1. Introduction
Nanomaterials are the cornerstone in the rapidly advancing field of nanotechnology, playing a crucial role in successful drug delivery at diseased sites [
1]. To date, many nanoplatforms have been applied for the delivery of temoporfin (5,10,15,20-tetra(m-hydroxyphenyl)chlorin, mTHPC), one of the most promising photosensitizers (PSs) used in the photodynamic therapy (PDT) of solid cancers. Temoporfin has been marketed in the European Union since 2001 under the trade name Foscan
® (biolitec pharma Ltd., Jena, Germany), and is indicated for the palliative treatment of head and neck squamous cell carcinoma [
2]. Nanodelivery systems were supposed to overcome or improve the major constraints of mTHPC, such as low solubility, unfavorable pharmacokinetic profiles, and side effects (pain upon injection, skin photosensitivity) [
3]. However, due to the complexity of drug distribution processes, the use of individual NPs offered neither optimal, leakage-free delivery of mTHPC to the tumor nor the local release of large amounts of mTHPC. At the same time, multifunctional nanomedicines featuring high drug loading capacity, controllable drug release, and real-time self-monitoring are attracting immense interest due to their potential to improve cancer therapy efficacy [
4,
5,
6].
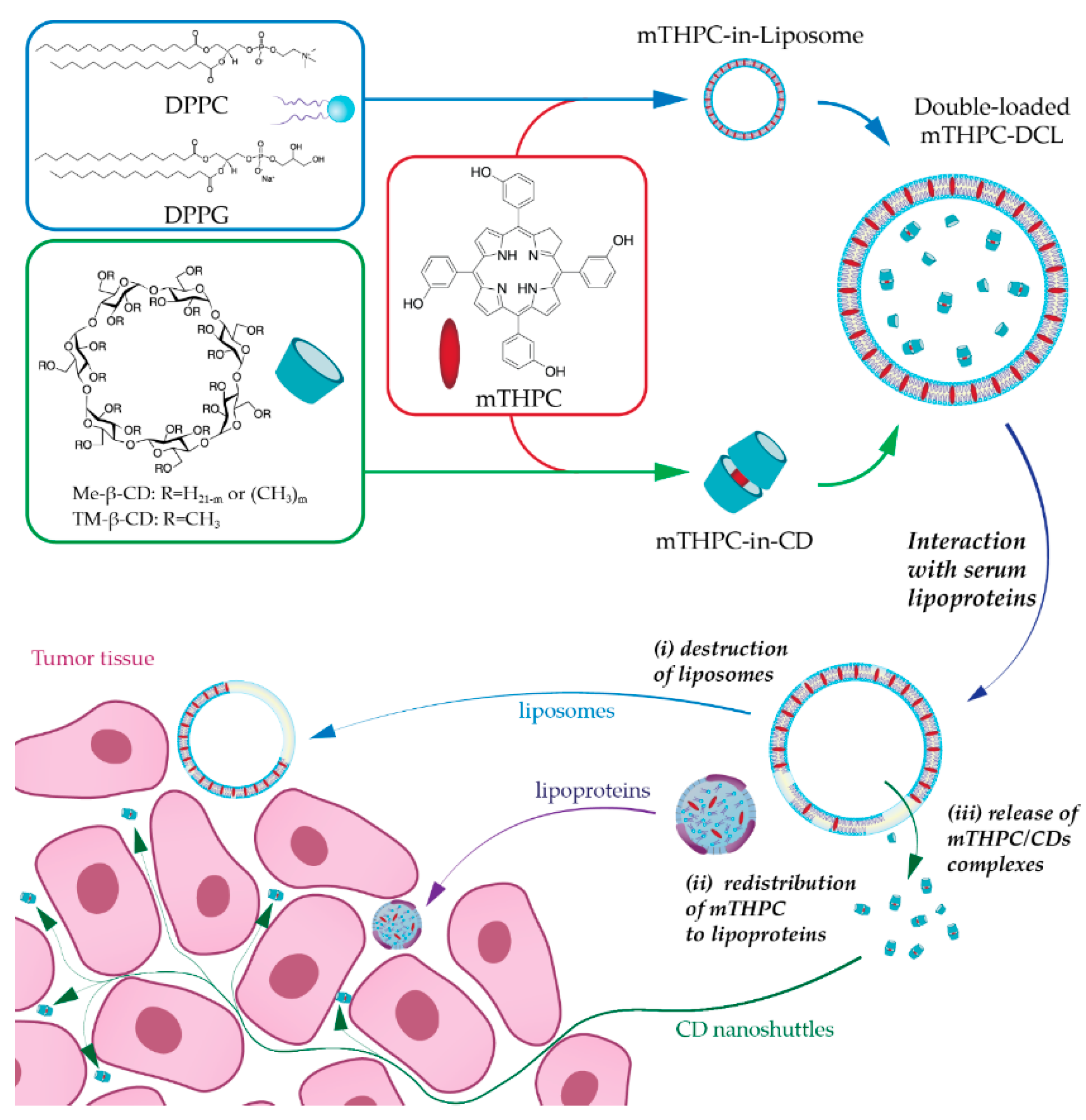
In the present study, we have suggested combining NPs into one nanoplatform as an advanced alternative strategy for mTHPC delivery. Recently, we reported the encapsulation of mTHPC-cyclodextrin (CD) supramolecular complex into liposomes, namely drug-in-cyclodextrin-in-liposome (DCL), as a prospective nanodelivery system for mTHPC [
7]. The upgraded double-loaded mTHPC-DCLs (DL-DCL) contained mTHPC in both lipid and aqueous compartments of lipid vesicles. We hypothesized that such “matryoshka-type” hybrid liposomes would combine the advantages of each delivery system (
Figure 1). Liposomes are effective containers for selective mTHPC delivery to target sites [
8]. However, liposomes have limited penetration into deep tissue layers [
9,
10]. Alternatively, mTHPC/CDs inclusion complexes easily penetrate tumor tissue, thereby significantly increasing PS accumulation [
11]. However, these supramolecular complexes are prone to dissociation in vivo, once diluted in the bloodstream [
12]. Thus, we suggested that the coupling of both delivery systems intp one DL-DCL could restrain the dissociation of drug–mTHPC complexes, avoid rapid drug release, and favorably alter PS penetration into tumor tissues.
