Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score
Abstract
1. Introduction
2. Results
2.1. Analysis at Baseline
2.2. Prospective Analysis—Outcome
2.3. Predictors of Outcome
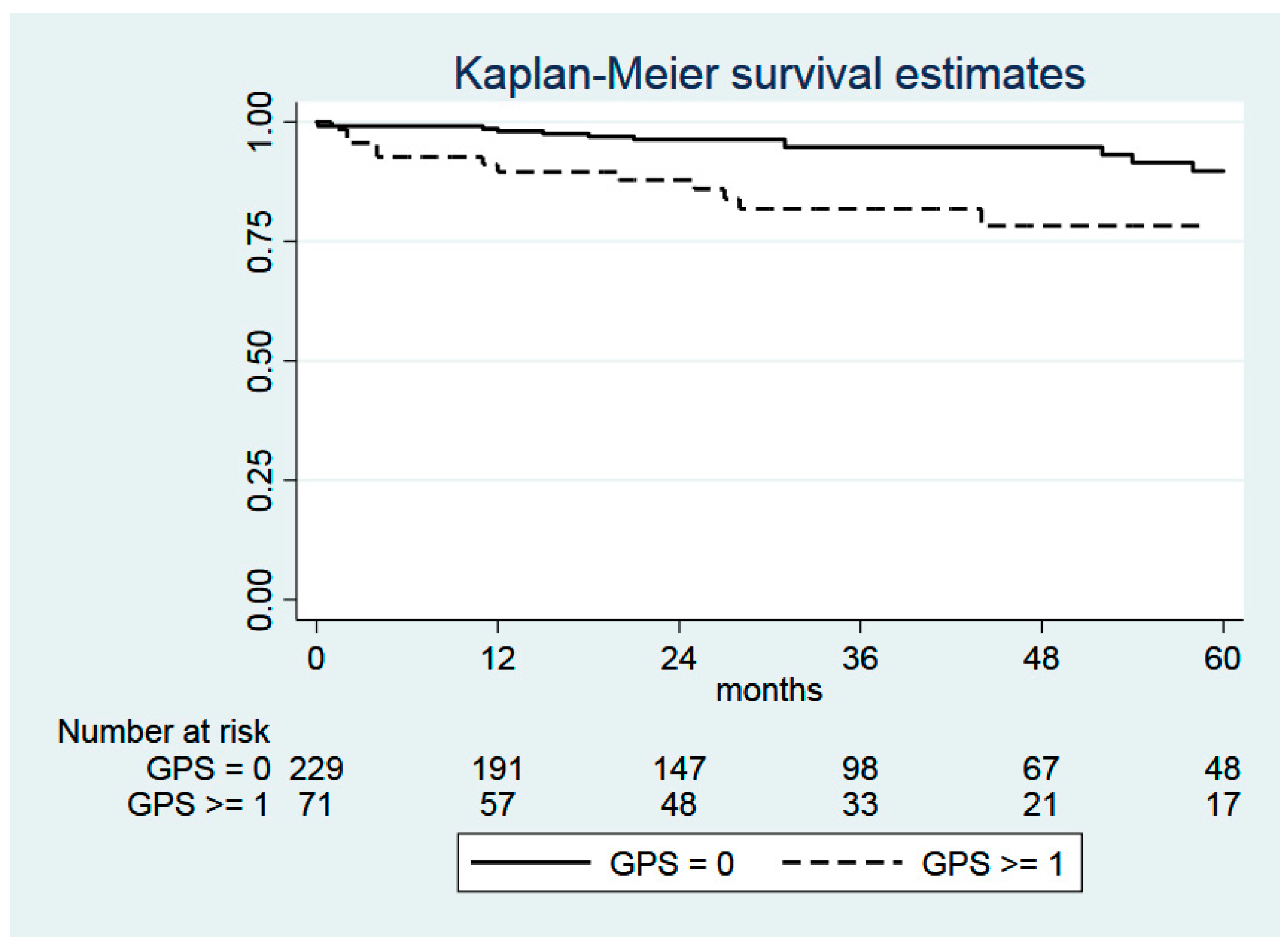
2.4. Glasgow Prognostic Score (GPS)
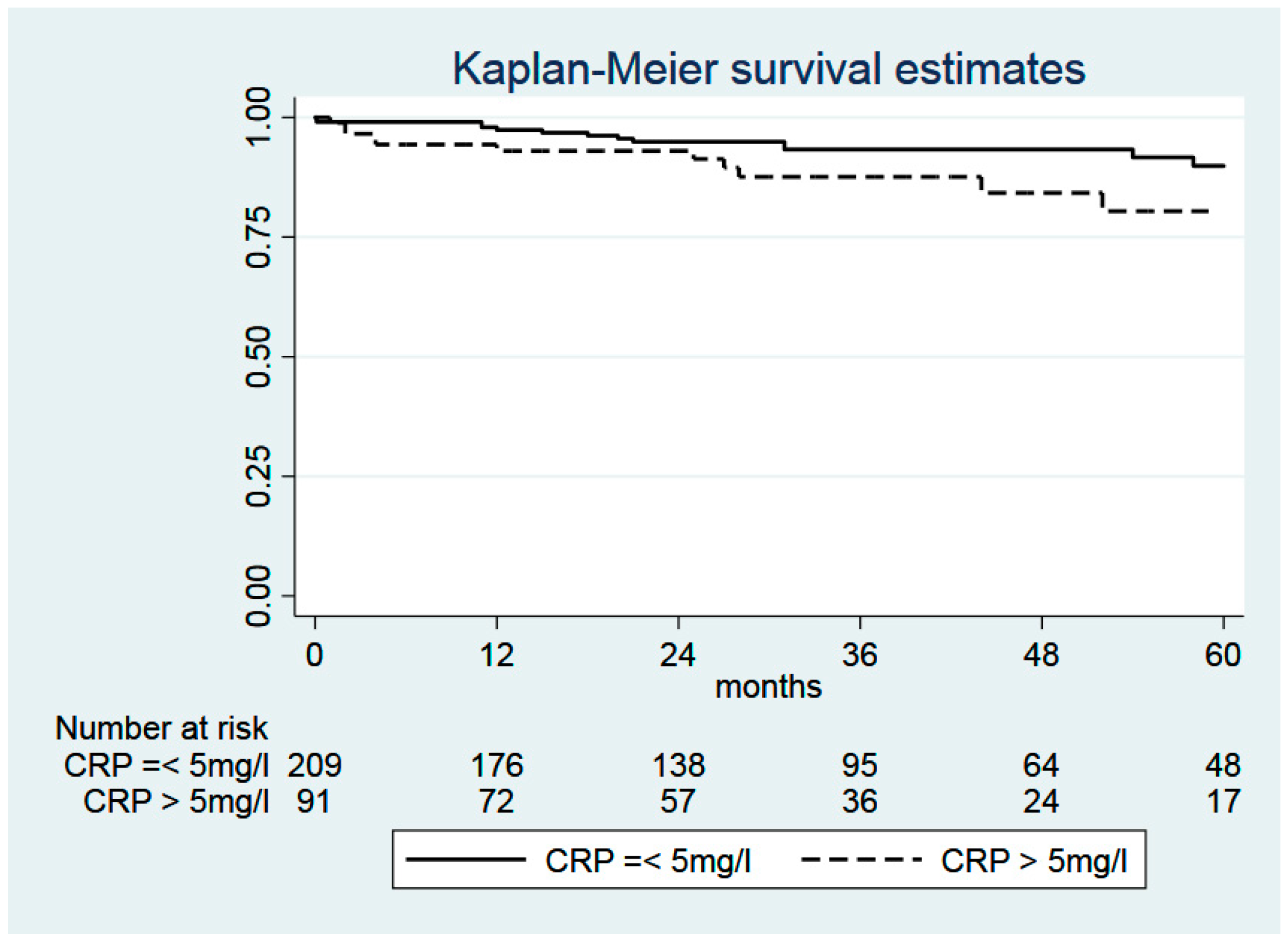
2.5. C-Reactive Protein (CRP)
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar] [PubMed]
- Goya, T.; Asamura, H.; Yoshimura, H.; Kato, H.; Shimokata, K.; Tsuchiya, R.; Sohara, Y.; Miya, T.; Miyaoka, E. Japanese Joint Committee of Lung Cancer Registry. Prognosis of 6644 resected non-small cell lung cancers in Japan: A Japanese lung cancer registry study. Lung Cancer 2005, 50, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, R.; Tsukada, H.; Nakazato, Y.; Takei, H.; Furuyashiki, G.; Koshi-ishi, Y.; Goya, T. Early recurrence after surgical resection in patients with pathological stage I non-small cell lung cancer. Thorac. Cardiovasc. Surg. 2009, 57, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Lewis, S.Z.; Diekemper, R.; Addrizzo-Harris, D.; Alberts, W.M. Executive Summary: Diagnosis and Management of Lungcancer, 3rd ed.; American College of ChestPhysicians evidence-based clinical practice guidelines. Chest 2013, 143, 7S–37S. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, S.; Ma, S.; Zhang, S. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: A meta-analysis. Springerplus 2016, 5, 439. [Google Scholar] [CrossRef]
- Jiang, A.G.; Chen, H.L.; Lu, H.Y. The relationship between Glasgow Prognostic Score and serum tumor markers in patients with advanced non-small cell lung cancer. BMC Cancer 2015, 15, 386. [Google Scholar] [CrossRef][Green Version]
- Lindenmann, J.; Fink-Neuboeck, N.; Koesslbacher, M.; Pichler, M.; Stojakovic, T.; Roller, R.E.; Maier, A.; Anegg, U.; Smolle, J.; Smolle-Juettner, F.M. The influence of elevated levels of C-reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment. J. Surg. Oncol. 2014, 110, 645–650. [Google Scholar] [CrossRef]
- Lindenmann, J.; Fink-Neuboeck, N.; Avian, A.; Pichler, M.; Habitzruther, M.; Maier, A.; Smolle-Juettner, F.M. Preoperative Glasgow Prognostic Score as additional independent prognostic parameter for patients with esophageal cancer after curative esophagectomy. Eur. J. Surg. Oncol. 2017, 43, 445–453. [Google Scholar] [CrossRef]
