Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells
Abstract
1. Introduction
2. Results
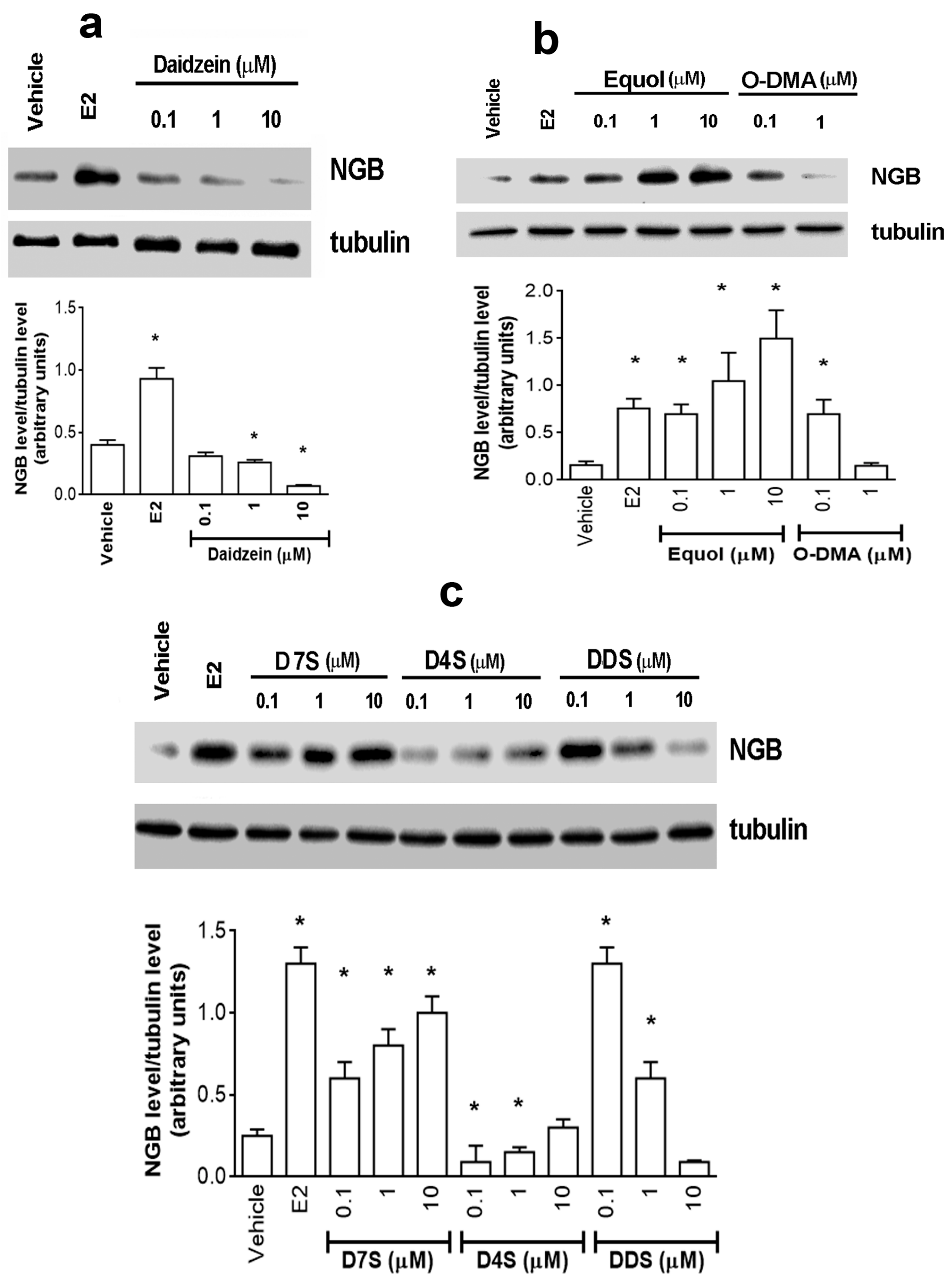
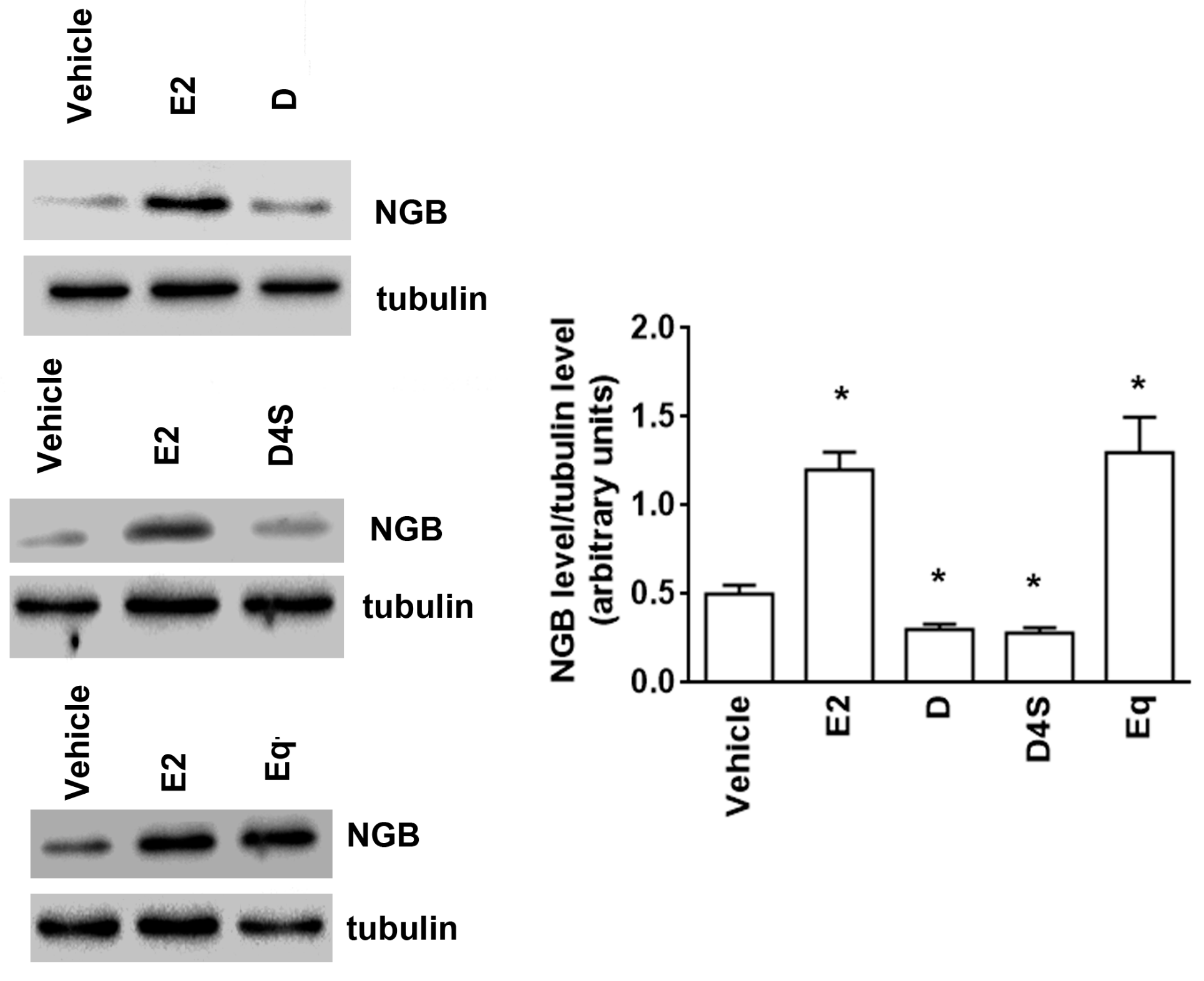
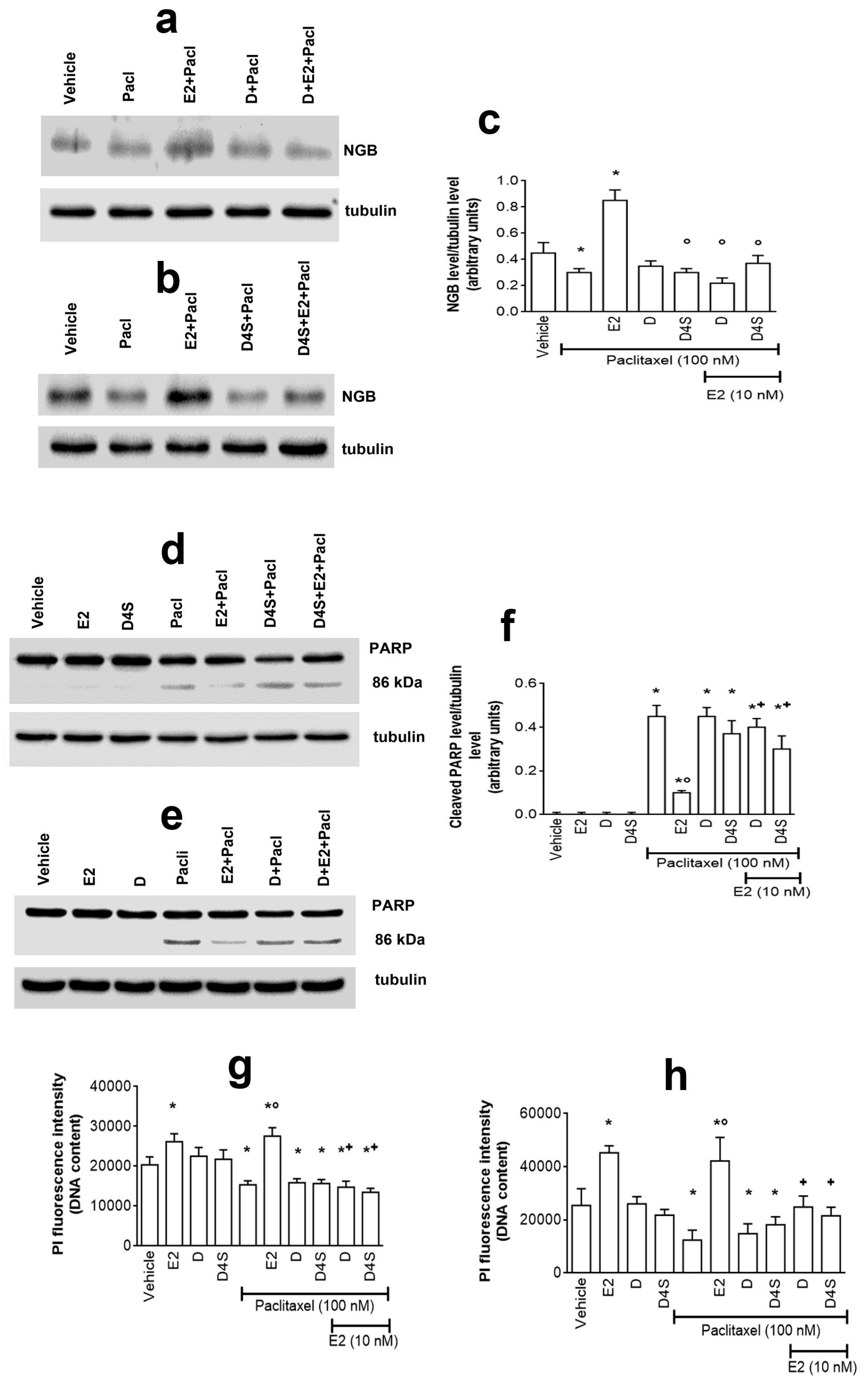
2.1. Effect of D and Its Metabolites on NGB Levels in Breast Cancer Cells
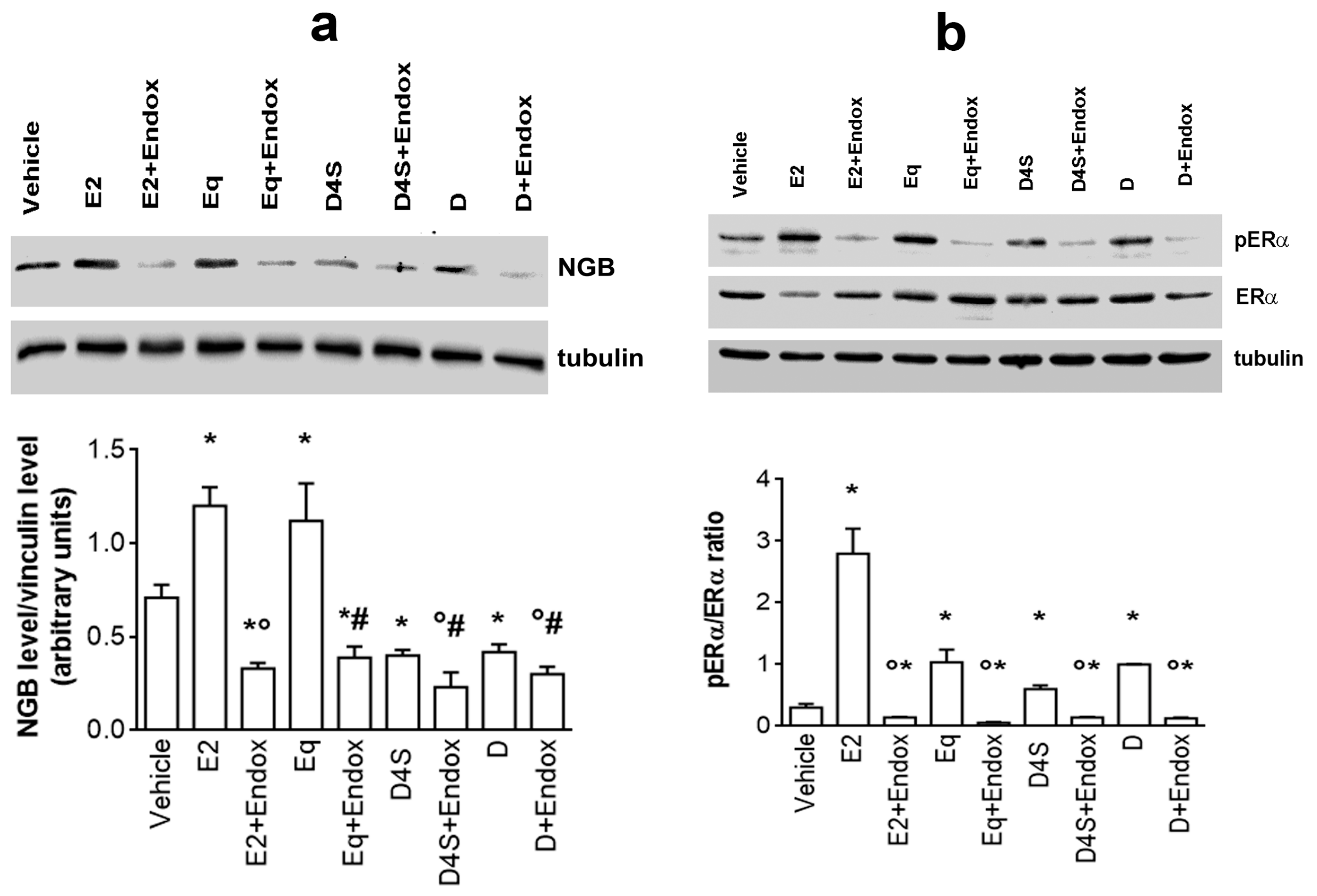
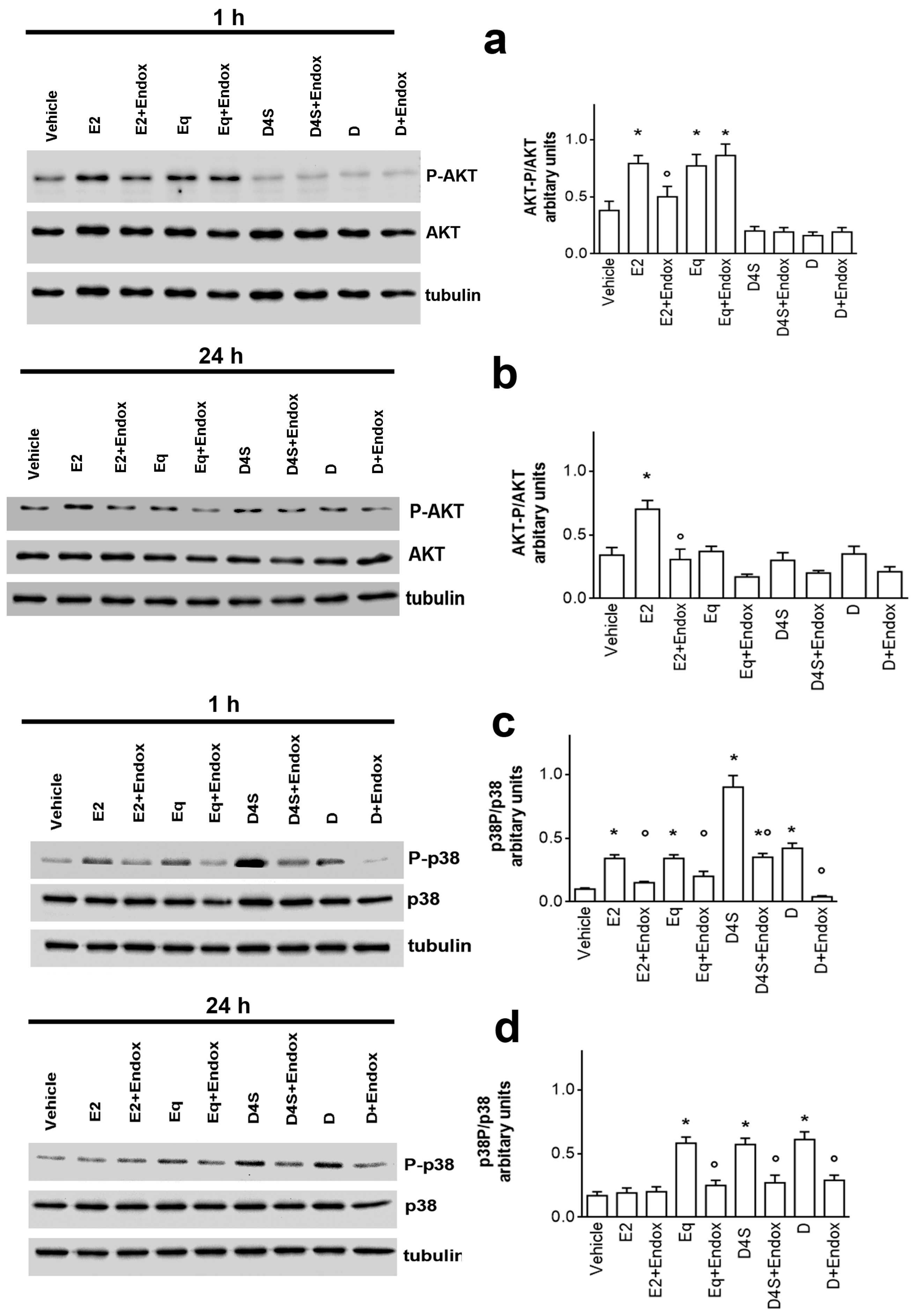
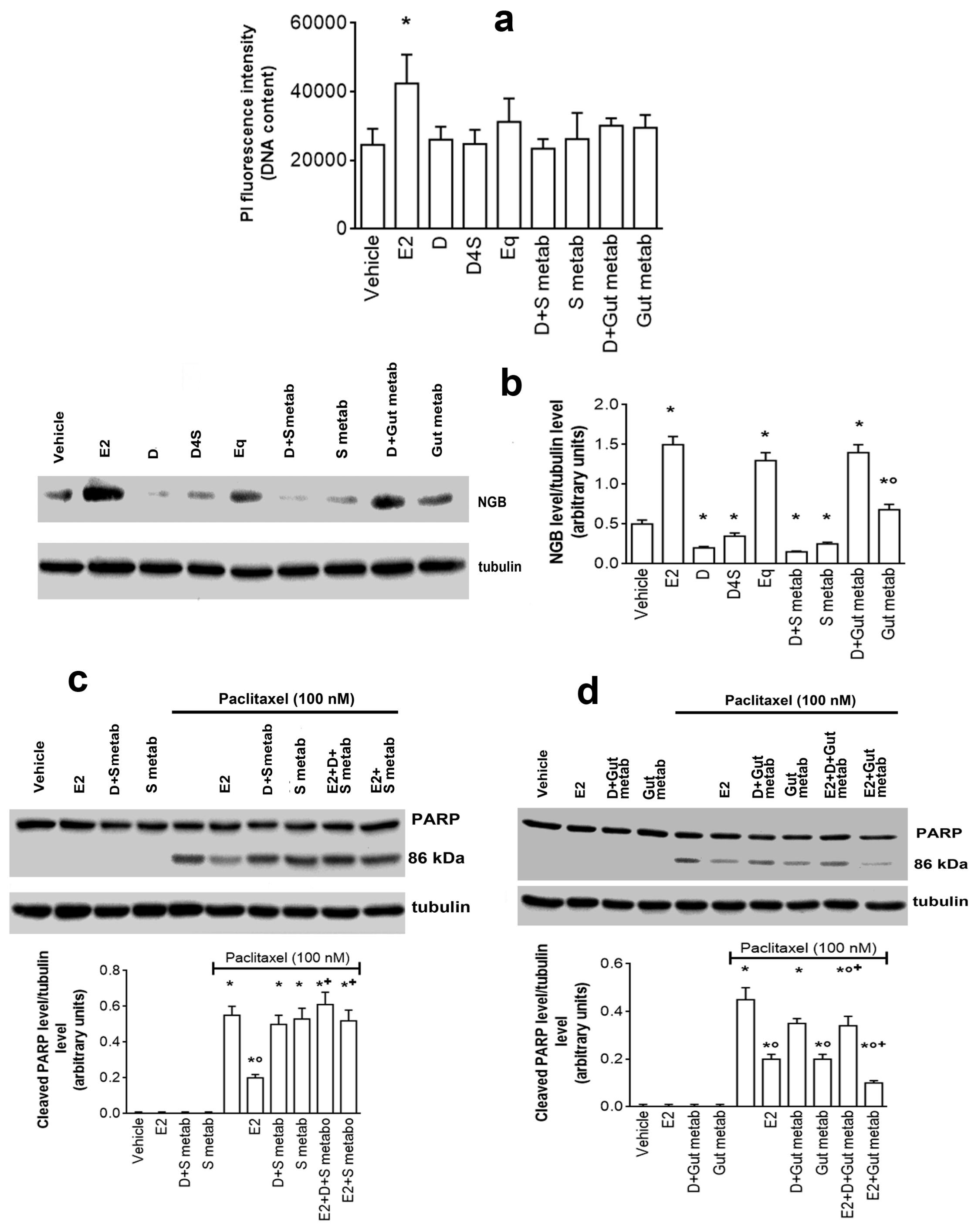
2.2. Mechanisms of D-, D4S-, and Eq Induced Modulation of NGB Levels
2.3. Physiological Outcomes of D- and D4S-Induced E2/ERα/NGB Pathway Avoidance
2.4. Physiological Outcomes of D in Mixture with Its Metabolites
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Western Blot Assay
4.4. Cellular DNA Content, Propidium Iodide (PI) Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A. Phytoestrogens, phytosteroids and saponins in vegetables: Biosynthesis, functions, health effects and practical applications. Adv. Food Nutr. Res. 2019, 90, 351–421. [Google Scholar]
- Lacroix, S.; Klicic Badoux, J.; Scott-Boyer, M.P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A computationally driven analysis of the polyphenol-protein interactome. Sci. Rep. 2018, 8, 2232. [Google Scholar] [CrossRef] [PubMed]