In the present work, we prepared and characterized DL-DCLs and tested these complexes in 2D and 3D tumor cell cultures. We focused on the study of double-loaded mTHPC-DCLs in 3D multicellular tumor spheroids in terms of PS penetration and accumulation. Finally, we conducted a preliminary study on double-loaded mTHPC-DCL efficacy in vivo in tumor-xenografted mice.
3. Discussion
To date, hybrid NPs, combining several nanomaterials in one, have attracted increasing attention as anti-cancer delivery systems [
15]. The careful selection of the combined nanomaterials could result in a considerable synergistic effect overcoming the individual nanostructures’ limitations. In the case of mTHPC, a synergy could be achieved by a coupling of CD-based nanoshuttles and liposomes. mTHPC exhibits an extremely strong affinity to methylated β-CDs (10
6–10
7 M
−1 for Me-β-CD and TM-β-CD, respectively [
16]) leading to the unique possibility of altering mTHPC biodistribution via a nanoshuttle mechanism [
11,
17]. At the same time, liposomal formulations of mTHPC display selective delivery of PS to tumor tissue [
8]. CDs could be regarded as an added value to mTHPC-based liposomes, and as such, could increase drug loading capacity, entrapment efficiency, prolong the circulation time of the drug in the bloodstream, reduce toxicity, and finally provide controlled release [
18,