- Wang, C.S.; Sun, C.F. C-reactive Protein and Malignancy: Clinico-pathological Association and Therapeutic Implication. Chang. Gung Med. J. 2009, 32, 471–482. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Mantovani, A. Cancer: Inflaming metastasis. Nature 2009, 457, 36–37. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Jiang, A.G.; Chen, H.L.; Lu, H.Y. Comparison of Glasgow prognostic score and prognostic index in patients with advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 563–568. [Google Scholar] [CrossRef]
- Fan, H.; Shao, Z.Y.; Xiao, Y.Y.; Xie, Z.H.; Chen, W.; Xie, H.; Qin, G.Y.; Zhao, N.Q. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1285–1297. [Google Scholar]
- Leung, E.Y.; Scott, H.R.; McMillan, D.C. Clinical utility of the pretreatment glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J. Thorac. Oncol. 2012, 7, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yamasaki, N.; Tsuchiya, T.; Matsumoto, K.; Kunizaki, M.; Taniguchi, D.; Nagayasu, T. Inflammation-based scoring is a useful prognostic predictor of pulmonary resection for elderly patients with clinical stage I non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2015, 47, e140–e145. [Google Scholar] [CrossRef] [PubMed]
- Yotsukura, M.; Ohtsuka, T.; Kaseda, K.; Kamiyama, I.; Hayashi, Y.; Asamura, H. Value of the Glasgow Prognostic Score as a Prognostic Factor in Resectable Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Cho, B.C.; Bae, M.K.; Lee, C.Y.; Park, I.K.; Kim, D.J.; Ahn, S.V.; Chung, K.Y. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer 2009, 63, 106–110. [Google Scholar] [CrossRef]
- Igai, H.; Matsuura, N.; Tarumi, S.; Chang, S.S.; Misaki, N.; Go, T.; Ishikawa, S.; Yokomise, H. Clinicopathological study of p-T1aN0M0 non-small-cell lung cancer, as defined in the seventh edition of the TNM classification of malignant tumors. Eur. J. Cardiothorac. Surg. 2011, 39, 963–967. [Google Scholar] [CrossRef]
