Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score
Abstract
:1. Introduction
2. Results
2.1. Analysis at Baseline
2.2. Prospective Analysis—Outcome
2.3. Predictors of Outcome
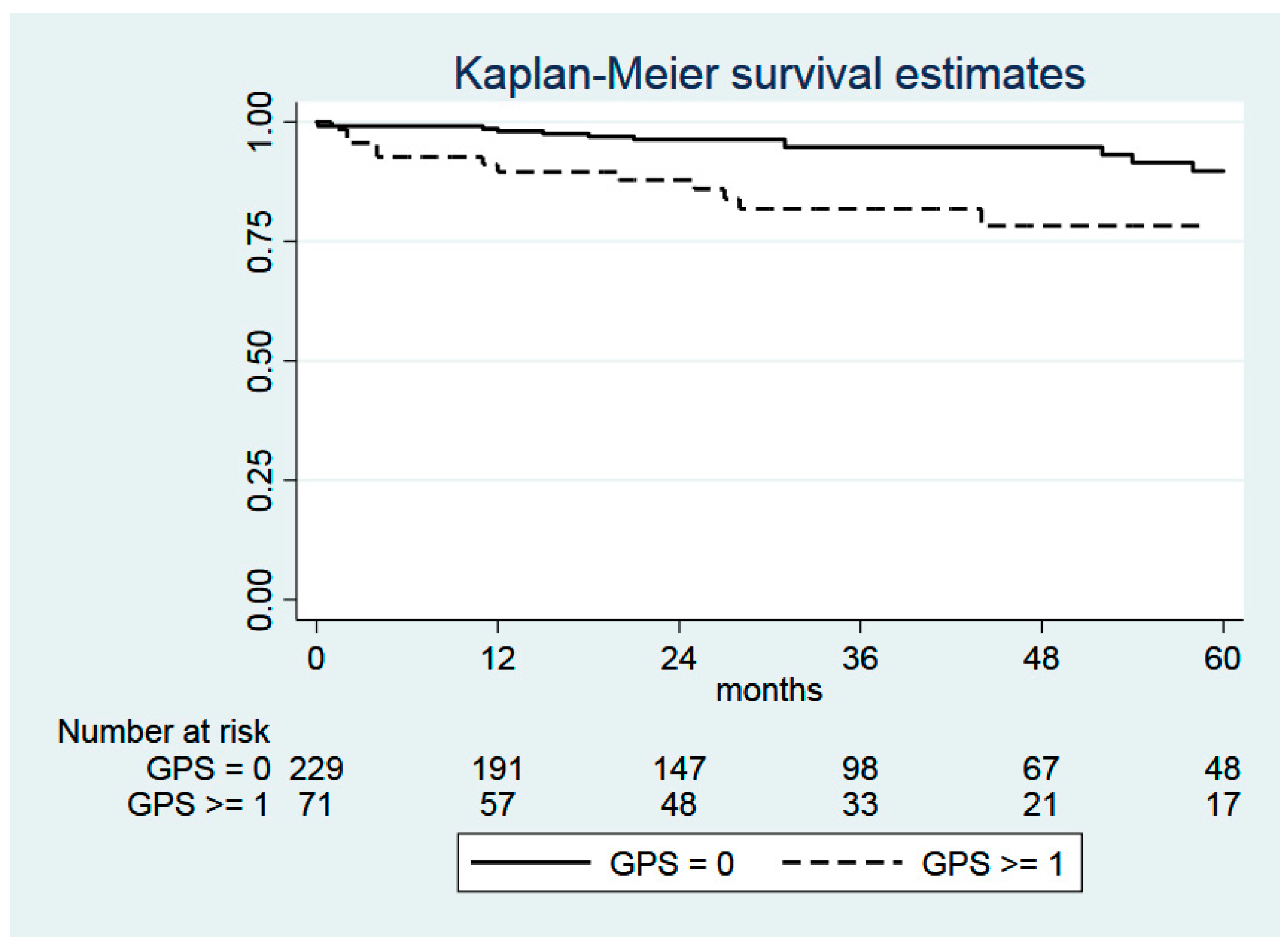
2.4. Glasgow Prognostic Score (GPS)
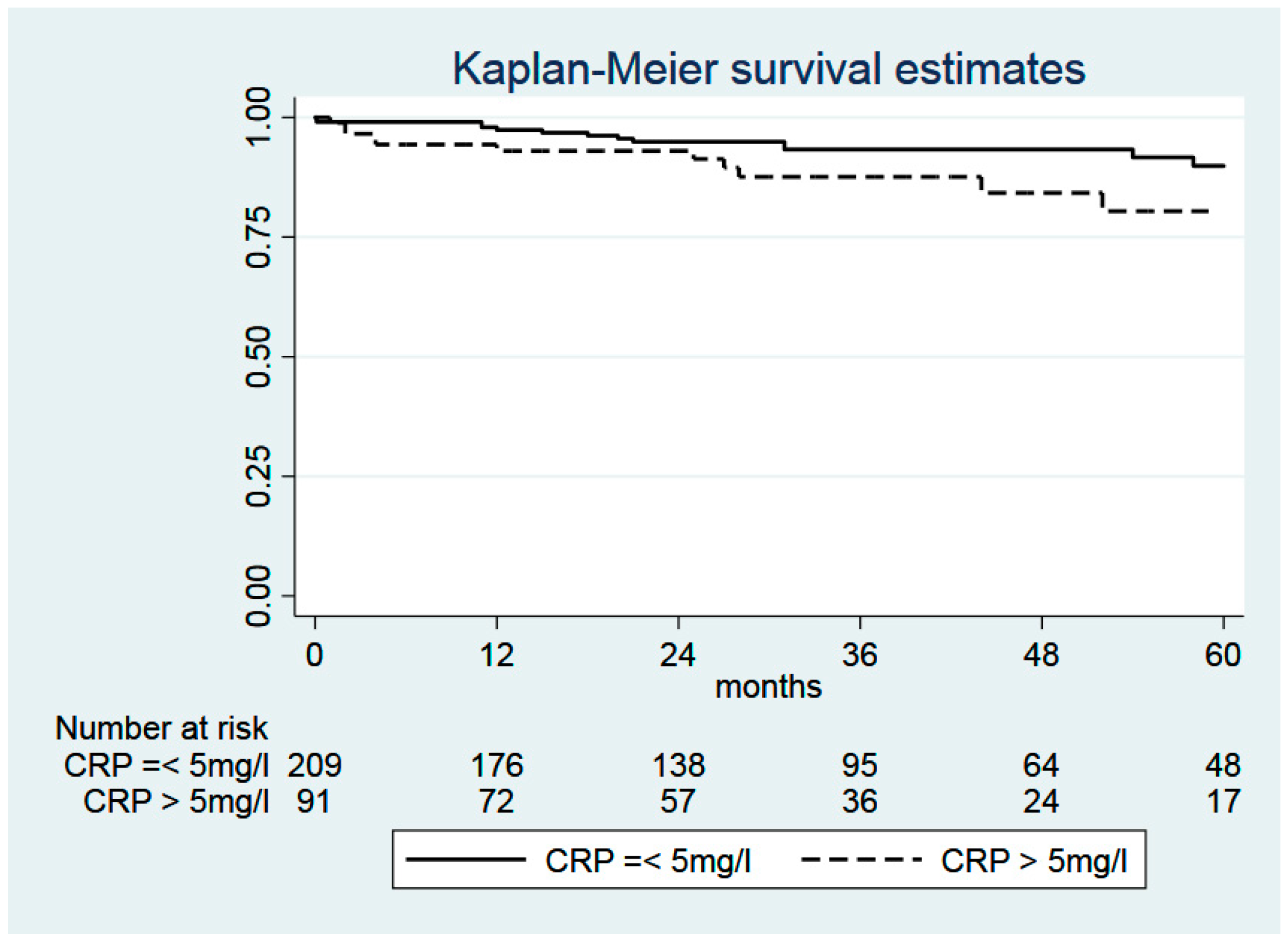
2.5. C-Reactive Protein (CRP)
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar] [PubMed]
- Goya, T.; Asamura, H.; Yoshimura, H.; Kato, H.; Shimokata, K.; Tsuchiya, R.; Sohara, Y.; Miya, T.; Miyaoka, E. Japanese Joint Committee of Lung Cancer Registry. Prognosis of 6644 resected non-small cell lung cancers in Japan: A Japanese lung cancer registry study. Lung Cancer 2005, 50, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, R.; Tsukada, H.; Nakazato, Y.; Takei, H.; Furuyashiki, G.; Koshi-ishi, Y.; Goya, T. Early recurrence after surgical resection in patients with pathological stage I non-small cell lung cancer. Thorac. Cardiovasc. Surg. 2009, 57, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Lewis, S.Z.; Diekemper, R.; Addrizzo-Harris, D.; Alberts, W.M. Executive Summary: Diagnosis and Management of Lungcancer, 3rd ed.; American College of ChestPhysicians evidence-based clinical practice guidelines. Chest 2013, 143, 7S–37S. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Chen, S.; Ma, S.; Zhang, S. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: A meta-analysis. Springerplus 2016, 5, 439. [Google Scholar] [CrossRef] [Green Version]
- Jiang, A.G.; Chen, H.L.; Lu, H.Y. The relationship between Glasgow Prognostic Score and serum tumor markers in patients with advanced non-small cell lung cancer. BMC Cancer 2015, 15, 386. [Google Scholar] [CrossRef] [Green Version]
- Lindenmann, J.; Fink-Neuboeck, N.; Koesslbacher, M.; Pichler, M.; Stojakovic, T.; Roller, R.E.; Maier, A.; Anegg, U.; Smolle, J.; Smolle-Juettner, F.M. The influence of elevated levels of C-reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment. J. Surg. Oncol. 2014, 110, 645–650. [Google Scholar] [CrossRef]
