The Regulation of the Metastatic Cascade by Physical Activity: A Narrative Review
Abstract
1. Introduction
2. Results
2.1. Whole Metastatic Development
2.2. Epithelial-Mesenchymal Transition and Extracellular Matrix Degradation
2.3. Intravasation
2.4. Survival in the Circulation
2.5. Extravasation
2.6. Seeding and Colonization
3. Discussion
3.1. Moderate Versus High Intensity Physical Activity
3.2. Acute Versus Chronic Physical Activity
3.3. Forced versus Voluntary Physical Activity
3.4. Lactate Levels
3.5. Limitations
3.6. Perspectives
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef]
- Liu, Z.J.; Semenza, G.L.; Zhang, H.F. Hypoxia-inducible factor 1 and breast cancer metastasis. J. Zhejiang Univ. Sci. B 2015, 16, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Country Profile; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Friedenreich, C.M.; Neilson, H.K.; Lynch, B.M. State of the epidemiological evidence on physical activity and cancer prevention. Eur. J. Cancer 2010, 46, 2593–2604. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Leitzmann, M.F. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014, 25, 1293–1311. [Google Scholar] [CrossRef] [PubMed]
- Jadeski, L.; Hoffman-Goetz, L. Exercise and in vivo natural cytotoxicity against tumour cells of varying metastatic capacity. Clin. Exp. Metastasis 1996, 14, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.; Gil da Costa, R.M.; Faustino-Rocha, A.I.; Ferreira, R.; Lopes, C.; Oliveira, P.A.; Colaco, B. Effects of exercise training on breast cancer metastasis in a rat model. Int. J. Exp. Pathol. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Assi, M.; Kenawi, M.; Ropars, M.; Rebillard, A. Interleukin-6, C/EBP-beta and PPAR-gamma expression correlates with intramuscular liposarcoma growth in mice: The impact of voluntary physical activity levels. Biochem. Biophys. Res. Commun. 2017, 490, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.V.; Lam, C.; Dalmia, S.; Gao, P.; Young, J.; Middleton, K.; Liu, C.; Xu, H.; You, L. Mechanical regulation of breast cancer migration and apoptosis via direct and indirect osteocyte signaling. J. Cell Biochem. 2018, 119, 5665–5675. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.V.; Xu, L.; Mei, X.; Middleton, K.; You, L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J. Cell Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Fu, A.; Luo, K.Q. High Shear Stresses under Exercise Condition Destroy Circulating Tumor Cells in a Microfluidic System. Sci. Rep. 2017, 7, 39975. [Google Scholar] [CrossRef] [PubMed]
- Faustino-Rocha, A.I.; Silva, A.; Gabriel, J.; Gil da Costa, R.M.; Moutinho, M.; Oliveira, P.A.; Gama, A.; Ferreira, R.; Ginja, M. Long-term exercise training as a modulator of mammary cancer vascularization. Biomed. Pharmacother. 2016, 81, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; May, K.M.; Arumugam, Y. Exercise training and mouse mammary tumour metastasis. Anticancer Res. 1994, 14, 2627–2631. [Google Scholar]
- Hoffmann-Goetz, L.; MacNeil, B.; Arumugam, Y. Tissue distribution of radiolabelled tumor cells in wheel exercised and sedentary mice. Int. J. Sports Med. 1994, 15, 249–253. [Google Scholar] [CrossRef]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Moon, E.J.; Schroeder, T.; Herndon, J.E., 2nd; et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef]
- Jones, L.W.; Antonelli, J.; Masko, E.M.; Broadwater, G.; Lascola, C.D.; Fels, D.; Dewhirst, M.W.; Dyck, J.R.; Nagendran, J.; Flores, C.T.; et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J. Appl. Physiol. 2012, 113, 263–272. [Google Scholar] [CrossRef]
