Isoliquiritigenin Suppresses E2-Induced Uterine Leiomyoma Growth through the Modulation of Cell Death Program and the Repression of ECM Accumulation
Abstract
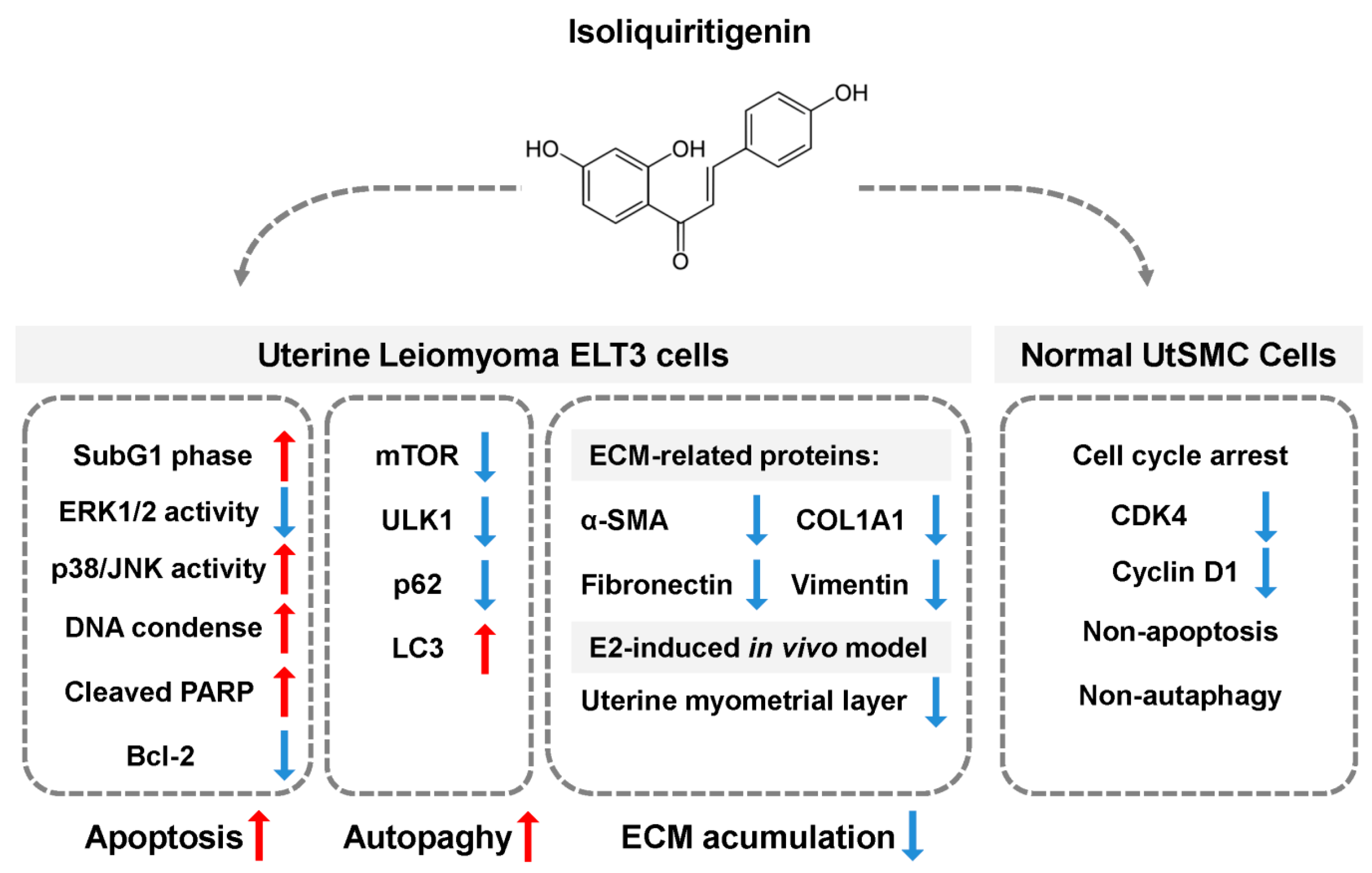
1. Introduction
2. Results
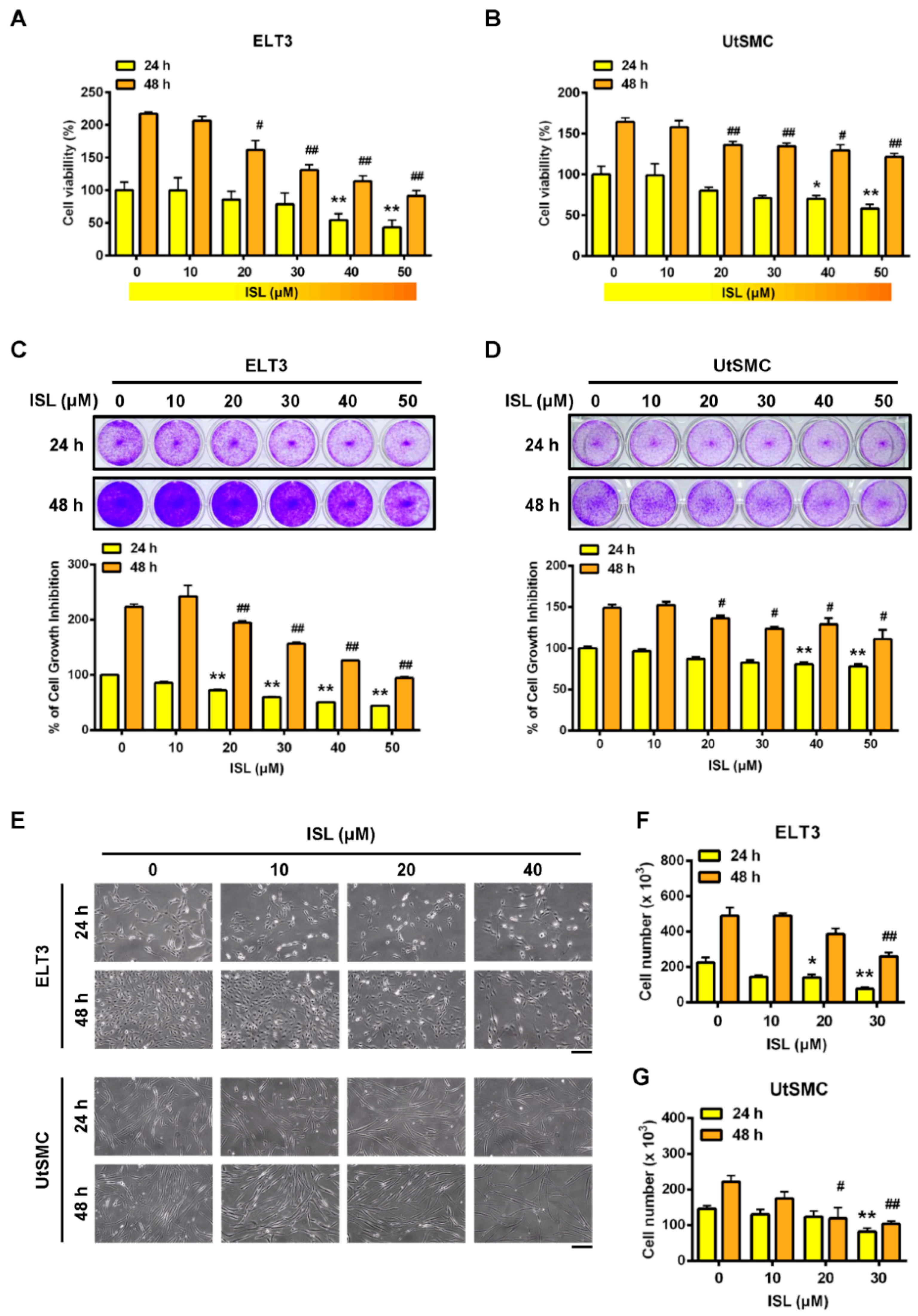