- Kudo, Y.; Saji, H.; Shimada, Y.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; Kajiwara, N.; Ohira, T.; Ikeda, N. Proposal on incorporating blood vessel invasion into the T classification parts as a practical staging system for stage I non-small cell lung cancer. Lung Cancer 2013, 81, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Y.; Lu, J.; Luo, Q.; Wu, C.; Liao, M.; Zheng, Y.; Ai, X.; Gu, L.; Lu, S. Analysis of the T descriptors and other prognosis factors in pathologic stage I non-small cell lung cancer in China. J. Thorac. Oncol. 2009, 4, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, G.; Galeone, C.; Gisabella, M.; Duranti, L.; Taverna, F.; Suatoni, P.; Morelli, D.; Pastorino, U. Baseline C-reactive protein level predicts survival of early-stage lung cancer: Evidence from a systematic review and meta-analysis. Tumori J. 2016, 102, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Shiner, R.J.; Seckl, M.J.; Stebbing, J.; Sharma, R.; Mauri, F.A. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br. J. Cancer 2014, 110, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Lerut, T.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- UICC International Union against Cancer. Lung and pleural tumours. In TNM Classification of Malignant Tumours, 7th ed.; Sobin, L.H., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 136–146. [Google Scholar]
- Crinò, L.; Weder, W.; van Meerbeeck, J.; Felip, E.; ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small cell lung cancer: ESMO Clinical PracticeGuidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v103–v115. [Google Scholar]
| Criterion | Value |
|---|---|
| Number of patients | 300 |
| Age | 65.4 ± 10.0 (20–87) |
| Male/Female | 187 (62.3%)/113 (37.7%) |
| BMI | 26.3 ± 4.3 (15.7–9.5) |
| Death | 73 (24.3%) |
| Death due to tumor | 38 (12.7%) |
| Death not tumor related | 35 (11.7%) |
| Tumor recurrence | 59 (19.7%) |
| Postoperative tumor size | |
| T1a | 116 (38.7%) |
| T1b | 78 (26.0%) |
| T2 | 106 (35.3%) |
| Albumin (g/dL) | 4.29 ± 0.49 (2.3–5.4) |
| CRP (mg/L) | 7.88 ± 15.80 (0.5–135.6) |
| GPS | |
| 0 | 229 (76.3%) |
| 1 | 68 (22.7%) |
| 2 | 3 (1.0%) |
| ASA | |
| 1 | 7 (2.3%) |
| 2 | 101 (33.7%) |
| 3 | 173 (57.7%) |