19]. Thus, we supposed that such a “matryoshka-doll” nanostructure could be a potent delivery system, providing both passive tumor targeting by liposomes and deep penetration of mTHPC/CD complexes into the tumor tissue (
Figure 1).
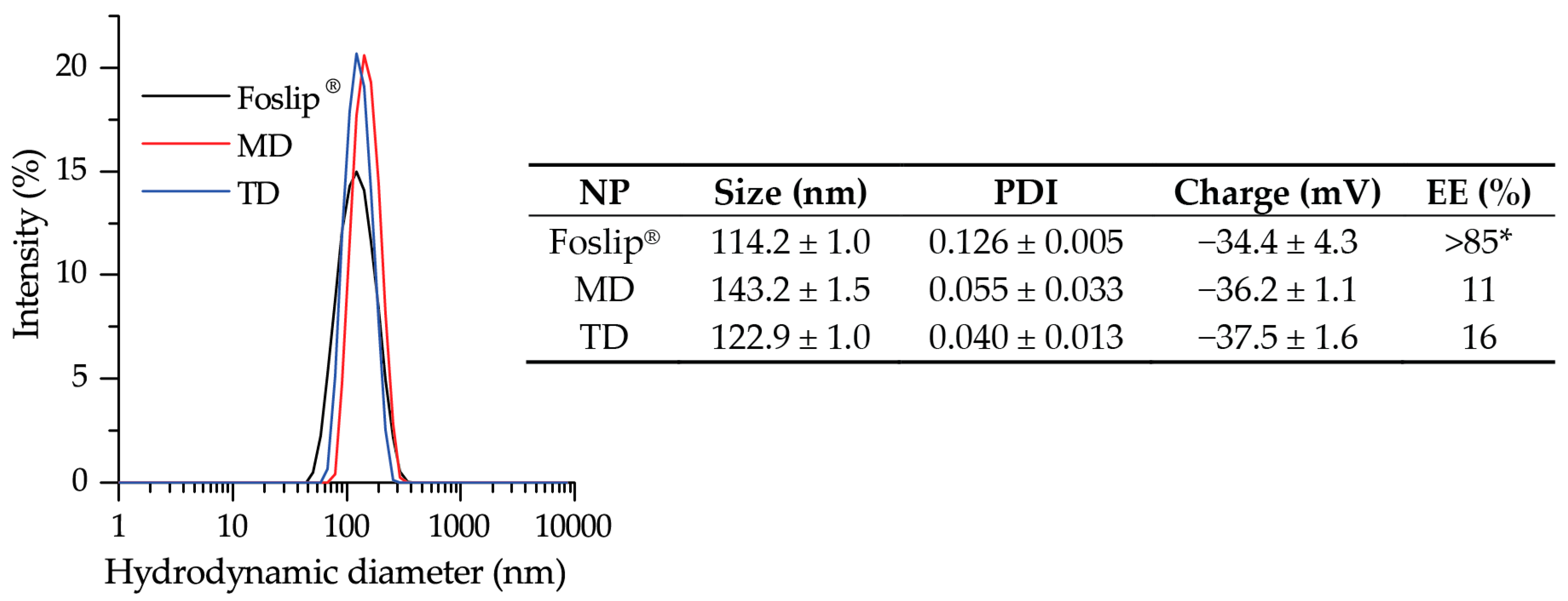
Recently, we optimized the composition of mTHPC-DCLs, selecting two of the most potent DCLs based on methylated β-CDs [
7]. In the present study, the lipid bilayers of selected DCLs were additionally loaded with lipophilic mTHPC, achieving DL-DCLs. Physico-chemical characteristics (size, PDI, charge, and colloidal stability) of DL-DCLs were similar to those of single-loaded DCLs [
7], and comparable with liposomal mTHPC formulation Foslip
® (
Figure 2). According to the preparation technique, the ratio between aqueous volume of liposomes and void volume during the liposome preparation was low, explaining the low EE values of mTHPC in DL-DCLs [
7,
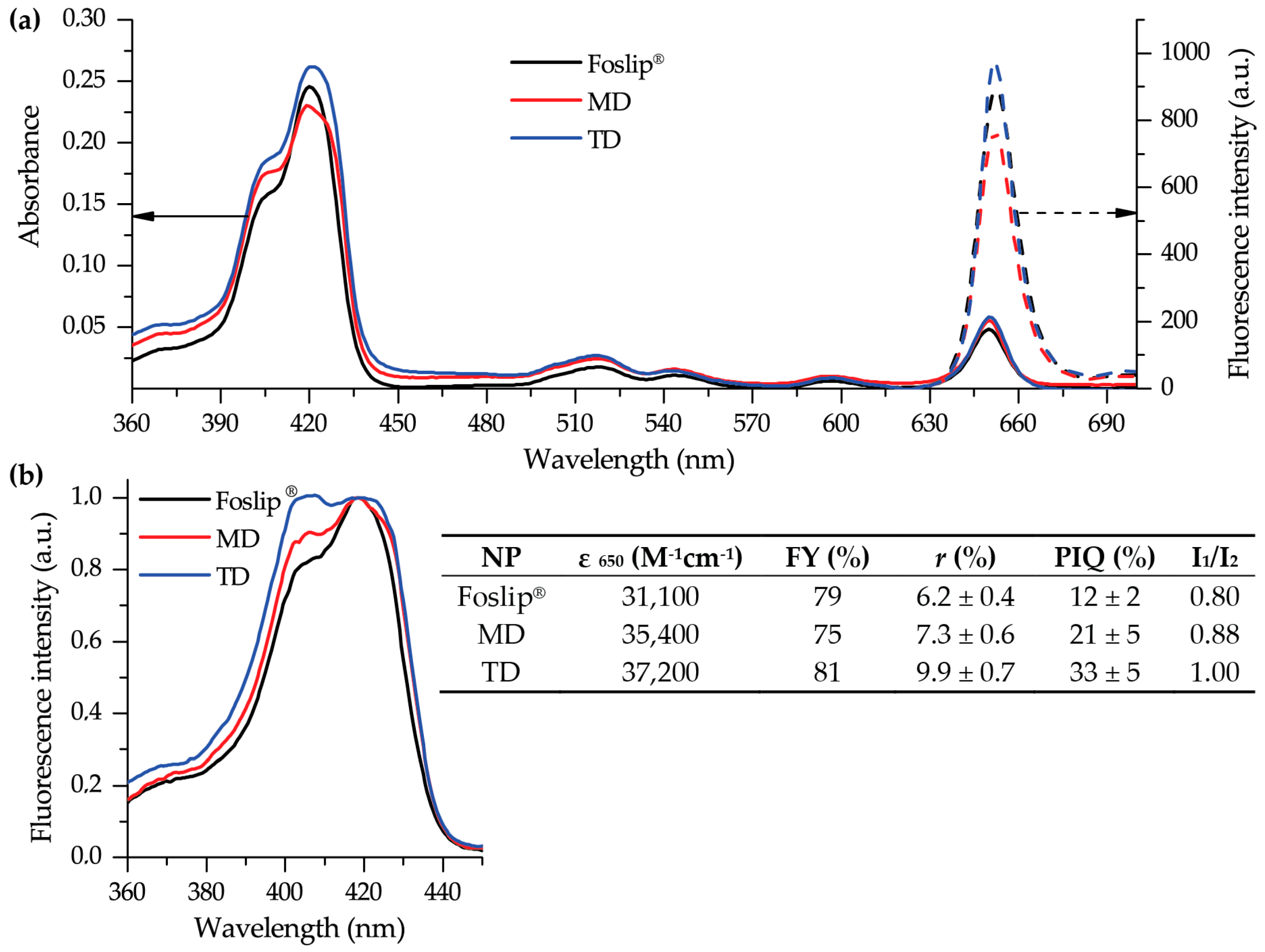
20]. The complete monomerization of mTHPC in all NPs was confirmed by absorption spectra (
Figure 3a), and double encapsulation of mTHPC in DCLs was established using fluorescence spectroscopy (
Figure 3b). The presence of mTHPC/CD complexes led to the increase of I
1/I
2 ratio [
14], demonstrating I
1/I
2 = 0.88 and 1.00 for MD and TD, respectively, while in the case of Foslip
®, I
1/I
2 was 0.8 (
Figure 3b). At the same time, the high loading of mTHPC in the lipid bilayer resulted in fluorescence quenching [
13,
21], causing the decrease of FY, PIQ, and
r values (
