Impact of Surgery on Long-Term Results of Hearing in Neurofibromatosis Type-2 Associated Vestibular Schwannomas
Abstract
1. Introduction
2. Results
2.1. Patients, Tumors, and Operation
2.2. Post-Operative Facial Function
2.3. Postoperative Deafness
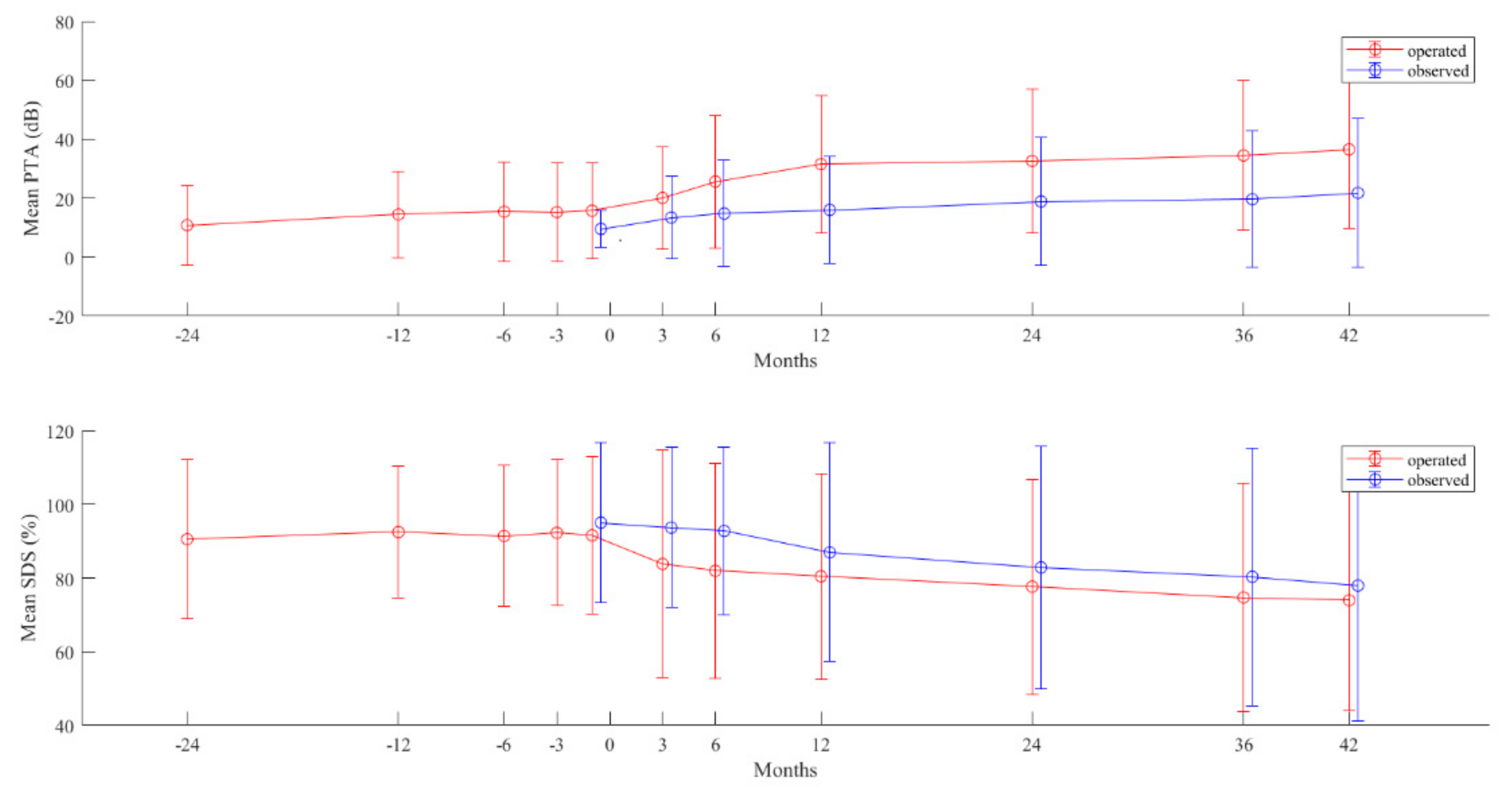
2.4. Postoperative Hearing
2.5. Postoperative Hearing in Long-Term
3. Discussion
4. Material and Methods
4.1. Patients and Clinical Characteristics
4.2. Hearing Evaluation
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, D.G.; Moran, A.; King, A.; Saeed, S.; Gurusinghe, N.; Ramsden, R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: Higher incidence than previously thought. Otol. Neurotol. 2005, 26, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.; Lalloo, F. Birth incidence and prevalence of tumour prone syndromes: Estimates from UK genetic family register service. Am. J. Med. Gen. 2009, 152, 327–332. [Google Scholar]
- Evans, D.G.; Huson, S.M.; Donnai, D.; Neary, W.; Blair, V.; Newton, V.; Harris, R. A clinical study of type 2 neurofibromatosis. Int. J. Med. 1992, 84, 603–618. [Google Scholar]
- Mautner, V.F.; Lindenau, M.; Baser, M.E.; Hazim, W.; Tatagiba, M.; Haase, W.; Samii, M.; Wais, R.; Pulst, S.M. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery 1996, 38, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.M.; Eldridge, R.; Kaiser-Kupfer, M.I.; Bouzas, E.A.; Pikus, A.; Patronas, N. Neurofibromatosis 2 (NF2): Clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am. J. Med. Genet. 1994, 52, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Gugel, I.; Mautner, V.F.; Kluwe, L.; Tatagiba, M.S.; Schuhmann, M.U. Cerebrovascular Insult as Presenting Symptom of Neurofibromatosis Type 2 in Children, Adolescents, and Young Adults. Front. Neurol. 2018, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, G.A.; Merel, P.; Lutchman, M.; Sanson, M.; Zucman, J.; Marineau, C.; Hoang-Xuan, K.; Demczuk, S.; Desmaze, C.; Plougastel, B.; et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 1993, 363, 515–521. [Google Scholar] [CrossRef]
- Trofatter, J.A.; MacCollin, M.M.; Rutter, J.L.; Murrell, J.R.; Duyao, M.P.; Parry, D.M.; Eldridge, R.; Kley, N.; Menon, A.G.; Pulaski, K.; et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 1993, 75, 826. [Google Scholar] [CrossRef]
- Halliday, D.; Emmanouil, B.; Pretorius, P.; MacKeith, S.; Painter, S.; Tomkins, H.; Evans, D.G.; Parry, A. Genetic Severity Score predicts clinical phenotype in NF2. J. Med. Genet. 2017, 54, 657–664. [Google Scholar] [CrossRef]
- Emmanouil, B.; Houston, R.; May, A.; Ramsden, J.D.; Hanemann, C.O.; Halliday, D.; Parry, A.; Mackeith, S. Progression of hearing loss in neurofibromatosis type 2 according to genetic severity. Laryngoscope 2018, 129, 974–980. [Google Scholar] [CrossRef]