- Lindenmann, J.; Fink-Neuboeck, N.; Avian, A.; Pichler, M.; Habitzruther, M.; Maier, A.; Smolle-Juettner, F.M. Preoperative Glasgow Prognostic Score as additional independent prognostic parameter for patients with esophageal cancer after curative esophagectomy. Eur. J. Surg. Oncol. 2017, 43, 445–453. [Google Scholar] [CrossRef]
- Wang, C.S.; Sun, C.F. C-reactive Protein and Malignancy: Clinico-pathological Association and Therapeutic Implication. Chang. Gung Med. J. 2009, 32, 471–482. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Mantovani, A. Cancer: Inflaming metastasis. Nature 2009, 457, 36–37. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Jiang, A.G.; Chen, H.L.; Lu, H.Y. Comparison of Glasgow prognostic score and prognostic index in patients with advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 563–568. [Google Scholar] [CrossRef]
- Fan, H.; Shao, Z.Y.; Xiao, Y.Y.; Xie, Z.H.; Chen, W.; Xie, H.; Qin, G.Y.; Zhao, N.Q. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1285–1297. [Google Scholar]
- Leung, E.Y.; Scott, H.R.; McMillan, D.C. Clinical utility of the pretreatment glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J. Thorac. Oncol. 2012, 7, 655–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, T.; Yamasaki, N.; Tsuchiya, T.; Matsumoto, K.; Kunizaki, M.; Taniguchi, D.; Nagayasu, T. Inflammation-based scoring is a useful prognostic predictor of pulmonary resection for elderly patients with clinical stage I non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2015, 47, e140–e145. [Google Scholar] [CrossRef] [PubMed]
- Yotsukura, M.; Ohtsuka, T.; Kaseda, K.; Kamiyama, I.; Hayashi, Y.; Asamura, H. Value of the Glasgow Prognostic Score as a Prognostic Factor in Resectable Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.G.; Cho, B.C.; Bae, M.K.; Lee, C.Y.; Park, I.K.; Kim, D.J.; Ahn, S.V.; Chung, K.Y. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer 2009, 63, 106–110. [Google Scholar] [CrossRef]
- Igai, H.; Matsuura, N.; Tarumi, S.; Chang, S.S.; Misaki, N.; Go, T.; Ishikawa, S.; Yokomise, H. Clinicopathological study of p-T1aN0M0 non-small-cell lung cancer, as defined in the seventh edition of the TNM classification of malignant tumors. Eur. J. Cardiothorac. Surg. 2011, 39, 963–967. [Google Scholar] [CrossRef] [Green Version]
