MicroRNA-361: A Multifaceted Player Regulating Tumor Aggressiveness and Tumor Microenvironment Formation
Abstract
1. Introduction
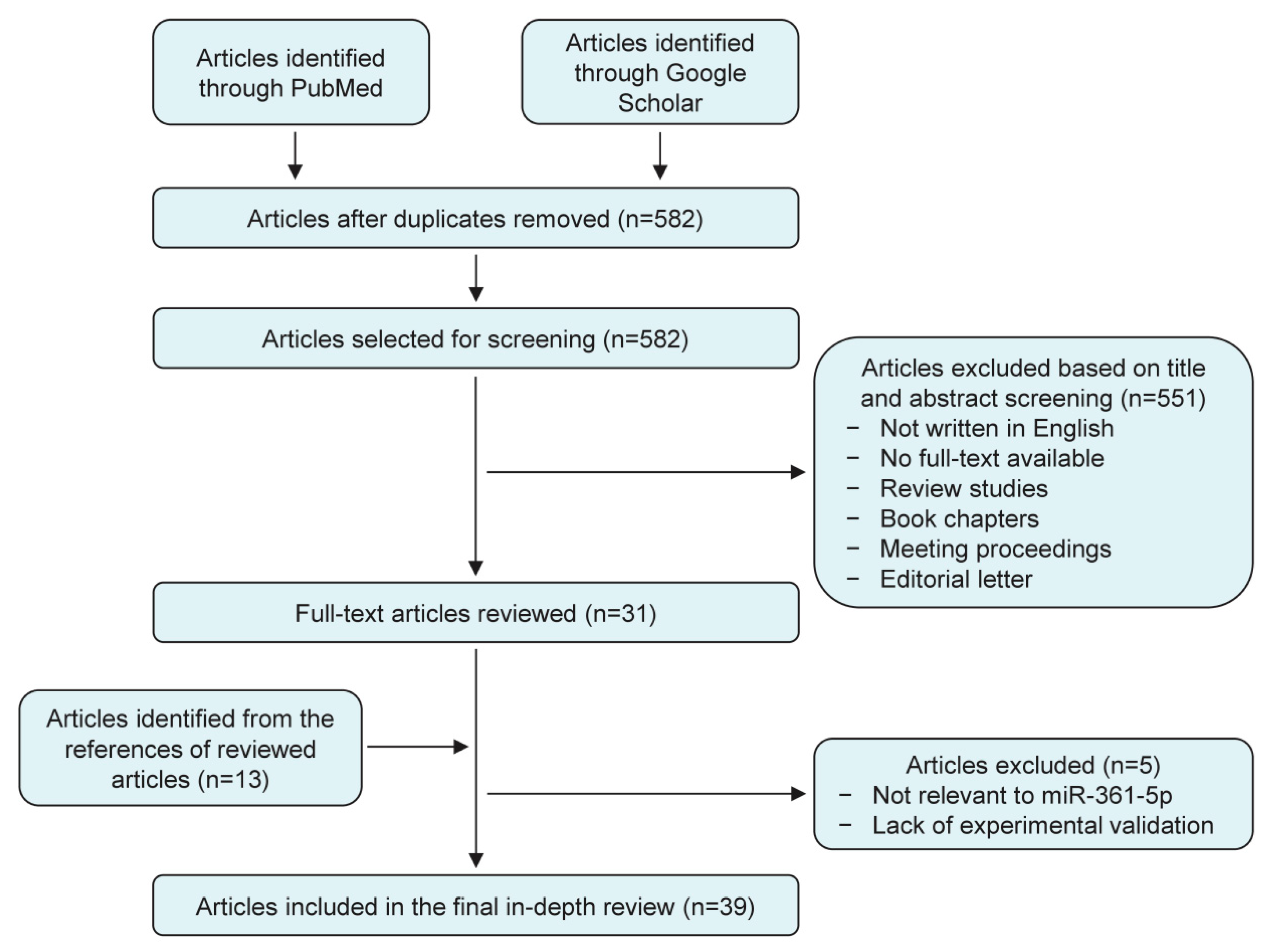
2. Evidence Acquisition
3. Dysregulation of miR-361 in Tumor
4. Mechanisms of miR-361 Regulation in Tumor
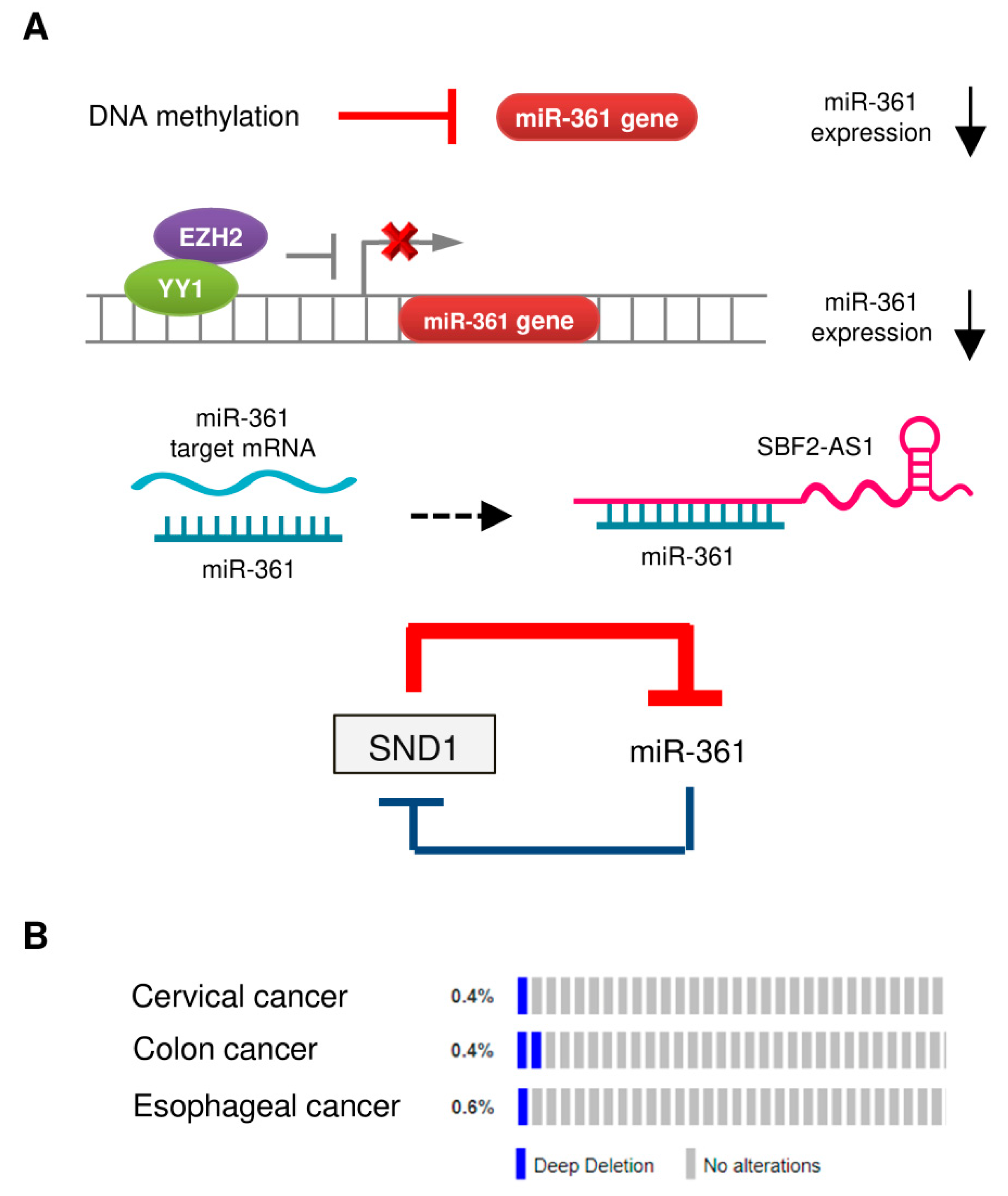
4.1. DNA Hypermethylation
4.2. Transcriptional Control of miR-361 Expression
4.3. Long Non-Coding RNA (lncRNA) SBF2-AS1 Acts as a Sponge for miR-361
4.4. The SND1/miR-361 Feedback Loop Controls miR-361 Expression
4.5. Deletion of the Human miR-361 Gene
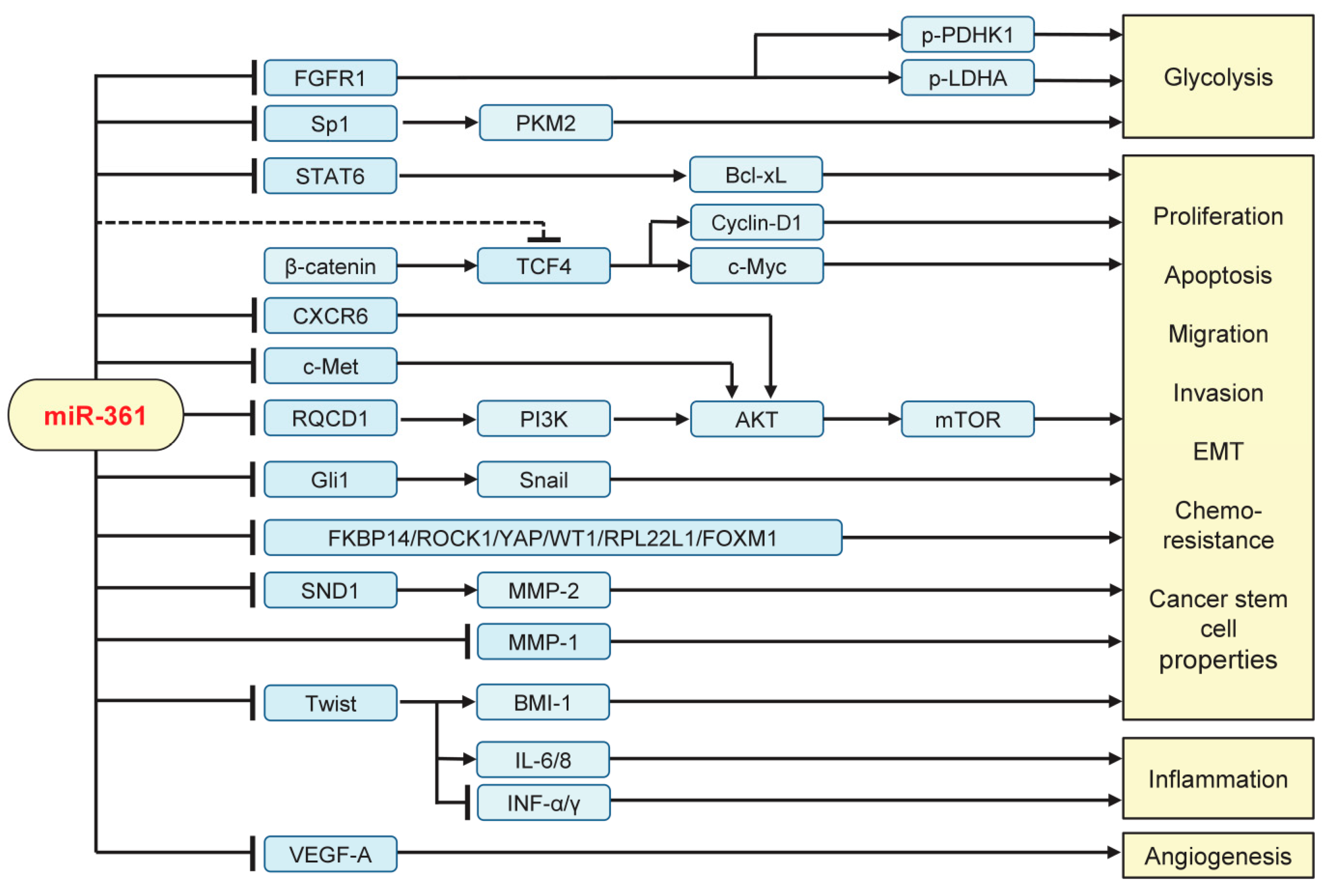
5. The Impact of miR-361 on the Aggressive Properties of Tumor Cells and Tumor Microenvironment Remodeling
5.1. Inhibiting Tumor Growth, Invasion, EMT, Metastasis, and Glycolysis
5.2. Suppressing Angiogenesis and Inflammation
6. Diagnostic and Prognostic Value of miR-361 in Tumor
7. Treating Cancer with miR-361 Replacement Therapy
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, R.A. MicroRNAs: Regulators of gene expression and cell differentiation. Blood 2006, 108, 3646–3653. [Google Scholar] [CrossRef] [PubMed]
- Da Sacco, L.; Masotti, A. Recent Insights and Novel Bioinformatics Tools to Understand the Role of MicroRNAs Binding to 5′ Untranslated Region. Int. J. Mol. Sci. 2012, 14, 480–495. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, A.E.; Reinhart, B.J.; Slack, F.; Martindale, M.Q.; Kuroda, M.I.; Maller, B.; Hayward, D.C.; Ball, E.E.; Degnan, B.; Müller, P.; et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000, 408, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Ruvkun, G. Glimpses of a Tiny RNA World. Science 2001, 294, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C. An Extensive Class of Small RNAs in Caenorhabditis elegans. Science 2001, 294, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.S.; Sharp, P.A. Roles for microRNAs in conferring robustness to biological processes. Cell 2012, 149, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Król, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Ihira, K.; Dong, P.; Xiong, Y.; Watari, H.; Konno, Y.; Hanley, S.J.; Noguchi, M.; Hirata, N.; Suizu, F.; Yamada, T.; et al. EZH2 inhibition suppresses endometrial cancer progression via miR-361/Twist axis. Oncotarget 2017, 8, 13509–13520. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal. Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Nishida, N.; Calin, G.A.; Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Bouchie, A. First microRNA mimic enters clinic. Nat. Biotechnol. 2013, 31, 577. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yu, J.; Chen, L.; Tao, T.; Yi, S.; Hanley, S.J.B.; Yue, J.; Watari, H.; Sakuragi, N. Control of PD-L1 expression by miR-140/142/340/383 and oncogenic activation of the OCT4–miR-18a pathway in cervical cancer. Oncogene 2018, 37, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Calin, G.A.; Lopez-Berestein, G.; Sood, A.K. MicroRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016, 6, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kanitz, A.; Imig, J.; Dziunycz, P.J.; Primorac, A.; Galgano, A.; Hofbauer, G.F.L.; Gerber, A.P.; Detmar, M. The Expression Levels of MicroRNA-361-5p and Its Target VEGFA Are Inversely Correlated in Human Cutaneous Squamous Cell Carcinoma. PLoS ONE 2012, 7, e49568. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qi, X.; Liu, H.; Su, H. MiR-361 inhibits osteosarcoma cell lines invasion and proliferation by targeting FKBP14. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, L.; Ma, L.; Zhang, Y.; Zhang, J.; Guo, B. MiR-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J. Exp. Clin. Cancer Res. 2017, 36, 158. [Google Scholar] [CrossRef]
- Chang, J.T.H.; Wang, F.; Chapin, W.; Huang, R.S. Identification of MicroRNAs as Breast Cancer Prognosis Markers through the Cancer Genome Atlas. PLoS ONE 2016, 11, e0168284. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.G.; Huang, Y.N.; Yao, L.; Liu, Y.R.; Hu, X.; Hou, Y.F.; Shao, Z.M. Positive expression of miR-361-5p indicates better prognosis for breast cancer patients. J. Thorac. Dis. 2016, 8, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yu, J.; Dai, Y.; Li, J.; Guo, M.; Song, J.; Zhou, X. Overexpression of miR-361-5p in triple-negative breast cancer (TNBC) inhibits migration and invasion by targeting RQCD1 and inhibiting the EGFR/PI3K/Akt pathway. Bosn. J. Basic Med. Sci. 2019, 19, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Qu, J.; Zhang, X.; Li, J.; Liu, J. MicroRNA-361-5p inhibits epithelial-to-mesenchymal transition of glioma cells through targeting Twist1. Oncol. Rep. 2017, 37, 1849–1856. [Google Scholar]