2.1. Inhibition of Cell Viability in ELT3 and Uterine Smooth Muscle (UtSMC) Cells by ISL
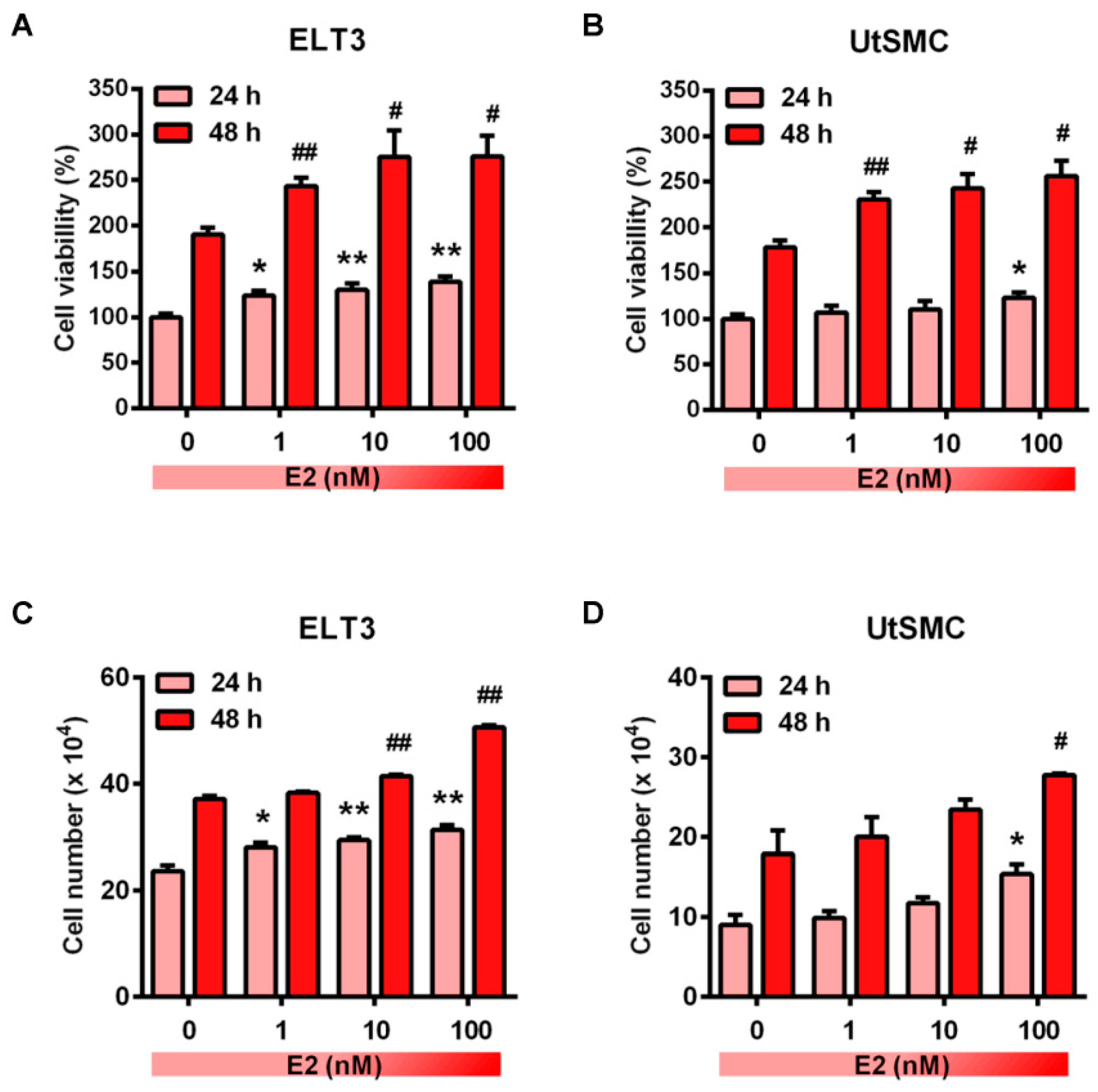
