.; Poisson, J.; Sheppard, D.; Beaulieu, J.F. Expression of the alpha9beta1 integrin in human colonic epithelial cells: Resurgence of the fetal phenotype in a subset of colon cancers and adenocarcinoma cell lines. Int. J. Cancer 1998, 75, 738–743. [Google Scholar] [CrossRef]
- Desloges, N.; Basora, N.; Perreault, N.; Bouatrouss, Y.; Sheppard, D.; Beaulieu, J.F. Regulated expression of the integrin alpha9beta1 in the epithelium of the developing human gut and in intestinal cell lines: Relation with cell proliferation. J. Cell. Biochem. 1998, 71, 536–545. [Google Scholar] [CrossRef]
- Cantor, D.I.; Cheruku, H.R.; Nice, E.C.; Baker, M.S. Integrin alphavbeta6 sets the stage for colorectal cancer metastasis. Cancer Metastasis Rev. 2015, 34, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Pelillo, C.; Bergamo, A.; Mollica, H.; Bestagno, M.; Sava, G. Colorectal Cancer Metastases Settle in the Hepatic Microenvironment Through alpha5beta1 Integrin. J. Cell. Biochem. 2015, 116, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Benoit, Y.D.; Lussier, C.; Ducharme, P.A.; Sivret, S.; Schnapp, L.M.; Basora, N.; Beaulieu, J.F. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol. Cell 2009, 101, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Benoit, Y.D.; Larrivee, J.F.; Groulx, J.F.; Stankova, J.; Vachon, P.H.; Beaulieu, J.F. Integrin alpha8beta1 confers anoikis susceptibility to human intestinal epithelial crypt cells. Biochem. Biophys. Res. Commun. 2010, 399, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, N.I.; Morozevich, G.E.; Chubukina, A.N.; Berman, A.E. Integrin alphavbeta3 promotes anchorage-dependent apoptosis in human intestinal carcinoma cells. Oncogene 2001, 20, 4710–4717. [Google Scholar] [CrossRef] [PubMed]
- Morozevich, G.E.; Kozlova, N.I.; Chubukina, A.N.; Berman, A.E. Role of integrin alphavbeta3 in substrate-dependent apoptosis of human intestinal carcinoma cells. Biochemistry (Moscow) 2003, 68, 416–423. [Google Scholar] [CrossRef]
- Ramovs, V.; Te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57–58, 213–243. [Google Scholar] [CrossRef]
- Pouliot, N.; Kusuma, N. Laminin-511: A multi-functional adhesion protein regulating cell migration, tumor invasion and metastasis. Cell Adhes. Migr. 2013, 7, 142–149. [Google Scholar] [CrossRef]
- de Melker, A.A.; Sonnenberg, A. Integrins: Alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays 1999, 21, 499–509. [Google Scholar] [CrossRef]
- Stallmach, A.; von Lampe, B.; Matthes, H.; Bornhoft, G.; Riecken, E.O. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut 1992, 33, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Lohi, J.; Oivula, J.; Kivilaakso, E.; Kiviluoto, T.; Frojdman, K.; Yamada, Y.; Burgeson, R.E.; Leivo, I.; Virtanen, I. Basement membrane laminin-5 is deposited in colorectal adenomas and carcinomas and serves as a ligand for alpha3beta1 integrin. Apmis 2000, 108, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Turchi, V.; Vitullo, P.; Navarra, G.; Ficari, F.; Cavaliere, F.; Sacchi, A.; Marianicostantini, R. Integrin beta-4 expression in colorectal-cancer. Int. J. Oncol. 1994, 5, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Sordat, I.; Bosman, F.T.; Dorta, G.; Rousselle, P.; Aberdam, D.; Blum, A.L.; Sordat, B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J. Pathol. 1998, 185, 44–52. [Google Scholar] [CrossRef]
- Ni, H.; Dydensborg, A.B.; Herring, F.E.; Basora, N.; Gagne, D.; Vachon, P.H.; Beaulieu, J.F. Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene 2005, 24, 6820–6829. [Google Scholar] [CrossRef] [PubMed]