- Liu, J.; Yang, J.; Yu, L.; Rao, C.; Wang, Q.; Sun, C.; Shi, C.; Hua, D.; Zhou, X.; Luo, W.; et al. miR-361-5p inhibits glioma migration and invasion by targeting SND1. OncoTargets Ther. 2018, 11, 5239–5252. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dong, B.; Wang, Z.; Jiang, T.; Chen, G. MicroRNA-361-5p inhibits papillary thyroid carcinoma progression by targeting ROCK1. Biomed. Pharmacother. 2018, 102, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.W.; Sun, X.; Yu, Y.; Zhao, H.M.; Yang, Z.J.; Wang, X.; Cao, X.C. miR-361-5p suppresses lung cancer cell lines progression by targeting FOXM1. Neoplasma 2017, 64, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Z.; Wu, L.; Wang, Y. MiR-361 targets Yes-associated protein (YAP) mRNA to suppress cell proliferation in lung cancer. Biochem. Biophys. Res. Commun. 2017, 492, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shang, J.; Yang, S.; Zhang, Y.; Zhao, X. microRNA-361 targets Wilms’ tumor 1 to inhibit the growth, migration and invasion of non-small-cell lung cancer cells. Mol. Med. Rep. 2016, 14, 5415–5421. [Google Scholar]
- Ma, Y.; Bao, C.; Kong, R.; Xing, X.; Zhang, Y.; Li, S.; Zhang, W.; Jiang, J.; Zhang, J.; Qiao, Z.; et al. MicroRNA-361-5p suppresses cancer progression by targeting signal transducer and activator of transcription 6 in non-small cell lung cancer. Mol. Med. Rep. 2015, 12, 7367–7373. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Z.; Xie, L.; Zhu, J. MiR-361-5p inhibits the mobility of gastric cancer cells through suppressing epithelial-mesenchymal transition via the Wnt/β-catenin pathway. Gene 2018, 675, 102–109. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Z.; Xie, L.; Zhu, J. MiR-361-5p suppresses chemoresistance of gastric cancer cells by targeting FOXM1 via the PI3K/Akt/mTOR pathway. Oncotarget 2017, 9, 4886–4896. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Song, H.; Guo, B.; Zhang, Y.; Zheng, Y.; Lin, C.; Wu, Y.; Guan, G.; Sha, R.; Zhou, Q.; et al. MiR-361-5p inhibits colorectal and gastric cancer growth and metastasis by targeting staphylococcal nuclease domain containing-1. Oncotarget 2015, 6, 17404–17416. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Chen, G.Y.; Xie, Z.T. MicroRNA-361-5p Inhibits Cancer Cell Growth by Targeting CXCR6 in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2016, 38, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jing, X.; Chen, Z.; Duan, Z.; Zhang, Y. MiR-361-5p decreases the tumorigenicity of epithelial ovarian cancer cells by targeting at RPL22L1 and c-Met signaling. Int. J. Clin. Exp. Pathol. 2018, 11, 2588–2596. [Google Scholar]