2.2. Effects of ISL Treatment on E2-Induced Cell Proliferation in ELT3 and UtSMC Cells
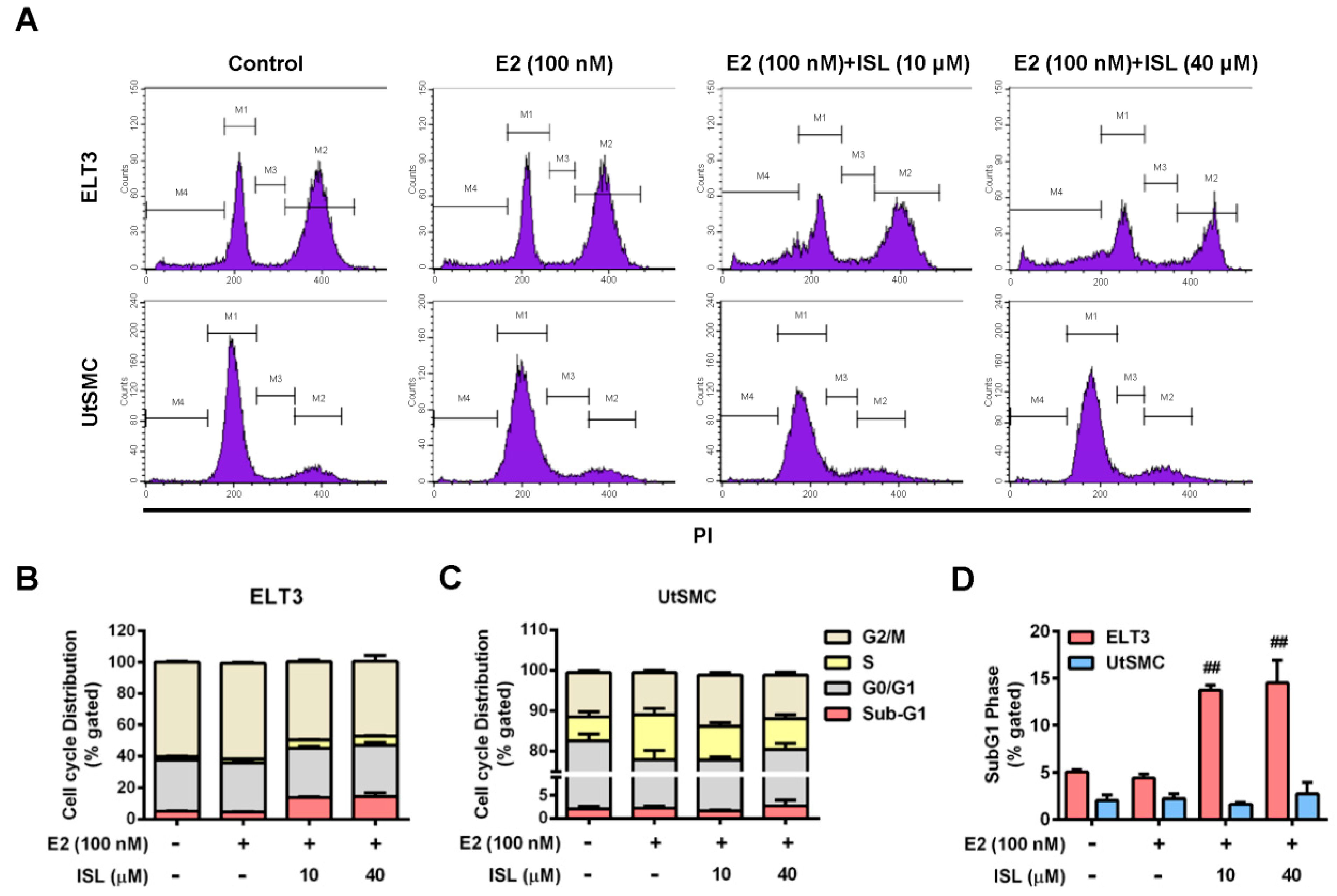