Figure 3b, insert). Overall, 30% of mTHPC was encapsulated in CD complexes, while the fraction of PS in the lipid bilayer was 70%.
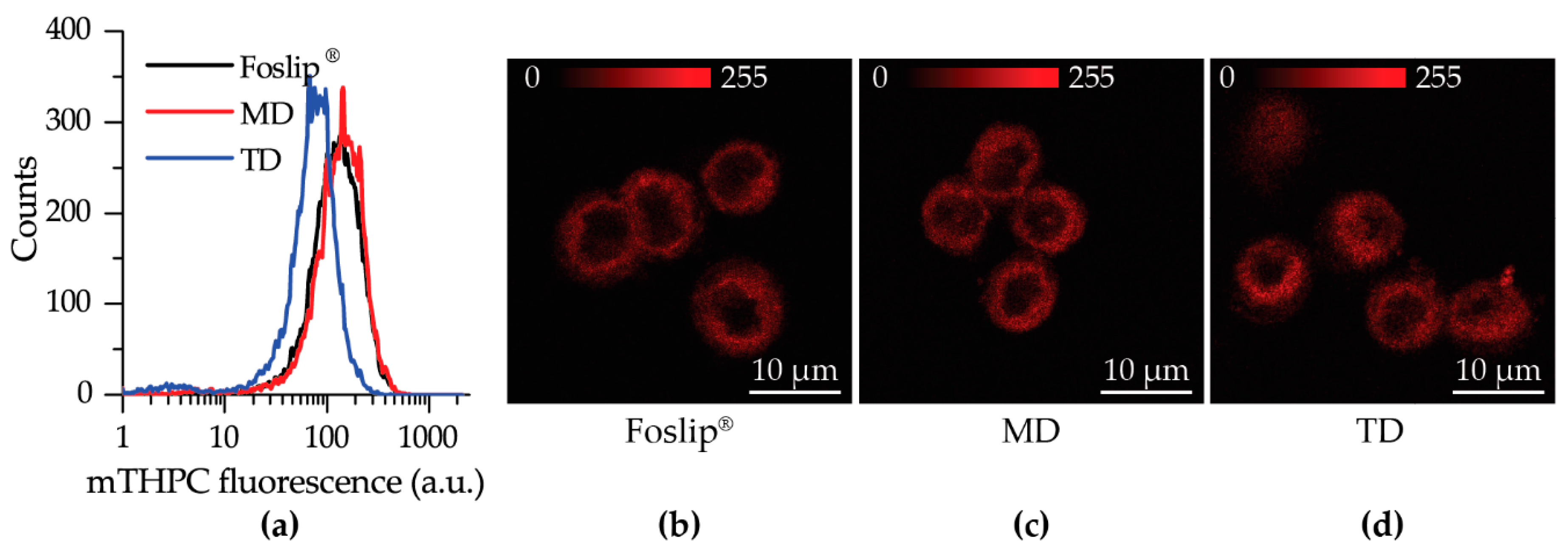
We further tested DL-DCLs in 2D and 3D in vitro tumor models. In 2D monolayer cell cultures, the behavior of both double-loaded mTHPC-DCLs was similar to that of Foslip
® (
Figure 4 and
Figure S1), while in 3D multicellular spheroids, we clearly demonstrated the benefits of matryoshka-type hybrid liposomes against conventional lipid vesicles. The 3D multicellular tumor spheroids more closely mimicked the native tumor environment, representing the tumor stroma tissue and offering better prediction potential when testing the penetration ability of nanomedicines [
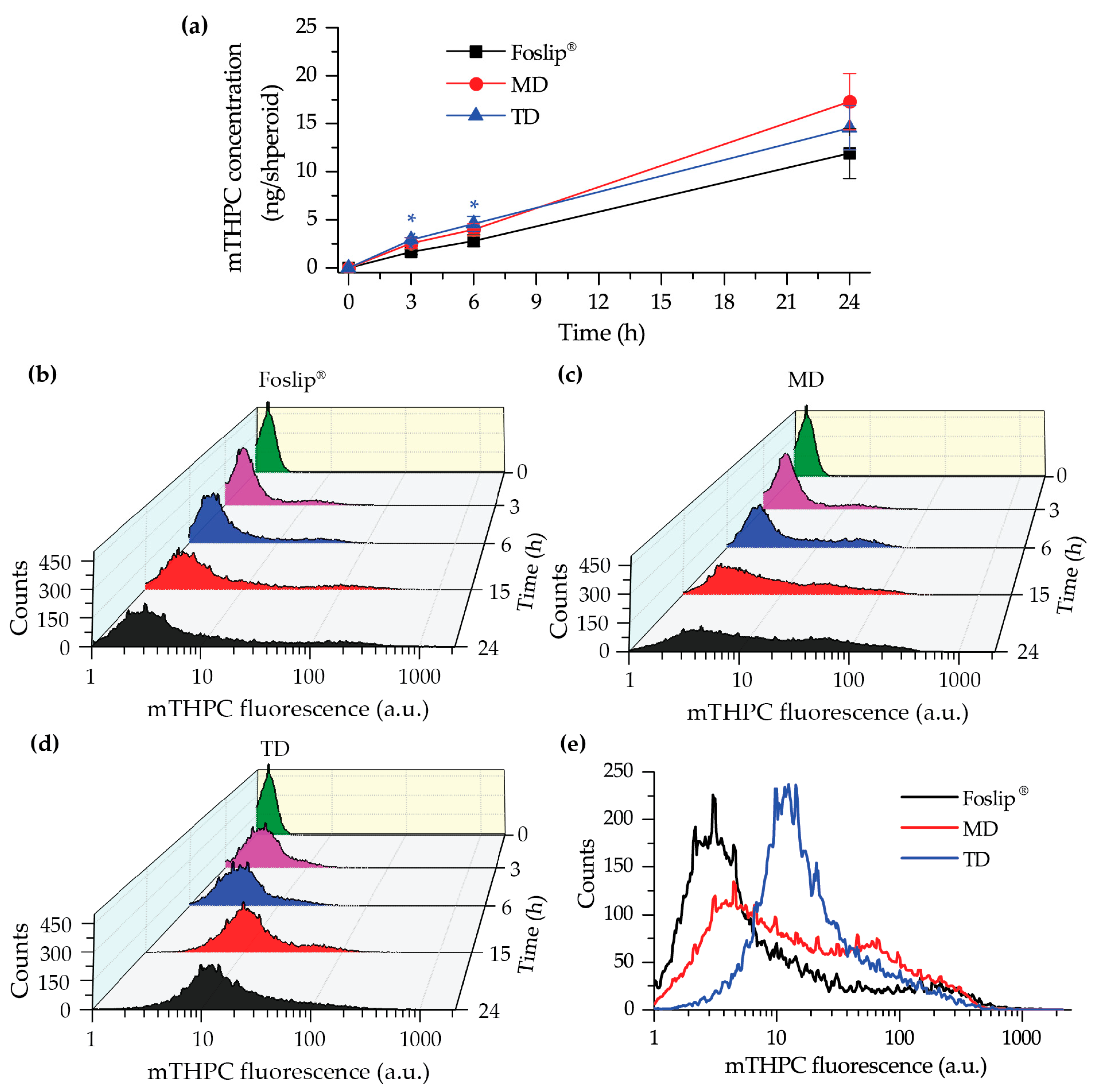
22]. The data of chemical extraction of mTHPC from spheroids demonstrated an almost similar amount of mTHPC at various incubation times (
Figure 5a). However, detailed study using flow cytometry showed different profiles of mTHPC accumulation in the spheroid cells for DL-DCLs and Foslip
®. The spheroids treated with TD displayed a homogeneous distribution between all cells at all time points for both HT29 (
Figure 5d) and FaDu spheroids (
Figure S2), while for MD and Foslip
®, the accumulation of mTHPC was mainly associated with the PS uptake in a small fraction of cells (
Figure 5b,c and
Figure S2). At 24 h post-incubation, MD exhibited a slightly more homogeneous PS distribution in HT29 spheroids than Foslip
® (
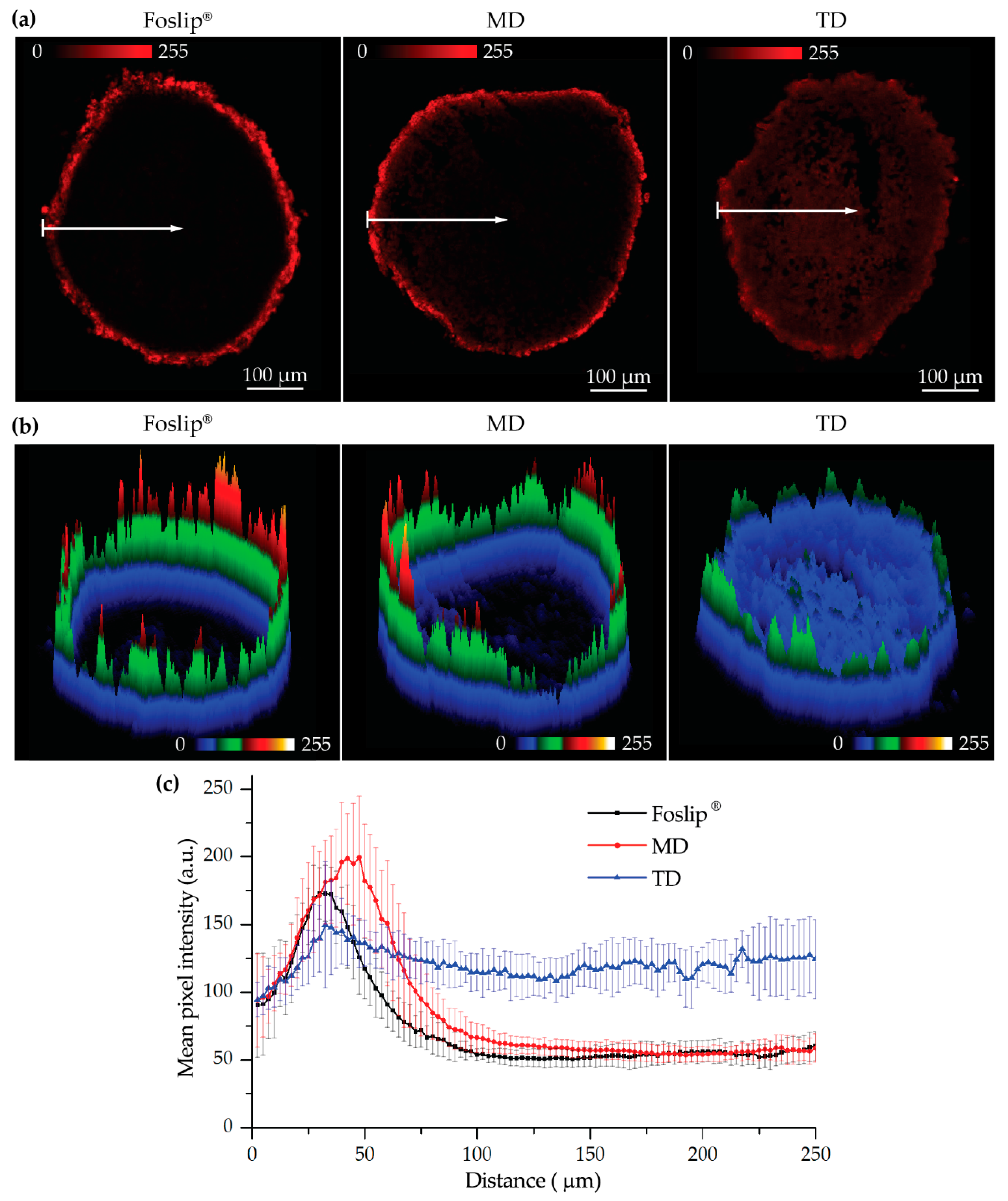