- Basora, N.; Herring-Gillam, F.E.; Boudreau, F.; Perreault, N.; Pageot, L.P.; Simoneau, M.; Bouatrouss, Y.; Beaulieu, J.F. Expression of functionally distinct variants of the beta4A integrin subunit in relation to the differentiation state in human intestinal cells. J. Biol. Chem. 1999, 274, 29819–29825. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.S.; Yamada, T.; Gotoh, M.; Kanai, Y.; Imai, K.; Hirohashi, S. Cloning and characterization of the human beta4-integrin gene promoter and enhancers. J. Biol. Chem. 1998, 273, 33848–33855. [Google Scholar] [CrossRef]
- Phillips, J.L.; Taberlay, P.C.; Woodworth, A.M.; Hardy, K.; Brettingham-Moore, K.H.; Dickinson, J.L.; Holloway, A.F. Distinct mechanisms of regulation of the ITGA6 and ITGB4 genes by RUNX1 in myeloid cells. J. Cell. Physiol. 2018, 233, 3439–3453. [Google Scholar] [CrossRef]
- An, X.Z.; Zhao, Z.G.; Luo, Y.X.; Zhang, R.; Tang, X.Q.; Hao, D.; Zhao, X.; Lv, X.; Liu, D. Netrin-1 suppresses the MEK/ERK pathway and ITGB4 in pancreatic cancer. Oncotarget 2016, 7, 24719–24733. [Google Scholar] [CrossRef][Green Version]
- Ma, B.; Zhang, L.; Zou, Y.; He, R.; Wu, Q.; Han, C.; Zhang, B. Reciprocal regulation of integrin beta4 and KLF4 promotes gliomagenesis through maintaining cancer stem cell traits. J. Exp. Clin. Cancer Res. 2019, 38, 23. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Wu, Q.; Boyd, D.D. Unbiased screening for transcriptional targets of ZKSCAN3 identifies integrin beta 4 and vascular endothelial growth factor as downstream targets. J. Biol. Chem. 2008, 283, 35295–35304. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, X.; Wang, G.; Zhai, C.; Liu, Y.; Li, H.; Zhang, Y.; Yu, W.; Zhao, Z. ITGB4 is a novel prognostic factor in colon cancer. J. Cancer 2019, 10, 5223–5233. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.; Kontos, C.K.; Boni, T.; Bantounas, I.; Siakouli, D.; Kosmidou, V.; Vlassi, M.; Spyridakis, Y.; Tsipras, I.; Zografos, G.; et al. Epigenetic regulation of miR-21 in colorectal cancer: ITGB4 as a novel miR-21 target and a three-gene network (miR-21-ITGBeta4-PDCD4) as predictor of metastatic tumor potential. Epigenetics 2014, 9, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Walko, G.; Castanon, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef]
- Stewart, R.L.; O’Connor, K.L. Clinical significance of the integrin alpha6beta4 in human malignancies. Lab. Investig. 2015, 95, 976–986. [Google Scholar] [CrossRef]
- Benoit, Y.D.; Groulx, J.F.; Gagne, D.; Beaulieu, J.F. RGD-Dependent Epithelial Cell-Matrix Interactions in the Human Intestinal Crypt. J. Signal Transduct. 2012, 2012. [Google Scholar] [CrossRef]
- Shaw, L.M.; Rabinovitz, I.; Wang, H.H.; Toker, A.; Mercurio, A.M. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 1997, 91, 949–960. [Google Scholar] [CrossRef]
- Beausejour, M.; Boutin, A.; Vachon, P.H. Anoikis regulation—Complexities, distinction, and cell differentiation. In Apoptosis and Beyond: The Many Ways Cells Die; Radosevich, J.A., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 145–182. [Google Scholar]
- Boudjadi, S.; Beaulieu, J.F. MYC and integrins interplay in colorectal cancer. Oncoscience 2016, 3, 50–51. [Google Scholar] [CrossRef]
- Hogervorst, F.; Admiraal, L.G.; Niessen, C.; Kuikman, I.; Janssen, H.; Daams, H.; Sonnenberg, A. Biochemical characterization and tissue distribution of the A and B variants of the integrin alpha 6 subunit. J. Cell. Biol. 1993, 121, 179–191. [Google Scholar] [CrossRef]