- Kudo, Y.; Saji, H.; Shimada, Y.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; Kajiwara, N.; Ohira, T.; Ikeda, N. Proposal on incorporating blood vessel invasion into the T classification parts as a practical staging system for stage I non-small cell lung cancer. Lung Cancer 2013, 81, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Y.; Lu, J.; Luo, Q.; Wu, C.; Liao, M.; Zheng, Y.; Ai, X.; Gu, L.; Lu, S. Analysis of the T descriptors and other prognosis factors in pathologic stage I non-small cell lung cancer in China. J. Thorac. Oncol. 2009, 4, 702–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuzzi, G.; Galeone, C.; Gisabella, M.; Duranti, L.; Taverna, F.; Suatoni, P.; Morelli, D.; Pastorino, U. Baseline C-reactive protein level predicts survival of early-stage lung cancer: Evidence from a systematic review and meta-analysis. Tumori J. 2016, 102, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Shiner, R.J.; Seckl, M.J.; Stebbing, J.; Sharma, R.; Mauri, F.A. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br. J. Cancer 2014, 110, 1930–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Lerut, T.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef] [Green Version]
- UICC International Union against Cancer. Lung and pleural tumours. In TNM Classification of Malignant Tumours, 7th ed.; Sobin, L.H., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 136–146. [Google Scholar]
- Crinò, L.; Weder, W.; van Meerbeeck, J.; Felip, E.; ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small cell lung cancer: ESMO Clinical PracticeGuidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v103–v115. [Google Scholar]
| Criterion | Value |
|---|---|
| Number of patients | 300 |
| Age | 65.4 ± 10.0 (20–87) |
| Male/Female | 187 (62.3%)/113 (37.7%) |
| BMI | 26.3 ± 4.3 (15.7–9.5) |
| Death | 73 (24.3%) |
| Death due to tumor | 38 (12.7%) |
| Death not tumor related | 35 (11.7%) |
| Tumor recurrence | 59 (19.7%) |
| Postoperative tumor size | |
| T1a | 116 (38.7%) |
| T1b | 78 (26.0%) |
| T2 | 106 (35.3%) |
| Albumin (g/dL) | 4.29 ± 0.49 (2.3–5.4) |
| CRP (mg/L) | 7.88 ± 15.80 (0.5–135.6) |
| GPS | |
| 0 | 229 (76.3%) |
| 1 | 68 (22.7%) |
| 2 | 3 (1.0%) |
| ASA | |
| 1 | 7 (2.3%) |