- MacNeil, B.; Hoffman-Goetz, L. Exercise training and tumour metastasis in mice: Influence of time of exercise onset. Anticancer Res. 1993, 13, 2085–2088. [Google Scholar]
- MacNeil, B.R.I.; Hoffman-Goetz, L.A.U. Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Med. Sci. Sports Exerc. 1993, 25, 922–928. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, B.; Hoffman-Goetz, L. Chronic exercise enhances in vivo and in vitro cytotoxic mechanisms of natural immunity in mice. J. Appl. Physiol. 1993, 74, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Davis, J.M.; Brown, A.S.; Carmichael, M.D.; Mayer, E.P.; Ghaffar, A. Effects of moderate exercise and oat beta-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J. Appl. Physiol. 2004, 97, 955–959. [Google Scholar] [CrossRef][Green Version]
- Smeda, M.P.K.; Proniewski, B.; Zakrzewska, A.; Kaczor, D.; Stojak, M.; Buczek, E.; Nieckarz, Z.; Zoladz, J.A.; Wietrzyk, J.; Chlopicki, S. Breast cancer pulmonary metastasis is increased in mice undertaking spontaneous physical training in the running wheel; a call for revising beneficial effects of exercise on cancer progression. Am. J. Cancer Res. 2017, 7, 1926–1936. [Google Scholar] [PubMed]
- Tsai, M.S.; Kuo, M.L.; Chang, C.C.; Wu, Y.T. The effects of exercise training on levels of vascular endothelial growth factor in tumor-bearing mice. Cancer Biomark. 2013, 13, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Balke, J.E.; Andras, I.E.; Park, M.; Toborek, M. Exercise modulates redox-sensitive small GTPase activity in the brain microvasculature in a model of brain metastasis formation. PLoS ONE 2014, 9, e97033. [Google Scholar] [CrossRef]
- Wolff, G.; Davidson, S.J.; Wrobel, J.K.; Toborek, M. Exercise maintains blood-brain barrier integrity during early stages of brain metastasis formation. Biochem. Biophys. Res. Commun. 2015, 463, 811–817. [Google Scholar] [CrossRef]
- Yan, L.; Demars, L.C. Effects of non-motorized voluntary running on experimental and spontaneous metastasis in mice. Anticancer Res. 2011, 31, 3337–3344. [Google Scholar]
- Zhang, Q.B.; Zhang, B.H.; Zhang, K.Z.; Meng, X.T.; Jia, Q.A.; Zhang, Q.B.; Bu, Y.; Zhu, X.D.; Ma, D.N.; Ye, B.G.; et al. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: With reference to nervous system. Oncogene 2016, 35, 4122–4131. [Google Scholar] [CrossRef]
- Jones, L.W.; Fels, D.R.; West, M.; Allen, J.D.; Broadwater, G.; Barry, W.T.; Wilke, L.G.; Masko, E.; Douglas, P.S.; Dash, R.C.; et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev. Res. 2013, 6, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Chang, C.Y.; Chow, S.E.; Chen, Y.W.; Yang, C.M. Exercise modulates platelet-nasopharyngeal carcinoma cell aggregation and subsequent tissue factor and matrix metalloproteinase activities. J. Appl. Physiol. 2007, 103, 763–770. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L. Exercise, natural immunity, and tumor metastasis. Med. Sci. Sports Exerc. 1994, 26, 157–163. [Google Scholar] [CrossRef]
- Davis, J.M.; Kohut, M.L.; Jackson, D.A.; Colbert, L.H.; Mayer, E.P.; Ghaffar, A. Exercise effects on lung tumor metastases and in vitro alveolar macrophage antitumor cytotoxicity. Am. J. Physiol. 1998, 274, R1454–R1459. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Heilman, D.; Haorah, J.; Zelivyanskaya, M.; Persidsky, R.; Weber, G.A.; Shimokawa, H.; Kaibuchi, K.; Ikezu, T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood 2006, 107, 4770–4780. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Quail, D.F.; Zhang, X.; White, R.M.; Jones, L.W. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 2017, 17, 620–632. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Nindl, B.C. Recovery responses of testosterone, growth hormone, and IGF-1 after resistance exercise. J. Appl. Physiol. 2017, 122, 549–558. [Google Scholar] [CrossRef]