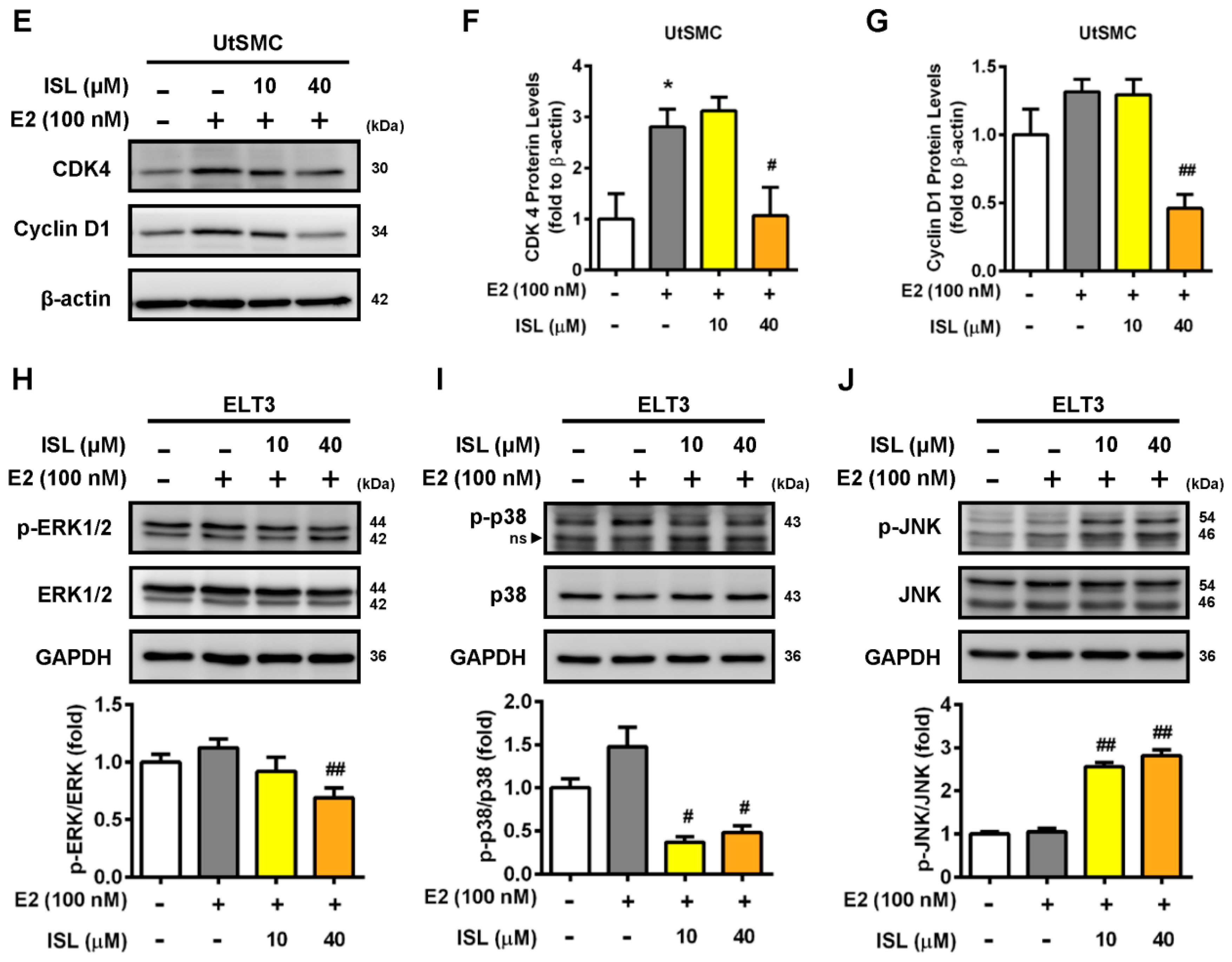
2.3. Effects of ISL Treatment on the Cell Cycle Progression of ELT3 and UtSMC Cells