Figure 6). The fluorescence imaging data of spheroid frozen-cut sections confirmed the almost homogeneous distribution of mTHPC delivered by TD in both types of spheroid cells (
Figure 6 and
Figure S3). In the case of TD, the mTHPC fluorescence signal was uniform for the whole spheroid, while for MD and Foslip
®, a strong peripheral fluorescence signal dropped to background values at 50 and 100 µM from the spheroid periphery, respectively. In all probability, the observed effect was related to the presence of mTHPC/CDs in the media due to DL-DCL disintegration, as has been previously observed for Foslip
® [
10,
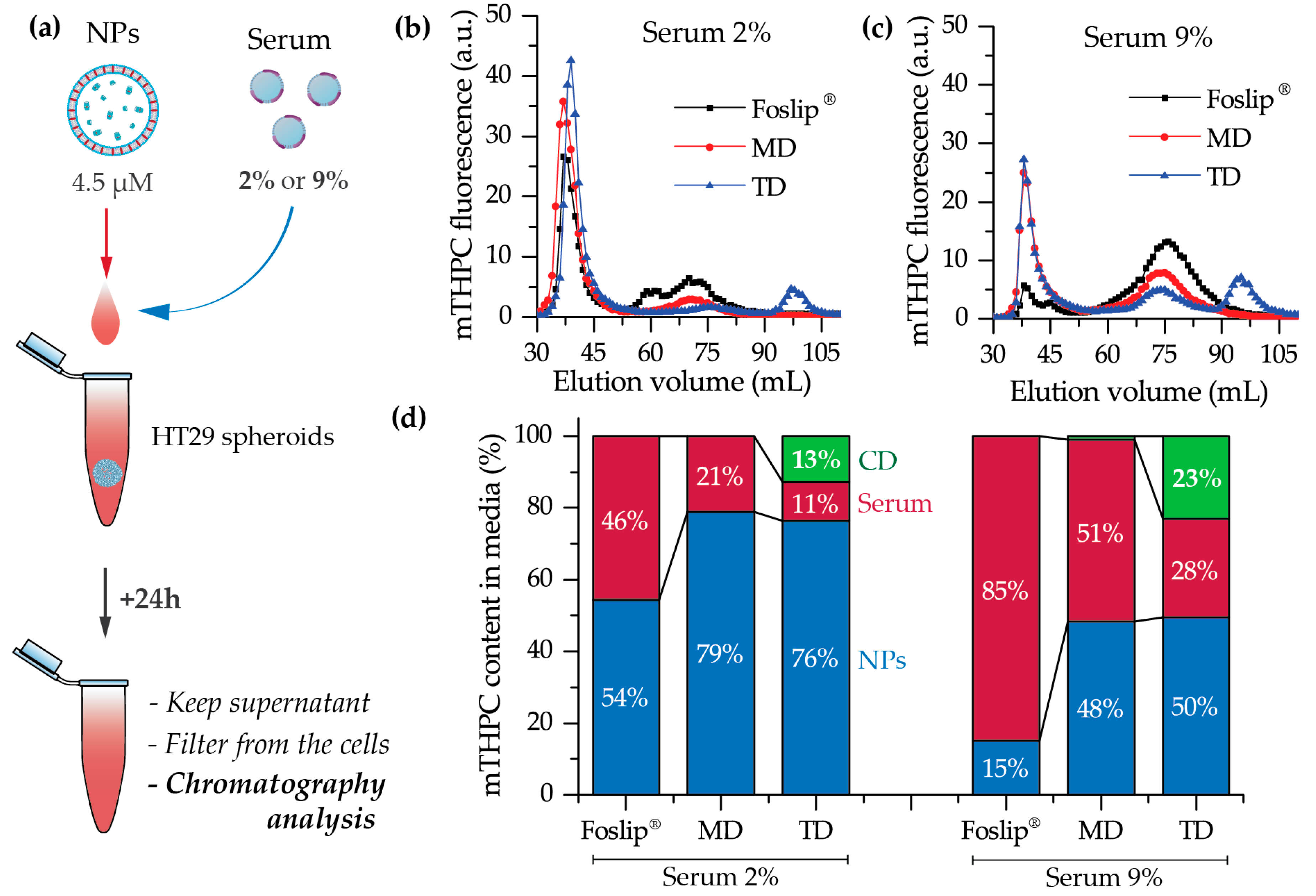
23]. Indeed, in the case of TD, chromatography analysis of culture media after 24 h incubation of NPs with tumor spheroids demonstrated the presence of mTHPC/CD complexes. To confirm the serum-induced destruction of DL-DCLs, we demonstrated the increase of the mTHPC-TM-β-CD fraction in the medium supplemented with 9% of serum compared to 2% (
Figure 7). It is worth noting that the MD-treated samples contained a similar amount of mTHPC in NPs, while all released mTHPC in the medium was localized only in serum proteins. Compared with TM-β-CD, Me-β-CDs possess less affinity to mTHPC [
17]; thus, the equilibrium distribution of mTHPC is shifted to serum proteins [