- Samii, M.; Matthies, C.; Tatagiba, M. Management of vestibular schwannomas (acoustic neuromas): Auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery 1997, 40, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Matthies, C.; Samii, M. Management of vestibular schwannomas (acoustic neuromas): The value of neurophysiology for evaluation and prediction of auditory function in 420 cases. Neurosurgery 1997, 40, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.A.; Goddard, J.C.; Wilkinson, E.P.; Schwartz, M.S.; Slattery, W.H., 3rd; Fayad, J.N.; Brackmann, D.E. Hearing preservation with the middle cranial fossa approach for neurofibromatosis type 2. Otol. Neurotol. 2011, 32, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Goutagny, S.; Bah, A.; Bernardeschi, D.; Larroque, B.; Sterkers, O.; Kalamarides, M. Conservative management of bilateral vestibular schwannomas in neurofibromatosis type 2 patients: Hearing and tumor growth results. Neurosurgery 2013, 72, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.; Robertson, J.H. Hearing preservation in unilateral acoustic neuroma surgery. Ann. Otol. Rhinol. Laryngol. 1988, 97, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Slattery, W.H., 3rd; Fisher, L.M.; Hitselberger, W.; Friedman, R.A.; Brackmann, D.E. Hearing preservation surgery for neurofibromatosis Type 2-related vestibular schwannoma in pediatric patients. J. Neurosurg. 2007, 106, 255–260. [Google Scholar] [CrossRef]
- Brackmann, D.E.; Fayad, J.N.; Slattery, W.H., 3rd; Friedman, R.A.; Day, J.D.; Hitselberger, W.E.; Owens, R.M. Early proactive management of vestibular schwannomas in neurofibromatosis type 2. Neurosurgery 2001, 49, 274–280. [Google Scholar]
- Bernardeschi, D.; Peyre, M.; Collin, M.; Smail, M.; Sterkers, O.; Kalamarides, M. Internal Auditory Canal Decompression for Hearing Maintenance in Neurofibromatosis Type 2 Patients. Neurosurgery 2015, 79, 370–377. [Google Scholar] [CrossRef]
- Slattery, W.H.; Hoa, M.; Bonne, N.; Friedman, R.A.; Schwartz, M.S.; Fisher, L.M.; Brackmann, D.E. Middle fossa decompression for hearing preservation: A review of institutional results and indications. Otol. Neurotol. 2011, 32, 1017–1024. [Google Scholar] [CrossRef]
- Samii, M.; Gerganov, V.; Samii, A. Microsurgery management of vestibular schwannomas in neurofibromatosis type 2: Indications and results. Prog. Neurol. Surg. 2008, 21, 169–175. [Google Scholar] [CrossRef]
- Tysome, J.R.; Macfarlane, R.; Durie-Gair, J.; Donnelly, N.; Mannion, R.; Knight, R.; Harris, F.; Vanat, Z.H.; Tam, Y.C.; Burton, K.; et al. Surgical management of vestibular schwannomas and hearing rehabilitation in neurofibromatosis type 2. Otol. Neurotol. 2012, 33, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Wigand, M.E.; Haid, T.; Goertzen, W.; Wolf, S. Preservation of Hearing in Bilateral Acoustic Neurinomas by Deliberate Partial Resection. Acta Oto Laryngol. 