- Gao, F.; Feng, J.; Yao, H.; Li, Y.; Xi, J.; Yang, J. LncRNA SBF2-AS1 promotes the progression of cervical cancer by regulating miR-361-5p/FOXM1 axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, G.; Yu, Q.; Zhang, X.; Han, G. MicroRNA-361 inhibited prostate carcinoma cell invasion by targeting Gli1. Int. J. Clin. Exp. Pathol. 2017, 10, 6108–6116. [Google Scholar]
- Liu, D.; Tao, T.; Xu, B.; Chen, S.; Liu, C.; Zhang, L.; Lu, K.; Huang, Y.; Jiang, L.; Zhang, X.; et al. MiR-361-5p acts as a tumor suppressor in prostate cancer by targeting signal transducer and activator of transcription-6(STAT6). Biochem. Biophys. Res. Commun. 2014, 445, 151–156. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, D.; Zhang, G.; Liang, Q.; Xiang, P.; Xu, Y.; Han, C.; Tao, T. miR-361-5p modulates metabolism and autophagy via the Sp1-mediated regulation of PKM2 in prostate cancer. Oncol. Rep. 2017, 38, 1621–1628. [Google Scholar] [CrossRef]
- Koutova, L.; Sterbova, M.; Pazourková, E.; Pospisilova, S.; Svobodová, I.; Horinek, A.; Lysak, D.; Korabečná, M. The impact of standard chemotherapy on miRNA signature in plasma in AML patients. Leuk. Res. 2015, 39, 1389–1395. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Kobayashi, N.; Todo, Y.; Watari, H. Exploring lncRNA-Mediated Regulatory Networks in Endometrial Cancer Cells and the Tumor Microenvironment: Advances and Challenges. Cancers 2019, 11, 234. [Google Scholar] [CrossRef]
- Wang, K.; Sun, T.; Li, N.; Wang, Y.; Wang, J.X.; Zhou, L.Y.; Long, B.; Liu, C.Y.; Liu, F.; Li, P.F. MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361. PLoS Genet. 2014, 10, e1004467. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Huang, X.; Guo, X.; Liu, Y.; Zhong, J.; Yuan, J. STAT3-induced upregulation of lncRNA MEG3 regulates the growth of cardiac hypertrophy through miR-361-5p/HDAC9 axis. Sci. Rep. 2019, 9, 460. [Google Scholar] [CrossRef]
- Ajiro, M.; Katagiri, T.; Ueda, K.; Nakagawa, H.; Fukukawa, C.; Lin, M.L.; Park, J.H.; Nishidate, T.; Daigo, Y.; Nakamura, Y. Involvement of RQCD1 overexpression, a novel cancer-testis antigen, in the Akt pathway in breast cancer cells. Int. J. Oncol. 2009, 35, 673–681. [Google Scholar]