- Lei, T.; Ling, X. IGF-1 promotes the growth and metastasis of hepatocellular carcinoma via the inhibition of proteasome-mediated cathepsin B degradation. World J. Gastroenterol. 2015, 21, 10137–10149. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Giganti, M.G.; Tresoldi, I.; Sorge, R.; Melchiorri, G.; Triossi, T.; Masuelli, L.; Lido, P.; Albonici, L.; Foti, C.; Modesti, A.; et al. Physical exercise modulates the level of serum MMP-2 and MMP-9 in patients with breast cancer. Oncol. Lett. 2016, 12, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A.; Davis, J.M.; Mayer, E.P.; Ghaffar, A.; Pate, R.R. Effects of exercise on macrophage activation for antitumor cytotoxicity. J. Appl. Physiol. 1994, 76, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; Kirk, E.A.; Lee, S.X.; Ladiges, W.C. Exercise, physical activity and breast cancer: The role of tumor-associated macrophages. Exerc. Immunol. Rev. 2012, 18, 158–176. [Google Scholar]
- Koh, Y.; Park, J. Cell adhesion molecules and exercise. J. Inflamm. Res. 2018, 11, 297–306. [Google Scholar] [CrossRef]
- Brown, J.C.; Rhim, A.D.; Manning, S.L.; Brennan, L.; Mansour, A.I.; Rustgi, A.K.; Damjanov, N.; Troxel, A.B.; Rickels, M.R.; Ky, B.; et al. Effects of exercise on circulating tumor cells among patients with resected stage I-III colon cancer. PLoS ONE 2018, 13, e0204875. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Trans. Sci. 2015, 135, 355–380. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Mirjam Kruijsen-Jaarsma, D.R.; Marc, B.; Bierings Laurien, M.; Buffart, T.T. Effects of Exercise on Immune Function in Patients with Cancer: A Systematic Review. Exerc. Immunol. Rev. 2013, 19, 120–143. [Google Scholar]
- Ju, J.; Nolan, B.; Cheh, M.; Bose, M.; Lin, Y.; Wagner, G.C.; Yang, C.S. Voluntary exercise inhibits intestinal tumorigenesis in ApcMin/+ mice and azoxymethane/dextran sulfate sodium-treated mice. BMC Cancer 2008, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto, J.A.; Akbarali, R.; Bellotti, L.; Dranitsaris, G. A structured group exercise program for patients with metastatic cancer receiving chemotherapy and CTNNB1 (beta-catenin) as a biomarker of exercise efficacy. Cancer Manag. Res. 2017, 9, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Meyerhardt, J.A.; Shima, K.; Nosho, K.; Chan, A.T.; Giovannucci, E.; Fuchs, C.S.; Ogino, S. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA 2011, 305, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- San-Millan, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Kurgan, N.; Tsakiridis, E.; Kouvelioti, R.; Moore, J.; Klentrou, P.; Tsiani, E. Inhibition of Human Lung Cancer Cell Proliferation and Survival by Post-Exercise Serum Is Associated with the Inhibition of Akt, mTOR, p70 S6K, and Erk1/2. Cancers 2017, 9, 46. [Google Scholar] [CrossRef]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
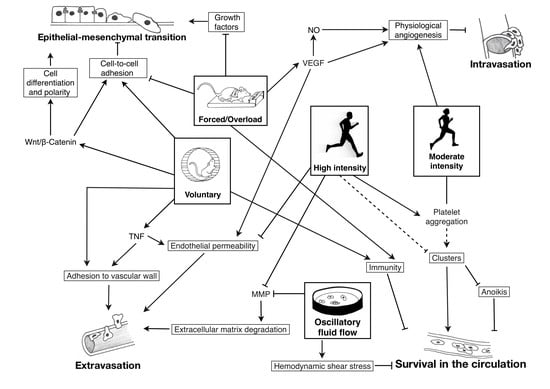
 : inhibition; plain lines ―: demonstrated; striped lines - - -: hypothetical.
: inhibition; plain lines ―: demonstrated; striped lines - - -: hypothetical.
 : inhibition; plain lines ―: demonstrated; striped lines - - -: hypothetical.