1992, 112, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Aihara, N.; Yamada, H.; Takahashi, M.; Inagaki, A.; Murakami, S.; Mase, M. Postoperative Headache after Undergoing Acoustic Neuroma Surgery via the Retrosigmoid Approach. Neurol. Med. chir. 2017, 57, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Matthies, C. Management of 1000 vestibular schwannomas (acoustic neuromas): Hearing function in 1000 tumor resections. Neurosurgery 1997, 40, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Gugel, I.; Grimm, F.; Teuber, C.; Kluwe, L.; Mautner, V.F.; Tatagiba, M.; Schuhmann, M.U. Management of NF2-associated vestibular schwannomas in children and young adults: Influence of surgery and clinical factors on tumor volume and growth rate. J. Neurosurg. Pediatr. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Abo-Dalo, B.; Kutsche, K.; Mautner, V.; Kluwe, L. Large intragenic deletions of the NF2 gene: Breakpoints and associated phenotypes. Genes Chromosomes Cancer 2010, 49, 171–175. [Google Scholar] [CrossRef] [PubMed]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef]
| Sex | |
| male | 8 |
| female | 15 |
| Operation side | |
| left | 19 |
| right | 20 |
| Family history for NF2 | |
| yes | 3 |
| no | 20 |
| Detected mutation types in 21 patients | |
| splicing mutations | 6 |
| nonsense mutations | 5 |
| frameshifting mutations | 4 |
| large genome alteration | 1 |
| Age at diagnosis in year (mean ± SD, range) | 12 ± 7, 1–22 |
| Age at first surgery in year (mean ± SD, range) | 16 ± 5, 8–26 |
| Age at second surgery in year (mean ± SD, range) | 16 ± 4, 11–24 |
| Follow-up period in months (mean ± SD, range) | 75 ± 6, 21–167 |
| Tumor volume in cm3 (mean ± SD, range) | |
| preoperative | 2 ± 2.6, 0.1–10.5 |
| postoperative | 1 ± 1.6, 0–18.6 |
| significance in difference | p = 0.002 |
| Growth rate in cm3/year (mean ± SD, range) | |
| preoperative | 0.6 ± 0.7, 0.03–3.4 |
| postoperative | 0.3 ± 0.4, −0.01–2.2 |
| significance in difference | p = 0.03 |
| Resection amount | |
| only bony decompression | 2 |
| decompression with laser coagulation | 1 |
| partial | 32 |
| subtotal | 2 |
| near total | 1 |
| total | 1 |
| PTA in dB (mean ± SD, range) | |
| preoperative | 17 ± 16, 1.3–80 |
| postoperative | 21 ± 18, 1.3–78 |
| significance in difference | p = 0.009 |
| SDS in % (mean ± SD, range) | |
| preoperative | 85 ± 27, 0–100 |
| postoperative | 81 ± 32, 0–100 |
| significance in difference | p = 0.043 |
| G–R Scale [15] | Postoperation Class (No) | ||||
| Preoperation Class (No) | I (26) | II (3) | III (3) | IV (0) | V (7) |
| I (32) II (4) III (3) IV (0) V (0) | 26 0 0 0 0 | 1 2 0 0 0 | 1 1 1 0 0 | 0 0 0 0 0 | 4 1 2 0 0 |
| AAO–HNS Classification | Postoperation Class (No) | ||||
| Preoperation Class (No) | A (26) | B (2) | C (0) | D (11) | |