- Wang, J.; Lu, Y.; Wang, J.; Koch, A.E.; Zhang, J.; Taichman, R.S. CXCR6 Induces Prostate Cancer Progression by the AKT/Mammalian Target of Rapamycin Signaling Pathway. Cancer Res. 2008, 68, 10367–10376. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Huo, W.; Zhao, G.; Yin, J.; Ouyang, X.; Wang, Y.; Yang, C.; Wang, B.; Dong, P.; Wang, Z.; Watari, H.; et al. Lentiviral CRISPR/Cas9 vector mediated miR-21 gene editing inhibits the epithelial to mesenchymal transition in ovarian cancer cells. J. Cancer 2017, 8, 57–64. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Watari, H.; Hanley, S.J.; Konno, Y.; Ihira, K.; Suzuki, F.; Yamada, T.; Kudo, M.; Yue, J.; et al. Suppression of iASPP-dependent aggressiveness in cervical cancer through reversal of methylation silencing of microRNA. Sci. Rep. 2016, 6, 35480. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef]
- Liao, G.B.; Li, X.Z.; Zeng, S.; Liu, C.; Yang, S.M.; Yang, L.; Hu, C.J.; Bai, J.Y. Regulation of the master regulator FOXM1 in cancer. Cell Commun. Signal. 2018, 16, 57. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, P.; Wang, X.-F. Microenvironmental regulation of cancer metastasis by miRNAs. Trends Cell Biol. 2014, 24, 153–160. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, S.; Lv, X.; Qiao, Y.; Liu, Y.J.; Chen, J. MicroRNAs: Pleiotropic Regulators in the Tumor Microenvironment. Front. Immunol. 2018, 9, 2491. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Marchat, L.A.; Gallardo-Rincón, D.; Ruiz-García, E.; Astudillo-De La Vega, H.; Echavarría-Zepeda, R.; López-Camarillo, C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review). Int. J. Mol. Med. 2019, 43, 657–670. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef]
- Palena, C.; Hamilton, D.H.; Fernando, I.R. Influence of IL-8 on the epithelial–mesenchymal transition and the tumor microenvironment. Futur. Oncol. 2012, 8, 713–722. [Google Scholar] [CrossRef]
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- León-Cabrera, S.; Molina-Guzman, E.; Delgado-Ramírez, Y.; Vazquez-Sandoval, A.; Ledesma-Soto, Y.; Perez-Plasencia, C.; Chirino, I.Y.; Delgado-Buenrostro, N.L.; Rodriguez-Sosa, M.; Vaca-Paniagua, F.; et al. Lack of STAT6 Attenuates Inflammation and Drives Protection against Early Steps of Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2017, 5, 385–396. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Scholl, V.; Hassan, R.; Zalcberg, I.R. miRNA-451: A putative predictor marker of Imatinib therapy response in chronic myeloid leukemia. Leuk. Res. 2012, 36, 119–121. [Google Scholar] [CrossRef]