18]. Therefore, we suppose that the lifetime of mTHPC-Me-β-CDs was too short to penetrate the deep layers of spheroids, and mTHPC released from the MD was quickly redistributed to both serum proteins and the nearest tumor cells by Me-β-CDs, while long-living mTHPC-TM-β-CD complexes could easily penetrate to the deep tissue regions.
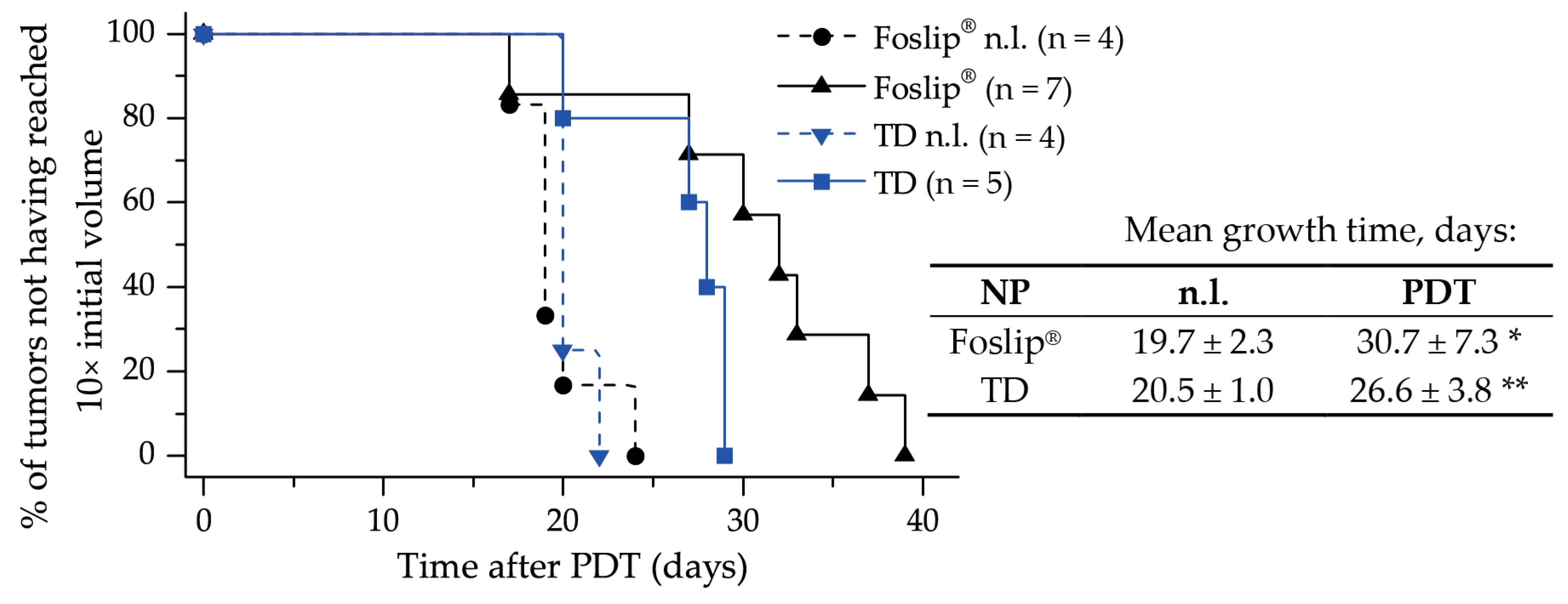
Finally, we assessed the PDT efficiency of TD and MD in 3D multicellular tumor spheroids in vitro, and of TD in xenografted mice in vivo. Despite the remarkable difference in penetration profiles, PDT-induced cell death in spheroids treated with TD and MD was similar to Foslip
®. The same tendency was observed in the xenografted mice model: the difference in mean tumor growth delay for both TD and Foslip
® was about 30 days (
Figure 8). We likely did not achieve the desired balance between the accumulation and distribution of PS in the tumor. We suggest that increasing the TD concentration could be helpful in exceeding the threshold of intracellular PS accumulation needed for efficient DCL-PDT photokilling.
4. Materials and Methods
4.1. Materials
mTHPC and its liposomal formulation (Foslip®) were kindly provided by biolitec research GmbH (Jena, Germany). The stock solution of mTHPC (2 mM) was prepared in methanol and kept at 4 °C in the dark. Foslip®, based on dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylglycerol (DPPG) liposomes with a mTHPC/lipid ratio of 1:12 (mol/mol), was prepared by solubilizing powder in ultrapure water (UPW, Milli-Q® Advantage A10® System, Millipore, Eschborn, Germany) to a final mTHPC concentration of 2 mM.