- Cipolletti, M.; Montalesi, E.; Nuzzo, M.T.; Fiocchetti, M.; Ascenzi, P.; Marino, M. Potentiation of paclitaxel effect by resveratrol in human breast cancer cells by counteracting the 17β-estradiol/estrogen receptor α/neuroglobin pathway. J. Cell. Physiol. 2019, 234, 3147–3157. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Bilir, B.; Ali, S.; Sahin, K.; Kucuk, O. Soy Isoflavones in Integrative Oncology: Increased Efficacy and Decreased Toxicity of Cancer Therapy. Integr. Cancer Ther. 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Q.; Zhang, Y.; Sun, W.; Zhang, S.; Li, Y.; Wang, J.; Bao, Y. Functional daidzein enhances the anticancer effect of topotecan and reverses BCRP-mediated drug resistance in breast cancer. Pharmacol. Res. 2019, 147, 104387. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol. Med. Rep. 2013, 7, 781–784. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, Y.; Shi, Z.; Everaert, N.; Ren, G. Synergistic Effect of Bioactive Anticarcinogens from Soybean on Anti-Proliferative Activity in MDA-MB-231 and MCF-7 Human Breast Cancer Cells In Vitro. Molecules 2018, 23, 1557. [Google Scholar] [CrossRef]
- Bao, C.; Namgung, H.; Lee, J.; Park, H.C.; Ko, J.; Moon, H.; Ko, H.W.; Lee, H.J. Daidzein suppresses tumor necrosis factor-α induced migration and invasion by inhibiting hedgehog/Gli1 signaling in human breast cancer cells. J. Agric. Food Chem. 2014, 62, 3759–3767. [Google Scholar] [CrossRef]