| 4 | 19 (6.3%) |
| Follow-up time (months) | 38.1 ± 28.3 (0–123) |
| Overall survival | |
| 1 year | 94.2% ± 1.4% |
| 3 year | 80.8% ± 2.7% |
| 5 year | 72.5% ± 3.5% |
| 10 year | 29.0% ± 9.2% |
| Criterion | All Cases | GPS 0 | GPS ≥ 1 | p Value |
|---|---|---|---|---|
| n = 300 (100%) | n = 229 (76%) | n = 71 (24%) | ||
| Age (years; mean) | 66.0 (60.1–72.2) | 65.3 | 68.3 | 0.243 |
| Weight (kg; mean) | 75 (65–85) | 75 | 75 | 0.995 |
| BMI (kg/m2; mean) | 26.2 (23.5–28.6) | 26.3 | 26.1 | 0.865 |
| Gender | ||||
| Male | 187 (62%) | 139 (61%) | 48 (68%) | 0.294 |
| Female | 113 (38%) | 90 (39%) | 23 (32%) | |
| Smoking Status | ||||
| Yes | 199 (66%) | 149 (65%) | 50 (70%) | 0.404 |
| No | 101 (34%) | 80 (35%) | 21 (30%) | |
| COPD | ||||
| Yes | 126 (42%) | 95 (41%) | 31 (44%) | 0.745 |
| No | 174 (58%) | 134 (59%) | 40 (56%) | |
| PAD | ||||
| Yes | 25 (8%) | 19 (8%) | 6 (8%) | 0.967 |
| No | 275 (92%) | 210 (92%) | 65 (92%) | |
| CKD | ||||
| Yes | 17 (6%) | 12 (5%) | 5 (7%) | 0.566 |
| No | 283 (94%) | 217 (95%) | 66 (93%) | |
| CHD | ||||
| Yes | 41 (14%) | 24 (10%) | 17 (24%) | 0.004 |
| No | 259 (86%) | 205 (90%) | 54 (76%) | |
| Alcohol abuse | ||||
| Yes | 136 (45%) | 96 (42%) | 40 (56%) | 0.033 |
| No | 164 (55%) | 133 (58%) | 31 (44%) | |
| Albumin (g/dL) | <0.0001 | |||
| Albumin < 3.5 g/dL | 21 (7%) | 0 (0%) | 21 (30%) | |
| Albumin ≥ 3.5 g/dL | 279 (93%) | 229 (100%) | 50 (70%) | |
| CRP (mg/L) | <0.0001 | |||
| CRP ≤ 10 mg/L | 247 (82%) | 229 (100%) | 18 (25%) | |
| CRP > 10 mg/L | 53 (18%) | 0 (0%) | 53 (75%) | |
| Histology | 0.101 | |||
| Adenocarcinoma | 191 (64%) | 154 (67%) | 37 (52%) | |
| Squamous cell | 95 (32%) | 64 (28%) | 31 (44%) | |
| Adenosquamous | 2 (1%) | 2 (1%) | 0 (0%) | |
| Large cell | 2 (1%) | 2 (1%) | 0 (0%) | |
| Other | 10 (3%) | 7 (3%) | 3 (4%) | |
| Grading | ||||
| G1 | 55 (18%) | 45 (19%) | 10 (14%) | 0.507 |
| G2 | 131 (44%) | 100 (44%) | 31 (44%) | |
| G3 | 114 (38%) | 84 (37%) | 30 (42%) | |
| Tumor size | ||||
| T1a | 116 (39%) | 96 (42%) | 20 (28%) | 0.024 |
| T1b | 78 (26%) | 61 (27%) | 17 (24%) | 0.367 |
| T2a | 106 (35%) | 72 (31%) | 34 (48%) | 0.002 |
| Tumor stage | 0.011 | |||