: inhibition; plain lines ―: demonstrated; striped lines - - -: hypothetical.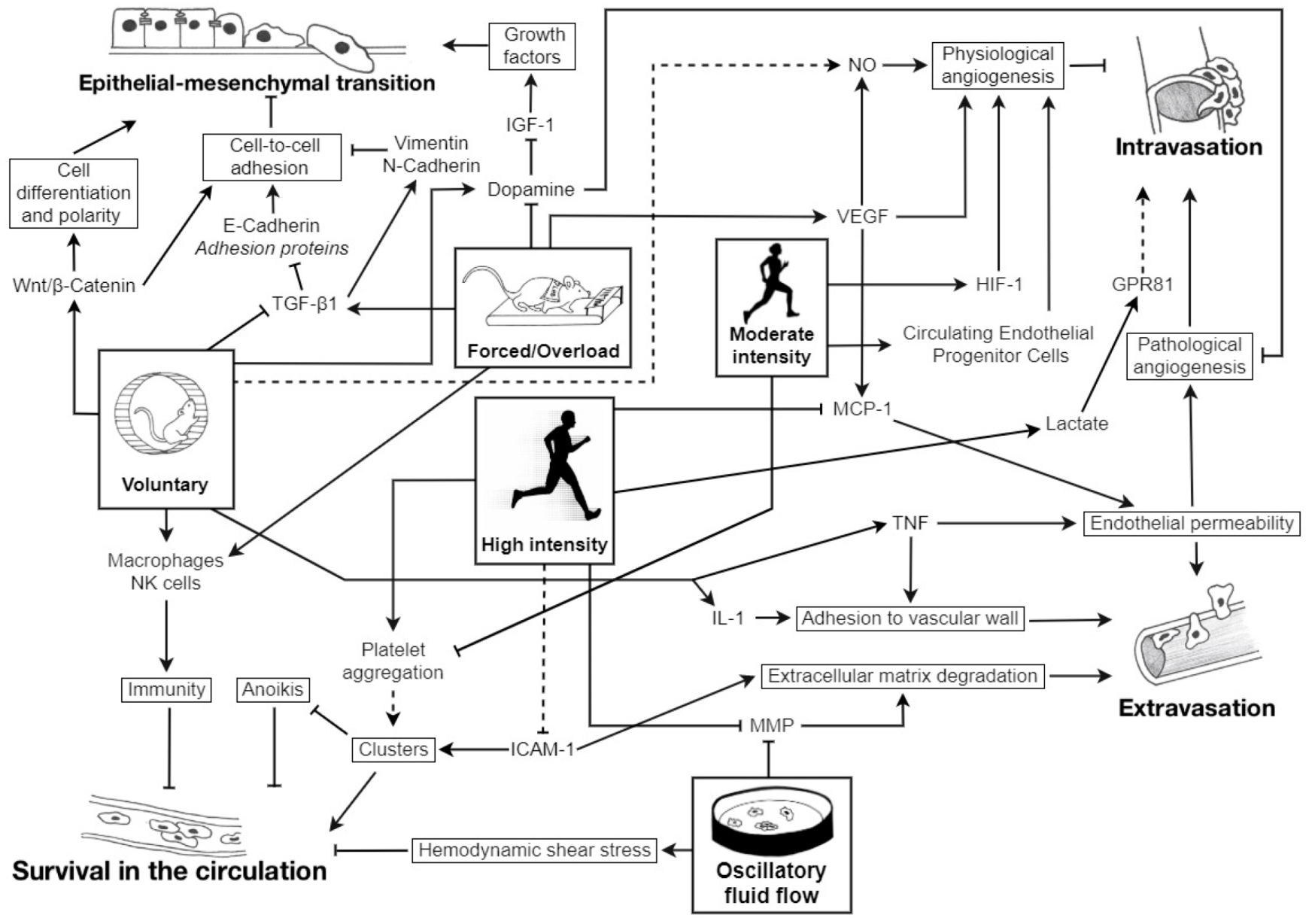
| Reference | Model | Tumor Type | Exercise Intervention | Measured Effects | Main Results (vs. Sedentary) | Conclusions |
|---|---|---|---|---|---|---|
| In vitro | ||||||
| Ma et al. (2018) [14] | In vitro | Breast cancer cells (non-metastatic and highly metastatic) | In vitro oscillatory fluid flow 1 Pa (mimicking mechanistic loading during mild exercise) | Osteocytes activity Osteoclasts activity IL-6 and ICAM-1 | Mimicked loading ↗ osteocytes activation Osteocytes directly ↗ cancer cell migration and ↘ apoptosis Osteocytes inhibit osteoclasts differentiation → ↘ migration and ↗ apoptosis Ostéocytes ↘ IL-6 expression → ↘ ICAM-1 → ↘ apoptosis | Opposite effects on breast cancer cell migration and apoptosis directly and indirectly mediated by osteocytes exposed to oscillatory fluid flow |
| Ma et al. (2018) [15] | In vitro | Bone metastatic breast cancer cells and osteocytes | Oscillatory Fluid Flow (2 h at 1 Hz and 1 peak shear stress at 1 Pa) | Endothelial permeability Adhesion on endothelial monolayer Cancer cells genes expression | ↘ Endothelial permeability ↘ Breast cancer cell adhesion onto endothelial monolayer (mediated by ICAM-1) Alteration of cancer cell genes expression via endothelial cells ↘ MMP-9 and FZD4 | Flow-stimulated osteocytes ↘ bone-metastatic potential of breast cancer cells by signaling through endothelial cells |
| Regmi et al. (2017) [16] | In vitro | Breast cancer (+ lung metastases) Ovarian lung cancer cells Leukemic cancer cells | Microfluidic circulatory system: Low shear stress of 15 dynes/cm2 (resting state) High shear stress of 60 dynes/cm2 (intensive ex) | CTC necrosis CTC apoptosis | ↗ CTC death in high shear stress 90% necrosis within 4h with high shear stress 10% apoptosis within 16–24h ↘ Viability of highly metastatic tumor cells in prolonged high shear stress treatment | Intensive exercise may be a good strategy for generating high shear stress that can destroy CTC and prevent cancer metastasis |
| Rodents | ||||||
| Alvarado et al. (2017) [12] | Female rats | Mammary cancer cells, ER and PR positive tumor cells | 35 weeks of moderate exercise training (treadmill running), 60 min/day, 5 days/week; post-tumor injection | Primary tumor and metastases development ER and PR immunoexpression | ↘ Mammary tumors numbers and masses No metastasis developed in exercising animals vs. 2 developed in the sedentary group ↗ ER and PR immuno-expression in neoplasms from sedentary and exercising groups | Long-term exercise training ↘ the risk of metastatic dissemination of breast cancer Anti-metastatic effects of exercise training are hormone-independent |