- Liu, X.; Suzuki, N.; Santosh Laxmi, Y.R.; Okamoto, Y.; Shibutani, S. Anti-breast cancer potential of daidzein in rodents. Life Sci. 2012, 91, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Wähälä, K.; Adlercreutz, H. Identification of isoflavone metabolites Dihydrodaidzein, Dihydrogenistein, 6-OH-O-DMA, and cis-4-OH-equol in human urine by gas chromatography–mass spectroscopy using authentic reference compounds. Anal. Biochem. 1999, 274, 211–219. [Google Scholar] [CrossRef] [PubMed]
- King, R.A.; Bursill, D.B. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am. J. Clin. Nutr. 1998, 67, 867–872. [Google Scholar] [CrossRef]
- Gardana, C.; Canzi, E.; Simonetti, P. The role of diet in the metabolism of daidzein by human faecal microbiota sampled from Italian volunteers. J. Nutr. Biochem. 2009, 20, 940–947. [Google Scholar] [CrossRef]
- Chang, Y.C.; Nair, M.G.; Nitiss, J.L. Metabolites of daidzein and genistein and their biological activities. J. Nat. Prod. 1995, 58, 1901–1905. [Google Scholar] [CrossRef]
- Soukup, S.T.; Helppi, J.; Müller, D.R.; Zierau, O.; Watzl, B.; Vollmer, G.; Diel, P.; Bub, A.; Kulling, S.E. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: A cross-species and sex comparison. Arch. Toxicol. 2016, 90, 1335–1347. [Google Scholar] [CrossRef]
- Chang, Y.C.; Nair, M.G. Metabolism of daidzein and genistein by intestinal bacteria. J. Nat. Prod. 1995, 58, 1892–1896. [Google Scholar] [CrossRef]
- Totta, P.; Acconcia, F.; Virgili, F.; Cassidy, A.; Weinberg, P.D.; Rimbach, G.; Marino, M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activities of estrogen receptor-beta in cultured human cancer cells. J. Nutr. 2005, 135, 2687–2693. [Google Scholar] [CrossRef]
- Lazennec, G.; Bresson, D.; Lucas, A.; Chauveau, C.; Vignon, F. ERβ inhibits proliferation and invasion of breast cancer cells. Endocrinology 2001, 142, 4120–4130. [Google Scholar] [CrossRef]
- Marino, M.; Ascenzi, P. Membrane association of estrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids 2008, 73, 853–858. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Cipolletti, M.; Leone, S.; Ascenzi, P.; Marino, M. Neuroglobin overexpression induced by the 17β-Estradiol-Estrogen receptor-α Pathway reduces the sensitivity of MCF-7 Breast cancer cell to paclitaxel. IUBMB Life 2016, 68, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J.