- Dydensborg, A.B.; Teller, I.C.; Basora, N.; Groulx, J.F.; Auclair, J.; Francoeur, C.; Escaffit, F.; Pare, F.; Herring, E.; Menard, D.; et al. Differential expression of the integrins alpha6Abeta4 and alpha6Bbeta4 along the crypt-villus axis in the human small intestine. Histochem. Cell Biol. 2009, 131, 531–536. [Google Scholar] [CrossRef]
- Dydensborg, A.B.; Teller, I.C.; Groulx, J.F.; Basora, N.; Pare, F.; Herring, E.; Gauthier, R.; Jean, D.; Beaulieu, J.F. Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation and c-Myc activity. BMC Cancer 2009, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Groulx, J.F.; Giroux, V.; Beausejour, M.; Boudjadi, S.; Basora, N.; Carrier, J.C.; Beaulieu, J.F. Integrin alpha6A splice variant regulates proliferation and the Wnt/beta-catenin pathway in human colorectal cancer cells. Carcinogenesis 2014, 35, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Kitazawa, R.; Mizuno, K.; Maeda, S.; Kitazawa, S. Identification of regulatory elements of human alpha 6 integrin subunit gene. Biochem. Biophys. Res. Commun. 1997, 241, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, M.; Vigneault, F.; Leclerc, S.; Guerin, S.L. Laminin reduces expression of the human alpha6 integrin subunit gene by altering the level of the transcription factors Sp1 and Sp3. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3490–3505. [Google Scholar] [CrossRef]
- Sikora, K.; Chan, S.; Evan, G.; Gabra, H.; Markham, N.; Stewart, J.; Watson, J. c-myc oncogene expression in colorectal cancer. Cancer 1987, 59, 1289–1295. [Google Scholar] [CrossRef]
- Erisman, M.D.; Rothberg, P.G.; Diehl, R.E.; Morse, C.C.; Spandorfer, J.M.; Astrin, S.M. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol. Cell. Biol. 1985, 5, 1969–1976. [Google Scholar] [CrossRef]
- Groulx, J.F.; Boudjadi, S.; Beaulieu, J.F. MYC Regulates alpha6 Integrin Subunit Expression and Splicing Under Its Pro-Proliferative ITGA6A form in Colorectal Cancer Cells. Cancers 2018, 10, 42. [Google Scholar] [CrossRef]
- Goel, H.L.; Gritsko, T.; Pursell, B.; Chang, C.; Shultz, L.D.; Greiner, D.L.; Norum, J.H.; Toftgard, R.; Shaw, L.M.; Mercurio, A.M. Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep. 2014, 7, 747–761. [Google Scholar] [CrossRef]
- Kretzschmar, K.; Clevers, H. Wnt/beta-catenin signaling in adult mammalian epithelial stem cells. Dev. Biol. 2017, 428, 273–282. [Google Scholar] [CrossRef]
- Beaulieu, J.F. Integrin alpha6beta4 in colorectal cancer. World J. Gastrointest. Pathophysiol. 2010, 1, 3–11. [Google Scholar] [CrossRef] [PubMed]
- De Arcangelis, A.; Hamade, H.; Alpy, F.; Normand, S.; Bruyere, E.; Lefebvre, O.; Mechine-Neuville, A.; Siebert, S.; Pfister, V.; Lepage, P.; et al. Hemidesmosome integrity protects the colon against colitis and colorectal cancer. Gut 2017, 66, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.F. Integrin α6 variants and colorectal cancer. Gut 2018, 67, 1747–1748. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.J.; Imperiale, T.F. Stool Testing for Colorectal Cancer Screening. Gastroenterology 2015, 149, 1286–1293. [Google Scholar] [CrossRef]
- Willyard, C. Screening: Early alert. Nature 2015, 521, S4–S5. [Google Scholar] [CrossRef]
- Brenner, H.; Hoffmeister, M.; Stegmaier, C.; Brenner, G.; Altenhofen, L.; Haug, U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007, 56, 1585–1589. [Google Scholar] [CrossRef]