Random methyl-β-cyclodextrin (Me-β-CD; product code CY-2004.1,29; substitution degree of 12, average molecular weight 1135 Da) and heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin (TM-β-CD; product code CY-2003,34; molecular weight 1429.6 Da) were purchased from CYCLOLAB R&D. Ltd., (Budapest, Hungary). DPPC and DPPG were purchased from Sigma (USA).
4.2. DL-DCL Preparation
DCLs were prepared by the thin lipid film hydration method, as described previously [
7]. Briefly, inclusion complexes of mTHPC with β-CDs were formed using the solvent co-evaporation method in UPW. DPPC/DPPG liposomes loaded with mTHPC were prepared by membrane extrusion technique according to the previously published procedure, yielding unilamellar liposomes [
24]. These liposomes contained DPPC and DPPG at a molar ratio of 9:1, with a final lipid concentration of 15 mg/mL. To obtain DL-DCLs, mTHPC was added at the step of the preparation of lipid mixture at a molar drug/lipid ratio of 1:15, and mTHPC/β-CD inclusion complexes were encapsulated at the lipid film hydration step. The purification of DCLs from the non-encapsulated mTHPC/β-CDs in the medium was performed using a minicolumn chromatography technique [
25].
4.3. Cell Lines
HT29 human colon adenocarcinoma cell line was purchased from ATCC (LGC Promochem, Molsheim, France). FaDu (human pharynx squamous cell carcinoma) cell line was purchased from ATCC (Cat. No: ATCC1 HTB-43™). Cells were cultured in phenol red-free Roswell Park Memorial Institute 1640 medium (RPMI-1640, Invitrogen™, Carlsbad, California, USA), supplemented with 9% (vol/vol) heat-inactivated fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), penicillin (10,000 IU) streptomycin (10,000 mg/mL) and 1% (vol/vol) 0.2 M glutamine (Invitrogen™, Carlsbad, California, USA). Cells were kept as a monolayer culture in a humidified incubator (5% CO2) at 37 °C. Cell culture was reseeded every week to ensure exponential growth.
4.4. Multicellular Tumor Spheroid Model
HT29 MCTSs were initiated as previously described [
26]. Briefly, flasks coated with 1% L-agarose were seeded with 5 × 10
4 HT29 cells/mL. After three days, cellular aggregates were transferred into spinner flasks (Integra Biosciences, Cergy Pontoise, France) containing 125 mL RPMI-1640 medium supplemented with 9% FBS. Spinner flasks were placed under constant agitation at 75 rpm at 37 °C (5% CO
2, humidified atmosphere) for 15 days. Spheroids were filtered to approximatively 500 µm in diameter before conducting experiments.
MCTSs were generated from FaDu cells using the liquid overlay technique (LOT), as described previously [
27]. Briefly, 100 µL of FaDu cells (5 × 10
4 cells/ml) and 100 µL of full RPMI medium were added to each well of a 96-well plate previously coated with 1% agarose (w/v in water), and cultured at 37 °C, 5% CO
2 for 5 days before being taken into experiments.
For dissociation, spheroids were transferred into a 12-well plate, washed twice with PBS and further incubated with 0.025% trypsin (GIBCO™, ThermoFisher, Waltham, MA, USA) and 0.01% ethylenediaminetetraacetic acid (GIBCO™, ThermoFisher, Waltham, MA, USA). For complete trypsinization, the plate with spheroids was placed on a rotatory shaker (60 rpm) for 30 min in subdued light. After dissociation, trypsin action was inhibited by the addition of 3 mL complete culture medium (9% FBS), and the cell suspension was centrifuged to a pellet and further re-suspended in a fresh medium.
4.5. Fluorescence Staining
For in vitro cell experiments, stock NP solutions were diluted in RPMI-1640 supplemented with 2% heat-inactivated fetal bovine serum (FBS, Life Technologies, Carlsbad, California, USA) to obtain the final mTHPC concentration of 1.5 µM for 2D monolayer cells and 4.5 µM for 3D tumor spheroid experimentation.
For the 2D monolayer cell culture, cells (5 × 104 cells/mL) were seeded in 24-well plates for 72 h and then incubated with Foslip®, MD, or TD (1.5 µM). In the case of 3D cell culture, before incubation with mTHPC NPs (4.5 µM), spheroids were washed with serum-free RPMI-1640 medium. PS incubation was performed in the dark at 37 °C in a humidified 5% CO2 air atmosphere.
4.6. Animal Model
All experiments were performed in accordance with animal care guidelines from the European Union and were approved by the appropriate authority. The animal project registered under the number (#2438) received a favorable assessment from the Ethics Committee and was approved by the French Higher Education and Research Minister. All procedures involving animals were performed under general anesthesia with inhaled isoflurane (Vetflurane; Virbac, France) using a Univentor 400 anesthesia unit (Genestil, Royaucourt, France). Mice were housed in filtered air cabinets with a 12 h light/dark cycle at 22–24 °C and 50% humidity, provided with food and water ad libitum, and manipulated following aseptic procedures. Female NMRInu/nu mice (Janvier, St Berthevin, France) aged 9–10 weeks were used, with a mean bodyweight of 30 ± 3 g. Mice were inoculated subcutaneously in the left flank with 8 × 106 exponentially growing HT29 cells and the experiments were initiated 5–7 days after inoculation when the tumors reached 50 mm3 in volume.
4.7. Analytical Techniques
4.7.1. Spectroscopy
Absorption measurements were recorded with a Lambda 35 spectrometer (Perkin Elmer, USA) and fluorescence measurements were conducted with a LS55B spectrofluorometer (PerkinElmer, USA) equipped with polarizers, thermostated cuvette compartments, and magnetic stirring for polarization experiments. The concentrations of mTHPC in DL-DCLs and Foslip
® were estimated spectroscopically (λem = 652 nm) by dissolving nanoparticles in methanol. DL-DCLs were previously purified by minicolumn chromatography. Fluorescence quantum yield and photoinduced fluorescence quenching were measured as previously described (λ
ex: 416 nm; λ
em: 652 nm) [
15]. The measurements of mTHPC fluorescence anisotropy were performed as described earlier (λ
ex: 430 nm; λ
em: 652 nm) [
15]. EE of mTHPC in DCLs was measured spectroscopically (λ
em: 652 nm) immediately after extrusion and purification, as previously described [
7].