- Sebio, A.; Paré, L.; Páez, D.; Salazar, J.; González, A.; Sala, N.; del Río, E.; Martín-Richard, M.; Tobeña, M.; Barnadas, A. The LCS6 polymorphism in the binding site of let-7 microRNA to the KRAS 3-untranslated region: Its role in the efficacy of anti-EGFR-based therapy in metastatic colorectal cancer patients. Pharm. Genom. 2013, 23, 142–147. [Google Scholar] [CrossRef]
- Pardini, B.; Rosa, F.; Barone, E.; Di Gaetano, C.; Slyskova, J.; Novotny, J.; Levy, M.; Garritano, S.; Vodickova, L.; Buchler, T.; et al. Variation within 3-UTRs of Base Excision Repair Genes and Response to Therapy in Colorectal Cancer Patients: A Potential Modulation of microRNAs Binding. Clin. Cancer Res. 2013, 19, 6044–6056. [Google Scholar] [CrossRef]
- Zhuang, Z.L.; Tian, F.M.; Sun, C.L. Downregulation of miR-361-5p associates with aggressive clinicopathological features and unfavorable prognosis in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5132–5136. [Google Scholar]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Kelly, R.; Newell, J.; Kerin, M.J. Systemic miRNA-195 Differentiates Breast Cancer from Other Malignancies and Is a Potential Biomarker for Detecting Noninvasive and Early Stage Disease. Oncologist 2010, 15, 673–682. [Google Scholar] [CrossRef]
- Cuk, K.; Zucknick, M.; Madhavan, D.; Schott, S.; Golatta, M.; Heil, J.; Marmé, F.; Turchinovich, A.; Sinn, P.; Sohn, C.; et al. Plasma MicroRNA Panel for Minimally Invasive Detection of Breast Cancer. PLoS ONE 2013, 8, e76729. [Google Scholar] [CrossRef]
- Inns, J.; James, V. Circulating microRNAs for the prediction of metastasis in breast cancer patients diagnosed with early stage disease. Breast 2015, 24, 364–369. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L.; et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non–Small Cell Lung Cancer Using Next-Generation Sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, H.C.; Chanock, S.J. SNPs in cancer research and treatment. Br. J. Cancer 2004, 90, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Alsarraj, J.; Hunter, K. Understanding susceptibility to breast cancer metastasis: The genetic approach. Breast Cancer Manag. 2014, 3, 165–172. [Google Scholar] [CrossRef]