| 2 | 101 (33.7%) |
| 3 | 173 (57.7%) |
| 4 | 19 (6.3%) |
| Follow-up time (months) | 38.1 ± 28.3 (0–123) |
| Overall survival | |
| 1 year | 94.2% ± 1.4% |
| 3 year | 80.8% ± 2.7% |
| 5 year | 72.5% ± 3.5% |
| 10 year | 29.0% ± 9.2% |
| Criterion | All Cases | GPS 0 | GPS ≥ 1 | p Value |
|---|---|---|---|---|
| n = 300 (100%) | n = 229 (76%) | n = 71 (24%) | ||
| Age (years; mean) | 66.0 (60.1–72.2) | 65.3 | 68.3 | 0.243 |
| Weight (kg; mean) | 75 (65–85) | 75 | 75 | 0.995 |
| BMI (kg/m2; mean) | 26.2 (23.5–28.6) | 26.3 | 26.1 | 0.865 |
| Gender | ||||
| Male | 187 (62%) | 139 (61%) | 48 (68%) | 0.294 |
| Female | 113 (38%) | 90 (39%) | 23 (32%) | |
| Smoking Status | ||||
| Yes | 199 (66%) | 149 (65%) | 50 (70%) | 0.404 |
| No | 101 (34%) | 80 (35%) | 21 (30%) | |
| COPD | ||||
| Yes | 126 (42%) | 95 (41%) | 31 (44%) | 0.745 |
| No | 174 (58%) | 134 (59%) | 40 (56%) | |
| PAD | ||||
| Yes | 25 (8%) | 19 (8%) | 6 (8%) | 0.967 |
| No | 275 (92%) | 210 (92%) | 65 (92%) | |
| CKD | ||||
| Yes | 17 (6%) | 12 (5%) | 5 (7%) | 0.566 |
| No | 283 (94%) | 217 (95%) | 66 (93%) | |
| CHD | ||||
| Yes | 41 (14%) | 24 (10%) | 17 (24%) | 0.004 |
| No | 259 (86%) | 205 (90%) | 54 (76%) | |
| Alcohol abuse | ||||
| Yes | 136 (45%) | 96 (42%) | 40 (56%) | 0.033 |
| No | 164 (55%) | 133 (58%) | 31 (44%) | |
| Albumin (g/dL) | <0.0001 | |||
| Albumin < 3.5 g/dL | 21 (7%) | 0 (0%) | 21 (30%) | |
| Albumin ≥ 3.5 g/dL | 279 (93%) | 229 (100%) | 50 (70%) | |
| CRP (mg/L) | <0.0001 | |||
| CRP ≤ 10 mg/L | 247 (82%) | 229 (100%) | 18 (25%) | |
| CRP > 10 mg/L | 53 (18%) | 0 (0%) | 53 (75%) | |
| Histology | 0.101 | |||
| Adenocarcinoma | 191 (64%) | 154 (67%) | 37 (52%) | |
| Squamous cell | 95 (32%) | 64 (28%) | 31 (44%) | |
| Adenosquamous | 2 (1%) | 2 (1%) | 0 (0%) | |
| Large cell | 2 (1%) | 2 (1%) | 0 (0%) | |
| Other | 10 (3%) | 7 (3%) | 3 (4%) | |
| Grading | ||||
| G1 | 55 (18%) | 45 (19%) | 10 (14%) | 0.507 |
| G2 | 131 (44%) | 100 (44%) | 31 (44%) | |
| G3 | 114 (38%) | 84 (37%) | 30 (42%) | |
| Tumor size | ||||
| T1a | 116 (39%) | 96 (42%) | 20 (28%) | 0.024 |
| T1b | 78 (26%) | 61 (27%) | 17 (24%) | 0.367 |
| T2a | 106 (35%) | 72 (31%) | 34 (48%) | 0.002 |