| IA | 194 (65%) | 157 (69%) | 37 (52%) | |
| IB | 106 (35%) | 72 (31%) | 34 (48%) | |
| Lymphatic invasion | ||||
| Yes | 80 (27%) | 58 (25%) | 22 (31%) | 0.346 |
| No | 220 (73%) | 171 (75%) | 49 (69%) | |
| Vascular invasion | ||||
| Yes | 28 (9%) | 17 (7%) | 11 (15%) | 0.041 |
| No | 272 (91%) | 212 (93%) | 60 (85%) | |
| Surgical procedure | 0.721 | |||
| Lobectomy | 279 (93%) | 214 (93%) | 65 (91%) | |
| Bilobectomy | 8 (3%) | 6 (3%) | 2 (3%) | |
| Sleeve Lobectomy | 11 (4%) | 7 (3%) | 4 (6%) | |
| Pneumonectomy | 2 (1%) | 2 (1%) | 0 (0%) |
| Criterion | All Cases | CRP ≤ 5 mg/L | CRP > 5 mg/L | p Value |
|---|---|---|---|---|
| n = 300 (100%) | n = 209 (70%) | n = 91 (30%) | ||
| Age (years; mean) | 66.0 (60.1–72.2) | 64.9 | 66.3 | 0.870 |
| Weight (kg; mean) | 75 (65–85) | 74.8 | 78.3 | 0.976 |
| BMI (kg/m2; mean) | 26.2 (23.5–28.6) | 26.0 | 27.1 | 0.979 |
| Gender | ||||
| Male | 187 (62%) | 124 (59%) | 63 (69%) | 0.104 |
| Female | 113 (38%) | 85 (41%) | 28 (31%) | |
| Smoking Status | ||||
| Yes | 199 (66%) | 137 (66%) | 62 (68%) | 0.664 |
| No | 101 (34%) | 72 (34%) | 29 (32%) | |
| COPD | ||||
| Yes | 126 (42%) | 84 (40%) | 42 (46%) | 0.336 |
| No | 174 (58%) | 125 (60%) | 49 (54%) | |
| PAD | ||||
| Yes | 25 (8%) | 18 (9%) | 7 (8%) | 0.791 |
| No | 275 (92%) | 191 (91%) | 84 (92%) | |
| CKD | ||||
| Yes | 17 (6%) | 11 (5%) | 6 (7%) | 0.647 |
| No | 283 (94%) | 198 (95%) | 85 (93%) | |
| CHD | ||||
| Yes | 41 (14%) | 18 (9%) | 23 (25%) | <0.0001 |
| No | 259 (86%) | 191 (91%) | 68 (75%) | |
| Alcohol abuse | ||||
| Yes | 136 (45%) | 91 (44%) | 45 (49%) | 0.345 |
| No | 164 (55%) | 118 (56%) | 46 (51%) | |
| Albumin (g/dL) | 0.422 | |||
| Albumin < 3.5 g/dL | 21 (7%) | 13 (6%) | 8 (9%) | |
| Albumin ≥ 3.5 g/dL | 279 (93%) | 196 (94%) | 83 (91%) | |
| Histology | ||||
| Adenocarcinoma | 191 (64%) | 140 (67%) | 51 (56%) | |
| Squamous cell | 95 (32%) | 57 (27%) | 38 (42%) | 0.013 |
| Adenosquamous | 2 (1%) | 1 (1%) | 1 (1%) | |
| Large cell | 2 (1%) | 2 (1%) | 0 (0%) | |
| Other | 10 (3%) | 9 (4%) | 1 (1%) | |
| Grading | ||||
| G1 | 55 (18%) | 44 (21%) | 11 (12%) | 0.172 |
| G2 | 131 (44%) | 87 (42%) | 44 (48%) | |
| G3 | 114 (38%) | 78 (37%) | 36 (40%) | |