-Å. Estrogen receptors: Therapies targeted to receptor subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Bocedi, A.; Marino, M. Structure-function relationship of estrogen receptor alpha and beta: Impact on human health. Mol. Asp. Med. 2006, 27, 299–402. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gustafsson, J.-Å. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Fiocchetti, M.; Solar Fernandez, V.; Montalesi, E.; Marino, M. Neuroglobin: A novel player in the oxidative stress response of cancer cells. Oxidative Med. Cell. Longev. 2019, 2019, 6315034. [Google Scholar] [CrossRef] [PubMed]
- Fiocchetti, M.; Cipolletti, M.; Leone, S.; Naldini, A.; Carraro, F.; Giordano, D.; Verde, C.; Ascenzi, P.; Marino, M. Neuroglobin in breast cancer cells: Effect of hypoxia and oxidative stress on protein level, localization, and anti-apoptotic function. PLoS ONE 2016, 11, e0154959. [Google Scholar] [CrossRef] [PubMed]
- Fiocchetti, M.; Cipolletti, M.; Ascenzi, P.; Marino, M. Dissecting the 17β-estradiol pathways necessary for neuroglobin anti-apoptotic activity in breast cancer. J. Cell. Physiol. 2018, 233, 5087–5103. [Google Scholar] [CrossRef]
- Galluzzo, P.; Ascenzi, P.; Bulzomi, P.; Marino, M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor alpha-palmitoylation. Endocrinology 2008, 149, 2567–2575. [Google Scholar] [CrossRef]