| Assi et al. (2017) [13] | Male mice | Liposarcoma | 6 weeks of spontaneous physical activity before tumor injection 8 weeks of voluntary wheel running post-tumor injection | Intramuscular tumor size FABP4 IL-6, C/EBP-β PPAR-γ Autophagy markers | Larger intramuscular tumors ↗ Expression of FAPB4, C/EBP-β, and PPAR-γ ↗ IL-6 levels in both active and inactive groups ↗ Expression of autophagy markers Beclin-1 and GABARAPL-1 | Physical activity ↗ liposarcoma development by ↗ autophagy in tumor mass |
| Faustino-Rocha et al. (2016) [17] | Female rats | Mammary cancer | 35 weeks of treadmill running (60 min/day, 5 days/week) | VEGF-A Vascularization Aggressiveness | ↗ VEGF-A expression ↗ Tumor vascularization ↘ Aggressiveness | Long-term exercise training: ↗ Tumor vascularization ↘ Tumor multiplicity and aggressiveness |
| Hoffman-Goetz et al. (1994) [18] | Female mice | Mammary tumor line 66 (in vivo) YAC-1 (in vitro) | 8 weeks before tumor inoculation: forced treadmill (T), voluntary wheel running (W) or sedentary (S) 3 weeks post-inoculation: continuation (TT/WW), cessation (TS/WS), start activity (ST/SW) or maintenance sedentary (SS) | NK cell activity LAK Pulmonary tumor density | Exercise before tumor injection: ↘ Basal NK activity (WS, TS) ↗ LAK activity (WS, TS) ↘ Pulmonary tumor density Exercise continued after tumor injection: ↘ LAK activity (TT, WW) ↗ Pulmonary tumor density (TT, WW) | ↗ LAK activity with endurance training or physical activity before tumor injection ↗ Natural immunity with exercise training but no significant difference in tumor burden and spread Exercise-induced changes depend on the tumor sensitivity to NK and LAK cells |
| Hoffmann-Goetz et al. (1994) [19] | Male mice | CIRAS 1 tumor cells | 9 weeks of voluntary wheel running before tumor cells injection | Tumor cell lung residency Tumor cell radioactivity | ↘ Lung tumor cell residency in exercise-trained animals at 5 min post-injection, up to 30 min ↘ Tumor cell radioactivity in liver, spleen, kidney of wheel running mice 30 min and 3 h post-injection | Exercise training ↘ tumor cell adherence to the vascular wall |
| Jadeski et al. (1996) [11] | Mice |
|
|
|
|
|
| Jones et al. (2010) [20] | Female mice | Human mammary adenocarcinoma |
|
|
| Aerobic exercise ↗ intratumoral vascularization → normalization of tumor microenvironment → ↘ rate of metastasis and ↗ cancer therapy efficiency |
| Jones et al. (2012) [21] | Male mice | Murine prostate cancer |
|
|