- Click, B.; Pinsky, P.F.; Hickey, T.; Doroudi, M.; Schoen, R.E. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA 2018, 319, 2021–2031. [Google Scholar] [CrossRef]
- Schroy, P.C., 3rd; Lal, S.; Glick, J.T.; Robinson, P.A.; Zamor, P.; Heeren, T.C. Patient preferences for colorectal cancer screening: How does stool DNA testing fare? Am. J. Manag. Care 2007, 13, 393–400. [Google Scholar]
- Allison, J.E.; Fraser, C.G.; Halloran, S.P.; Young, G.P. Population screening for colorectal cancer means getting FIT: The past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (FIT). Gut Liver 2014, 8, 117–130. [Google Scholar] [CrossRef]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef]
- Yu, Y.J.; Majumdar, A.P.; Nechvatal, J.M.; Ram, J.L.; Basson, M.D.; Heilbrun, L.K.; Kato, I. Exfoliated cells in stool: A source for reverse transcription-PCR-based analysis of biomarkers of gastrointestinal cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.S.; Baker, M.S.; Nice, E.C. Mass spectrometry-based analysis for the discovery and validation of potential colorectal cancer stool biomarkers. Methods Enzymol. 2017, 586, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014, 371, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Barnell, E.K.; Kang, Y.; Wurtzler, E.M.; Griffith, M.; Chaudhuri, A.A.; Griffith, O.L.; Geneoscopy, S. Noninvasive Detection of High-Risk Adenomas Using Stool-Derived Eukaryotic RNA Sequences as Biomarkers. Gastroenterology 2019, 157, 884–887 e883. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, Y.; Yoshida, K.; Takai, T.; Ikuma, M.; Hishida, A.; Kanaoka, S. Factors that contribute to faecal cyclooxygenase-2 mRNA expression in subjects with colorectal cancer. Br. J. Cancer 2010, 102, 916–921. [Google Scholar] [CrossRef]
- Kanaoka, S.; Yoshida, K.; Miura, N.; Sugimura, H.; Kajimura, M. Potential usefulness of detecting cyclooxygenase 2 messenger RNA in feces for colorectal cancer screening. Gastroenterology 2004, 127, 422–427. [Google Scholar] [CrossRef]
- Herring, E.; Kanaoka, S.; Tremblay, E.; Beaulieu, J.F. A stool multitarget mRNA assay for the detection of colorectal neoplasms. Methods Mol. Biol. 2018, 1765, 217–227. [Google Scholar] [CrossRef]
- Beaulieu, J.F.; Herring, E.; Kanaoka, S.; Tremblay, E. Use of integrin alpha 6 transcripts in a stool mRNA assay for the detection of colorectal cancers at curable stages. Oncotarget 2016, 7, 14684–14692. [Google Scholar] [CrossRef]
- Herring, E.; Kanaoka, S.; Tremblay, E.; Beaulieu, J.F. Droplet digital PCR for quantification of ITGA6 in a stool mRNA assay for the detection of colorectal cancers. World J. Gastroenterol. 2017, 23, 2891–2898. [Google Scholar] [CrossRef]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaulieu, J.-F. Integrin α6β4 in Colorectal Cancer: Expression, Regulation, Functional Alterations and Use as a Biomarker. Cancers 2020, 12, 41. https://doi.org/10.3390/cancers12010041
Beaulieu J-F. Integrin α6β4 in Colorectal Cancer: Expression, Regulation, Functional Alterations and Use as a Biomarker. Cancers. 2020; 12(1):41. https://doi.org/10.3390/cancers12010041
Chicago/Turabian StyleBeaulieu, Jean-François. 2020. "Integrin α6β4 in Colorectal Cancer: Expression, Regulation, Functional Alterations and Use as a Biomarker" Cancers 12, no. 1: 41. https://doi.org/10.3390/cancers12010041
APA StyleBeaulieu, J.-F. (2020). Integrin α6β4 in Colorectal Cancer: Expression, Regulation, Functional Alterations and Use as a Biomarker. Cancers, 12(1), 41. https://doi.org/10.3390/cancers12010041