- Crowe, K.M.; Francis, C. Position of the academy of nutrition and dietetics: Functional foods. J. Acad. Nutr. Diet. 2013, 113, 1096–1103. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Zakroczymski, M.A.; Naaz, A.; Mukai, M.; Ju, Y.H.; Doerge, D.R.; Katzenellenbogen, J.A.; Helferich, W.G.; Cooke, P.S. Estrogenicity of the isoflavone metabolite equol on reproductive and non-reproductive organs in mice. Biol. Reprod. 2004, 71, 966–972. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Fiocchetti, M.; Marino, M. Xenoestrogen regulation of ERα/ERβ balance in hormone-associated cancers. Mol. Cell. Endocrinol. 2017, 457, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bulzomi, P.; Galluzzo, P.; Bolli, A.; Leone, S.; Acconcia, F.; Marino, M. The pro-apoptotic effect of quercetin in cancer cell lines requires ERβ-dependent signals. J. Cell. Physiol. 2012, 227, 1891–1898. [Google Scholar] [CrossRef]
- Park, H.; Jin, U.H.; Orr, A.A.; Echegaray, S.P.; Davidson, L.A.; Allred, C.D.; Chapkin, R.S.; Jayaraman, A.; Lee, K.; Tamamis, P.; et al. Isoflavones as Ah Receptor Agonists in Colon-Derived Cell Lines: Structure-Activity Relationships. Chem. Res. Toxicol. 2019, 32, 2353–2364. [Google Scholar] [CrossRef]