| A (32) B (3) C (0) D (4) | 26 0 0 0 | 1 1 0 0 | 0 0 0 0 | 5 2 0 4 | |
| BAEP Classification System [12] | Postoperation Class (No) | ||||
| Preoperation Class (No) | I (5) | II (23) | III (4) | IV (0) | V (7) |
| I (11) II (23) III (1) IV (2) V (2) | 3 2 0 0 0 | 5 18 0 0 0 | 1 1 1 1 0 | 0 0 0 0 0 | 2 2 0 1 2 |
| Positive Correlation | |
| Preoperative BAEP correlated positively with Postoperative PTA | r = 0.3, p = 0.04 |
| Preoperative PTA correlated positively with Postoperative PTA | p < 0.001 |
| Preoperative SDS correlated positively with Postoperative SDS | p < 0.001 |
| Negative (Inversed) Correlation | |
| Truncation NF2 mutations correlate with worse PTA (compared to the splicing mutation) | p = 0.012 |
| Truncation NF2 mutations correlate with worse SDS (compared to the splicing mutation) | p = 0.008 |
| Larger preoperative tumor volume correlates with worse postoperative PTA | r = 0.3, p = 0.04 |
| Larger resection amount correlate with worse postoperative PTA | r = 0.354, p = 0.031 |
| Larger resection amount correlate with worse postoperative SDS | r = −0.386, p = 0.018 |
| Sex | |
| male | 4 |
| female | 11 |
| Family history for NF2 | |
| yes | 4 |
| no | 11 |
| Detected mutation types in 8 patients | |
| splicing mutations | 3 |
| nonsense mutations | 2 |
| frameshifting mutations | 3 |
| Mosaic (No) | 3 |
| No mutation detected (No) | 1 |
| No genetic analysis performed (No) | 3 |
| Age at diagnosis in year (mean ± SD, range) | 10 ± 7, 0−21 |
| Follow-up period in months (mean ± SD, range) | 60 ± 33, 14−117 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugel, I.; Grimm, F.; Liebsch, M.; Zipfel, J.; Teuber, C.; Kluwe, L.; Mautner, V.-F.; Tatagiba, M.; Schuhmann, M.U. Impact of Surgery on Long-Term Results of Hearing in Neurofibromatosis Type-2 Associated Vestibular Schwannomas. Cancers 2019, 11, 1376. https://doi.org/10.3390/cancers11091376
Gugel I, Grimm F, Liebsch M, Zipfel J, Teuber C, Kluwe L, Mautner V-F, Tatagiba M, Schuhmann MU. Impact of Surgery on Long-Term Results of Hearing in Neurofibromatosis Type-2 Associated Vestibular Schwannomas. Cancers. 2019; 11(9):1376. https://doi.org/10.3390/cancers11091376
Chicago/Turabian StyleGugel, Isabel, Florian Grimm, Marina Liebsch, Julian Zipfel, Christian Teuber, Lan Kluwe, Victor-Felix Mautner, Marcos Tatagiba, and Martin Ulrich Schuhmann. 2019. "Impact of Surgery on Long-Term Results of Hearing in Neurofibromatosis Type-2 Associated Vestibular Schwannomas" Cancers 11, no. 9: 1376. https://doi.org/10.3390/cancers11091376
APA StyleGugel, I., Grimm, F., Liebsch, M., Zipfel, J., Teuber, C., Kluwe, L., Mautner, V.-F., Tatagiba, M., & Schuhmann, M. U. (2019). Impact of Surgery on Long-Term Results of Hearing in Neurofibromatosis Type-2 Associated Vestibular Schwannomas. Cancers, 11(9), 1376. https://doi.org/10.3390/cancers11091376