| Exercise-induced stabilization of HIF-1α upregulates VEGF expression. This led to physiological tumor vascularization with a shift toward suppressed metastasis. |
| MacNeil et al. (1993) [22] | Mice | CIRAS 1 tumor cells with pulmonary metastases |
|
|
| Exercise training before, but not after tumor inoculation ↘ number of lung metastases |
| MacNeil et al. (1993) [23] | Male mice and in vitro |
|
|
|
|
|
| MacNeil et al. (1993) [24] | Male mice |
|
|
|
| Exercise training ↗ natural immunity and NK cell cytotoxicity against tumor cells and ↘ pulmonary tumor retention in wheel and treadmill running mice |
| Murphy et al. (2004) [25] | Male mice | B16 melanoma cells |
|
|
| Short-term moderate-intensity exercise training ↘ metastatic spread by ↗ macrophages function |
| Smeda et al. (2017) [26] | Female mice | Orthotopic breast cancer cells |
|
|
|
|
| Tsai et al. (2013) [27] | Male mice | Lewis lung carcinoma |
|
|
| No effect of moderate exercise on tumor growth despite higher plasma VEGF levels |
| Wolff et al. (2014) [28] | Male mice | Lewis lung carcinoma |
|
|
| Exercise can protect microvessels from blood-brain barrier instability by decreasing Rho activation |
| Wolff et al. (2015) [29] | Male mice | Murine Lewis lung carcinoma |
|
|
|
|
| Yan et al. (2011) [30] | Male mice | Lewis lung carcinoma |
|
|
|
|
| Zhang et al. (2016) [31] | Mice and in vitro | Liver cancer transplantation with lung metastasis |
|
|
| Divergent activation of dopamine system may explain the opposite effects on tumor growth and metastasis between moderate and overload swimming |
| Human | ||||||
| Jones et al. (2013) [32] | Women | Breast adenocarcinoma |
|
|
| Aerobic exercise training can modulate tumor progression and metastasis:
|
| Wang et al. (2007) [33] | Men | Nasopharyngeal carcinoma (NPC) |
|
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Doorslaer de ten Ryen, S.; Deldicque, L. The Regulation of the Metastatic Cascade by Physical Activity: A Narrative Review. Cancers 2020, 12, 153. https://doi.org/10.3390/cancers12010153
van Doorslaer de ten Ryen S, Deldicque L. The Regulation of the Metastatic Cascade by Physical Activity: A Narrative Review. Cancers. 2020; 12(1):153. https://doi.org/10.3390/cancers12010153
Chicago/Turabian Stylevan Doorslaer de ten Ryen, Sophie, and Louise Deldicque. 2020. "The Regulation of the Metastatic Cascade by Physical Activity: A Narrative Review" Cancers 12, no. 1: 153. https://doi.org/10.3390/cancers12010153
APA Stylevan Doorslaer de ten Ryen, S., & Deldicque, L. (2020). The Regulation of the Metastatic Cascade by Physical Activity: A Narrative Review. Cancers, 12(1), 153. https://doi.org/10.3390/cancers12010153