- Lee, J.E.; Safe, S. 3′,4′-dimethoxyflavone as an aryl hydrocarbon receptor antagonist in human breast cancer cells. Toxicol. Sci. 2000, 58, 235–242. [Google Scholar] [CrossRef]
- Marino, M.; Pellegrini, M.; La Rosa, P.; Acconcia, F. Susceptibility of estrogen receptor rapid responses to xenoestrogens: Physiological outcomes. Steroids 2012, 77, 910–917. [Google Scholar] [CrossRef]
- La Rosa, P.; Pellegrini, M.; Totta, P.; Acconcia, F.; Marino, M. Xenoestrogens alter estrogen receptor (ER) α intracellular levels. PLoS ONE 2014, 9, e88961. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Nuzzo, M.T.; Totta, P.; Acconcia, F.; Ascenzi, P.; Marino, M. Neuroglobin, a pro-survival player in estrogen receptor α-positive cancer cells. Cell Death Dis. 2014, 5, e1449. [Google Scholar] [CrossRef]
- Uifălean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy Isoflavones and Breast Cancer Cell Lines: Molecular Mechanisms and Future Perspectives. Molecules 2015, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Tseng, M.; Daly, M.B. Correlates of soy food consumption in women at increased risk for breast cancer. J. Am. Diet. Assoc. 