| Tumor stage | 0.011 | |||
| IA | 194 (65%) | 157 (69%) | 37 (52%) | |
| IB | 106 (35%) | 72 (31%) | 34 (48%) | |
| Lymphatic invasion | ||||
| Yes | 80 (27%) | 58 (25%) | 22 (31%) | 0.346 |
| No | 220 (73%) | 171 (75%) | 49 (69%) | |
| Vascular invasion | ||||
| Yes | 28 (9%) | 17 (7%) | 11 (15%) | 0.041 |
| No | 272 (91%) | 212 (93%) | 60 (85%) | |
| Surgical procedure | 0.721 | |||
| Lobectomy | 279 (93%) | 214 (93%) | 65 (91%) | |
| Bilobectomy | 8 (3%) | 6 (3%) | 2 (3%) | |
| Sleeve Lobectomy | 11 (4%) | 7 (3%) | 4 (6%) | |
| Pneumonectomy | 2 (1%) | 2 (1%) | 0 (0%) |
| Criterion | All Cases | CRP ≤ 5 mg/L | CRP > 5 mg/L | p Value |
|---|---|---|---|---|
| n = 300 (100%) | n = 209 (70%) | n = 91 (30%) | ||
| Age (years; mean) | 66.0 (60.1–72.2) | 64.9 | 66.3 | 0.870 |
| Weight (kg; mean) | 75 (65–85) | 74.8 | 78.3 | 0.976 |
| BMI (kg/m2; mean) | 26.2 (23.5–28.6) | 26.0 | 27.1 | 0.979 |
| Gender | ||||
| Male | 187 (62%) | 124 (59%) | 63 (69%) | 0.104 |
| Female | 113 (38%) | 85 (41%) | 28 (31%) | |
| Smoking Status | ||||
| Yes | 199 (66%) | 137 (66%) | 62 (68%) | 0.664 |
| No | 101 (34%) | 72 (34%) | 29 (32%) | |
| COPD | ||||
| Yes | 126 (42%) | 84 (40%) | 42 (46%) | 0.336 |
| No | 174 (58%) | 125 (60%) | 49 (54%) | |
| PAD | ||||
| Yes | 25 (8%) | 18 (9%) | 7 (8%) | 0.791 |
| No | 275 (92%) | 191 (91%) | 84 (92%) | |
| CKD | ||||
| Yes | 17 (6%) | 11 (5%) | 6 (7%) | 0.647 |
| No | 283 (94%) | 198 (95%) | 85 (93%) | |
| CHD | ||||
| Yes | 41 (14%) | 18 (9%) | 23 (25%) | <0.0001 |
| No | 259 (86%) | 191 (91%) | 68 (75%) | |
| Alcohol abuse | ||||
| Yes | 136 (45%) | 91 (44%) | 45 (49%) | 0.345 |
| No | 164 (55%) | 118 (56%) | 46 (51%) | |
| Albumin (g/dL) | 0.422 | |||
| Albumin < 3.5 g/dL | 21 (7%) | 13 (6%) | 8 (9%) | |
| Albumin ≥ 3.5 g/dL | 279 (93%) | 196 (94%) | 83 (91%) | |
| Histology | ||||
| Adenocarcinoma | 191 (64%) | 140 (67%) | 51 (56%) | |
| Squamous cell | 95 (32%) | 57 (27%) | 38 (42%) | 0.013 |
| Adenosquamous | 2 (1%) | 1 (1%) | 1 (1%) | |
| Large cell | 2 (1%) | 2 (1%) | 0 (0%) | |
| Other | 10 (3%) | 9 (4%) | 1 (1%) | |
| Grading | ||||
| G1 | 55 (18%) | 44 (21%) | 11 (12%) | 0.172 |
| G2 | 131 (44%) | 87 (42%) | 44 (48%) | |