| Tumor size | ||||
| T1a | 116 (39%) | 90 (43%) | 26 (29%) | 0.018 |
| T1b | 78 (26%) | 57 (27%) | 21 (23%) | 0.446 |
| T2a | 106 (35%) | 62 (30%) | 44 (48%) | 0.002 |
| Tumor stage | ||||
| IA | 194 (65%) | 147 (70%) | 47 (52%) | 0.002 |
| IB | 106 (35%) | 62 (30%) | 44 (48%) | |
| Lymphatic invasion | ||||
| Yes | 80 (27%) | 52 (25%) | 28 (31%) | 0.289 |
| No | 220 (73%) | 157 (75%) | 63 (69%) | |
| Vascular invasion | ||||
| Yes | 28 (9%) | 14 (7%) | 14 (15%) | 0.017 |
| No | 272 (91%) | 195 (93%) | 77 (85%) | |
| Surgical procedure | ||||
| Lobectomy | 279 (93%) | 195 (93%) | 84 (92%) | 0.756 |
| Bilobectomy | 8 (3%) | 4 (2%) | 4 (5%) | 0.220 |
| Sleeve Lobectomy | 11 (4%) | 8 (4%) | 3 (3%) | 0.822 |
| Pneumonectomy | 2 (1%) | 2 (1%) | 0 (0%) | 0.349 |
| Description | GPS |
|---|---|
| CRP ≤ 10 mg/L and albumin ≥ 3.5 g/dL | 0 |
| CRP ≤ 10 mg/L and albumin < 3.5 g/dL | 1 |
| CRP > 10 mg/L and albumin ≥ 3.5 g/dL | 1 |
| CRP > 10 mg/L and albumin < 3.5 g/dL | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindenmann, J.; Fink-Neuboeck, N.; Taucher, V.; Pichler, M.; Posch, F.; Brcic, L.; Smolle, E.; Koter, S.; Smolle, J.; Smolle-Juettner, F.M. Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score. Cancers 2020, 12, 152. https://doi.org/10.3390/cancers12010152
Lindenmann J, Fink-Neuboeck N, Taucher V, Pichler M, Posch F, Brcic L, Smolle E, Koter S, Smolle J, Smolle-Juettner FM. Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score. Cancers. 2020; 12(1):152. https://doi.org/10.3390/cancers12010152
Chicago/Turabian StyleLindenmann, Joerg, Nicole Fink-Neuboeck, Valentin Taucher, Martin Pichler, Florian Posch, Luka Brcic, Elisabeth Smolle, Stephan Koter, Josef Smolle, and Freyja Maria Smolle-Juettner. 2020. "Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score" Cancers 12, no. 1: 152. https://doi.org/10.3390/cancers12010152
APA StyleLindenmann, J., Fink-Neuboeck, N., Taucher, V., Pichler, M., Posch, F., Brcic, L., Smolle, E., Koter, S., Smolle, J., & Smolle-Juettner, F. M. (2020). Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score. Cancers, 12(1), 152. https://doi.org/10.3390/cancers12010152