2005, 105, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F.; Davis, C.; Park, M.; Heinze, T.M.; Beger, R.D. Variations in metabolism of the soy isoflavonoid daidzein by human intestinal microfloras from different individuals. Arch. Microbiol. 2003, 180, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hur, H.G.; Beger, R.D.; Heinze, T.M.; Lay, J.O., Jr.; Freeman, J.P.; Dore, J.; Rafii, F. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch. Microbiol. 2002, 178, 8–12. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalesi, E.; Cipolletti, M.; Cracco, P.; Fiocchetti, M.; Marino, M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers 2020, 12, 167. https://doi.org/10.3390/cancers12010167
Montalesi E, Cipolletti M, Cracco P, Fiocchetti M, Marino M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers. 2020; 12(1):167. https://doi.org/10.3390/cancers12010167
Chicago/Turabian StyleMontalesi, Emiliano, Manuela Cipolletti, Patrizio Cracco, Marco Fiocchetti, and Maria Marino. 2020. "Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells" Cancers 12, no. 1: 167. https://doi.org/10.3390/cancers12010167
APA StyleMontalesi, E., Cipolletti, M., Cracco, P., Fiocchetti, M., & Marino, M. (2020). Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers, 12(1), 167. https://doi.org/10.3390/cancers12010167