The hydrodynamic diameter of NPs, polydispersity index, and Z-potential were determined using photon-correlated spectroscopy by a Zetasizer Nano ZS (Malvern Instruments, UK) as previously reported [
7].
4.7.2. Flow Cytometry
Flow cytometry analysis was performed using a FACSCalibur (BD, Franklin Lakes, NJ, USA), equipped with lasers emitting at 488 nm and 633 nm. Flow cytometry histograms were obtained from the suspension of cells after dissociation of 50 NP-treated spheroids. The fluorescence of mTHPC was detected in the fluorescence channel FL4 with a 661 ± 16 nm filter under excitation at 633 nm. Propidium iodide (PI) fluorescence was detected in the FL2 channel with a 585 ± 42 nm filter (excitation at 488 nm). Data analysis was carried out using Flowing Software (Turku Centre for Biotechnology, Turku, Finland).
4.7.3. Fluorescence Microscopy
HT29 cells (3 × 104 cells/mL) were plated into Lab-Tek II chamber Slide (Roskilde, Denmark), incubated in the dark at 37 °C with 1.5 μM of mTHPC in different formulations for 3 h and then rinsed with PBS. mTHPC fluorescence was observed with a confocal laser-scanning microscope (Leica SP5 X AOBS LCSM, Leica microsystem, Wetzlar, Germany). For the 2D monolayer cell culture, fluorescence images were recorded using an oil immersion ×40 objective. The 3D tumor spheroids were embedded into a resin ShandonTM CryomatrixTM (ThermoFisher, Waltham, MA, USA), frozen, cut, and 20 µm thick cryosections were further analyzed by confocal microscopy (×10 objective). Fluorescence of mTHPC in FaDu 2D monolayer cells and frozen-cut cryosections of FaDu spheroids was analyzed using an upright epifluorescence microscope (AX-70 Provis, Olympus, France). The fluorescence images were obtained using a filter set at 405–445 nm excitation associated with a 570 nm dichroic mirror and a 590 nm long-pass emission filter for fluorescence measurements.
Analysis of the images was performed with ImageJ (NIH, USA) software. To estimate the penetration profile of dye in the spheroids, special macros were proposed. Briefly, the spheroid area was divided into 100 concentric rims, with the diameter decreasing in a linear way. After that we calculated the mean intensity of pixels in each rim. The final profiles were plotted as mean ± standard deviation from different cryo-sections (n = 7).
4.7.4. Uptake in Spheroids
mTHPC uptake in spheroids was measured by chemical extraction of mTHPC. After 3, 6, and 24 h incubation with NPs (4.5 µM), spheroids were dissociated and individual cells were subjected to the extraction of mTHPC with ethanol (99.6%) as previously described [
11]. Briefly, after sonication (15 min) and centrifugation (5 min, 1500 rpm), mTHPC fluorescence in the supernatant was assessed (λ
ex: 420 nm; λ
em: 652 nm).
4.7.5. Chromatography
Chromatographic experiments were performed on a Sigma 1.5 × 75 cm column filled with Sepharose CL-6B gel (GE Healthcare, USA) pre-equilibrated with PBS, with a total bed volume of 150 mL. HT29 tumor spheroids were incubated in RPMI-1640 media which was supplemented with 2% and 9% FBS. The NPs were added at final mTHPC concentration of 4.5 µM. After 24 h incubation, the supernatant was centrifuged (5 min, 1500 rpm), filtered using a 450 µm Millex
® – HV syringe driven filter unit (Sigma-Aldrich, St. Louis, MO, USA), and injected into the column using a three-way connector. Fractions of 1 mL were collected by an automated fraction collector. The column was stored at room temperature and separation was carried out in a partially light-protected environment to avoid mTHPC photobleaching. Fractions with elution volumes from 25 to 120 mL were collected and analyzed for mTHPC content using a SAFAS Xenius XM (SAFAS, Monaco, France) spectrofluorometer, as previosly reported [
28]. The mTHPC content in the chromatographic fractions was estimated by measurements the fluorescence intensity after the addition of 0.2% Triton
® X-100 to the samples. The analysis of chromatography histograms was performed using Origin software (OriginLab, Northampton, MA, USA).
4.8. Photoirradiation of Spheroids
HT29 spheroids were transferred from the spinner flask to 12-well plates and incubated with NPs for 24 h (4.5 µM). Spheroids were then washed and subjected to irradiation. Irradiation was performed at 652 nm with a Ceralas PDT diode laser (CeramOptec GmbH, Bonn, Germany) at 40 J/cm2 (fluence rate of 90 mW/cm2). Control spheroids were exposed to mTHPC only (drug, no light). The viability of the spheroid cells was assessed by propidium iodide probe. Spheroids were trypsinated 6 h post-PDT and the obtained cell suspension was stained with 1 µg/mL PI (Biolegend, San Diego, CA, USA) for 15 min in the dark at 37 °C, rinsed, and analyzed by flow cytometry.
4.9. Animal Experiments
NPs were administered intravenously by a tail vein injection at a dose of 0.15 mg/kg of mTHPC. Following the injection, mice were kept in the dark, and experiments were undertaken with minimal ambient light. Tumor irradiation was performed at 652 nm with a Ceralas PDT diode laser. The mice were treated 24 h post-administration at a fluence of 10 J/cm2 and the fluence rate of 100 mW/cm2. Just after PDT and 24 h later, mice received analgesia by subcutaneous injection of a mixture of 0.08 mg/kg buprenorphine (Axience, Pantin, France) and 1 mg/kg of the non-steroid anti-inflammatory Metacam (Boehringer Ingelhein, Ingelheim, Germany) Mice were kept in the dark for 7 days after PDT. Three times per week, the perpendicular diameters of the tumors were measured to document tumor growth. Tumor volume (V) was calculated using the equation: V = W2 × Y/2, where (W) and (Y) are the smaller and larger diameters. Mice were sacrificed when tumor volume reached the ethical size of 1000 mm3.
4.10. Statistics
The data from at least three independent experiments are presented as mean ± standard deviation. The data were evaluated using nonparametric Mann–Whitney U test (StatViewTM software) with a significance level of p < 0.05.