- Deng, N.; Zhou, H.; Fan, H.; Yuan, Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget 2017, 8, 110635–110649. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, C.; Wang, H.; Li, B.; Guo, Z. A miR-SNP of the XPO5 gene is associated with advanced non-small-cell lung cancer. OncoTargets Ther. 2013, 6, 877–881. [Google Scholar]
- Ziebarth, J.D.; Bhattacharya, A.; Cui, Y. Integrative Analysis of Somatic Mutations Altering MicroRNA Targeting in Cancer Genomes. PLoS ONE 2012, 7, e47137. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Ding, X.; Zhuo, Y.; Ren, P.; Zhou, C.; Zhou, J. A miR-200b/200c/429-Binding Site Polymorphism in the 3′ Untranslated Region of the AP-2α Gene Is Associated with Cisplatin Resistance. PLoS ONE 2011, 6, e29043. [Google Scholar] [CrossRef]
- Wu, R.; Li, F.; Zhu, J.; Tang, R.; Qi, Q.; Zhou, X.; Li, R.; Wang, W.; Hua, D.; Chen, W. A functional variant at miR-132-3p, miR-212-3p, and miR-361-5p binding site in CD80 gene alters susceptibility to gastric cancer in a Chinese Han population. Med. Oncol. 2014, 31, 60. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Dong, P.; Xiong, Y.; Yue, J.; Ihira, K.; Konno, Y.; Kobayashi, N.; Todo, Y.; Watari, H. MicroRNA-361: A Multifaceted Player Regulating Tumor Aggressiveness and Tumor Microenvironment Formation. Cancers 2019, 11, 1130. https://doi.org/10.3390/cancers11081130
Xu D, Dong P, Xiong Y, Yue J, Ihira K, Konno Y, Kobayashi N, Todo Y, Watari H. MicroRNA-361: A Multifaceted Player Regulating Tumor Aggressiveness and Tumor Microenvironment Formation. Cancers. 2019; 11(8):1130. https://doi.org/10.3390/cancers11081130
Chicago/Turabian StyleXu, Daozhi, Peixin Dong, Ying Xiong, Junming Yue, Kei Ihira, Yosuke Konno, Noriko Kobayashi, Yukiharu Todo, and Hidemichi Watari. 2019. "MicroRNA-361: A Multifaceted Player Regulating Tumor Aggressiveness and Tumor Microenvironment Formation" Cancers 11, no. 8: 1130. https://doi.org/10.3390/cancers11081130
APA StyleXu, D., Dong, P., Xiong, Y., Yue, J., Ihira, K., Konno, Y., Kobayashi, N., Todo, Y., & Watari, H. (2019). MicroRNA-361: A Multifaceted Player Regulating Tumor Aggressiveness and Tumor Microenvironment Formation. Cancers, 11(8), 1130. https://doi.org/10.3390/cancers11081130