| G3 | 114 (38%) | 78 (37%) | 36 (40%) | |
| Tumor size | ||||
| T1a | 116 (39%) | 90 (43%) | 26 (29%) | 0.018 |
| T1b | 78 (26%) | 57 (27%) | 21 (23%) | 0.446 |
| T2a | 106 (35%) | 62 (30%) | 44 (48%) | 0.002 |
| Tumor stage | ||||
| IA | 194 (65%) | 147 (70%) | 47 (52%) | 0.002 |
| IB | 106 (35%) | 62 (30%) | 44 (48%) | |
| Lymphatic invasion | ||||
| Yes | 80 (27%) | 52 (25%) | 28 (31%) | 0.289 |
| No | 220 (73%) | 157 (75%) | 63 (69%) | |
| Vascular invasion | ||||
| Yes | 28 (9%) | 14 (7%) | 14 (15%) | 0.017 |
| No | 272 (91%) | 195 (93%) | 77 (85%) | |
| Surgical procedure | ||||
| Lobectomy | 279 (93%) | 195 (93%) | 84 (92%) | 0.756 |
| Bilobectomy | 8 (3%) | 4 (2%) | 4 (5%) | 0.220 |
| Sleeve Lobectomy | 11 (4%) | 8 (4%) | 3 (3%) | 0.822 |
| Pneumonectomy | 2 (1%) | 2 (1%) | 0 (0%) | 0.349 |
| Description | GPS |
|---|---|
| CRP ≤ 10 mg/L and albumin ≥ 3.5 g/dL | 0 |
| CRP ≤ 10 mg/L and albumin < 3.5 g/dL | 1 |
| CRP > 10 mg/L and albumin ≥ 3.5 g/dL | 1 |
| CRP > 10 mg/L and albumin < 3.5 g/dL | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindenmann, J.; Fink-Neuboeck, N.; Taucher, V.; Pichler, M.; Posch, F.; Brcic, L.; Smolle, E.; Koter, S.; Smolle, J.; Smolle-Juettner, F.M. Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score. Cancers 2020, 12, 152. https://doi.org/10.3390/cancers12010152
Lindenmann J, Fink-Neuboeck N, Taucher V, Pichler M, Posch F, Brcic L, Smolle E, Koter S, Smolle J, Smolle-Juettner FM. Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score. Cancers. 2020; 12(1):152. https://doi.org/10.3390/cancers12010152
Chicago/Turabian StyleLindenmann, Joerg, Nicole Fink-Neuboeck, Valentin Taucher, Martin Pichler, Florian Posch, Luka Brcic, Elisabeth Smolle, Stephan Koter, Josef Smolle, and Freyja Maria Smolle-Juettner. 2020. "Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score" Cancers 12, no. 1: 152. https://doi.org/10.3390/cancers12010152
APA StyleLindenmann, J., Fink-Neuboeck, N., Taucher, V., Pichler, M., Posch, F., Brcic, L., Smolle, E., Koter, S., Smolle, J., & Smolle-Juettner, F. M. (2020). Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score. Cancers, 12(1), 152. https://doi.org/10.3390/cancers12010152