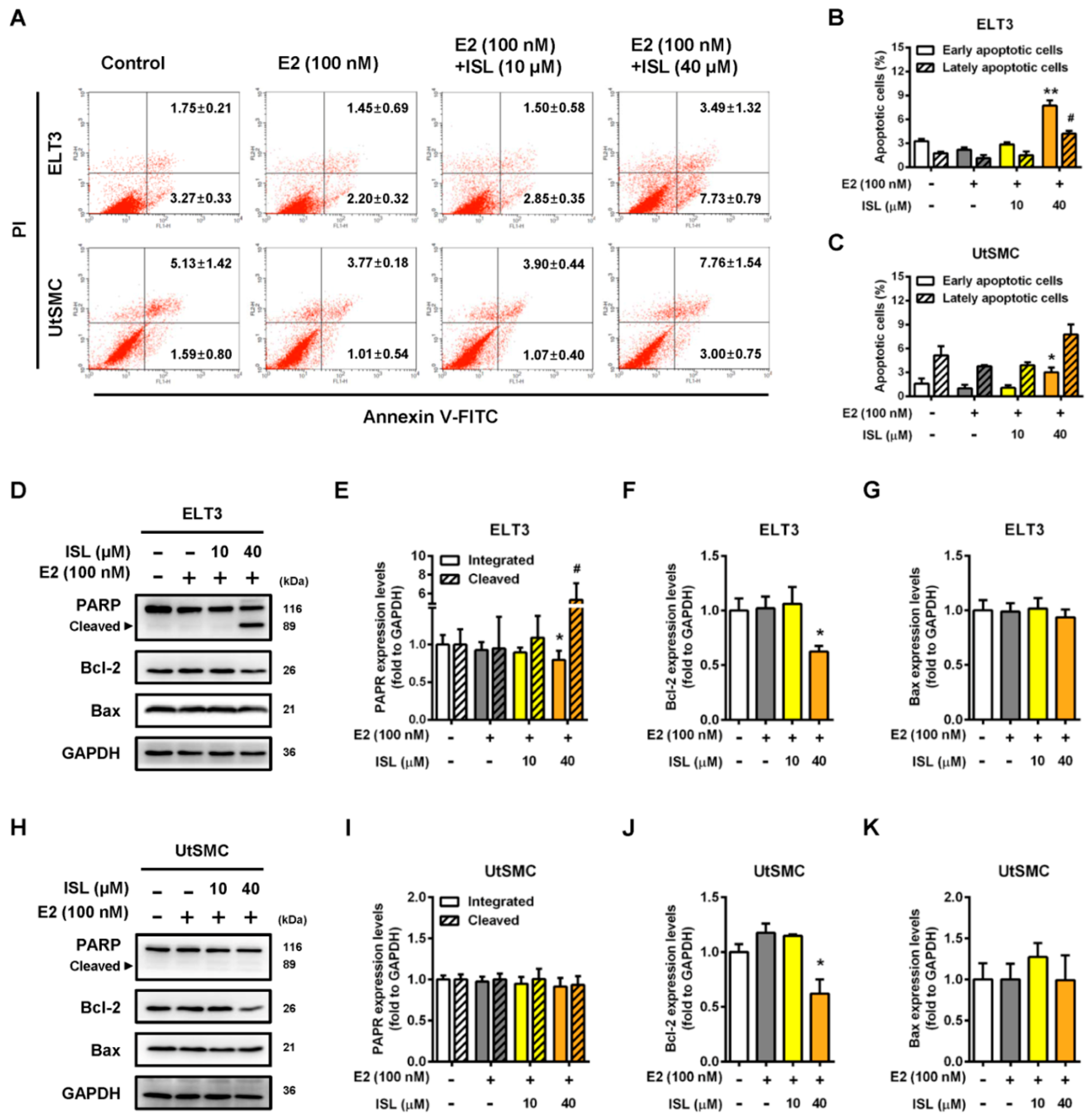
2.4. Effects of ISL Treatment on Apoptosis and Levels of Apoptosis-Associated Proteins in ELT3 and UtSMC Cells
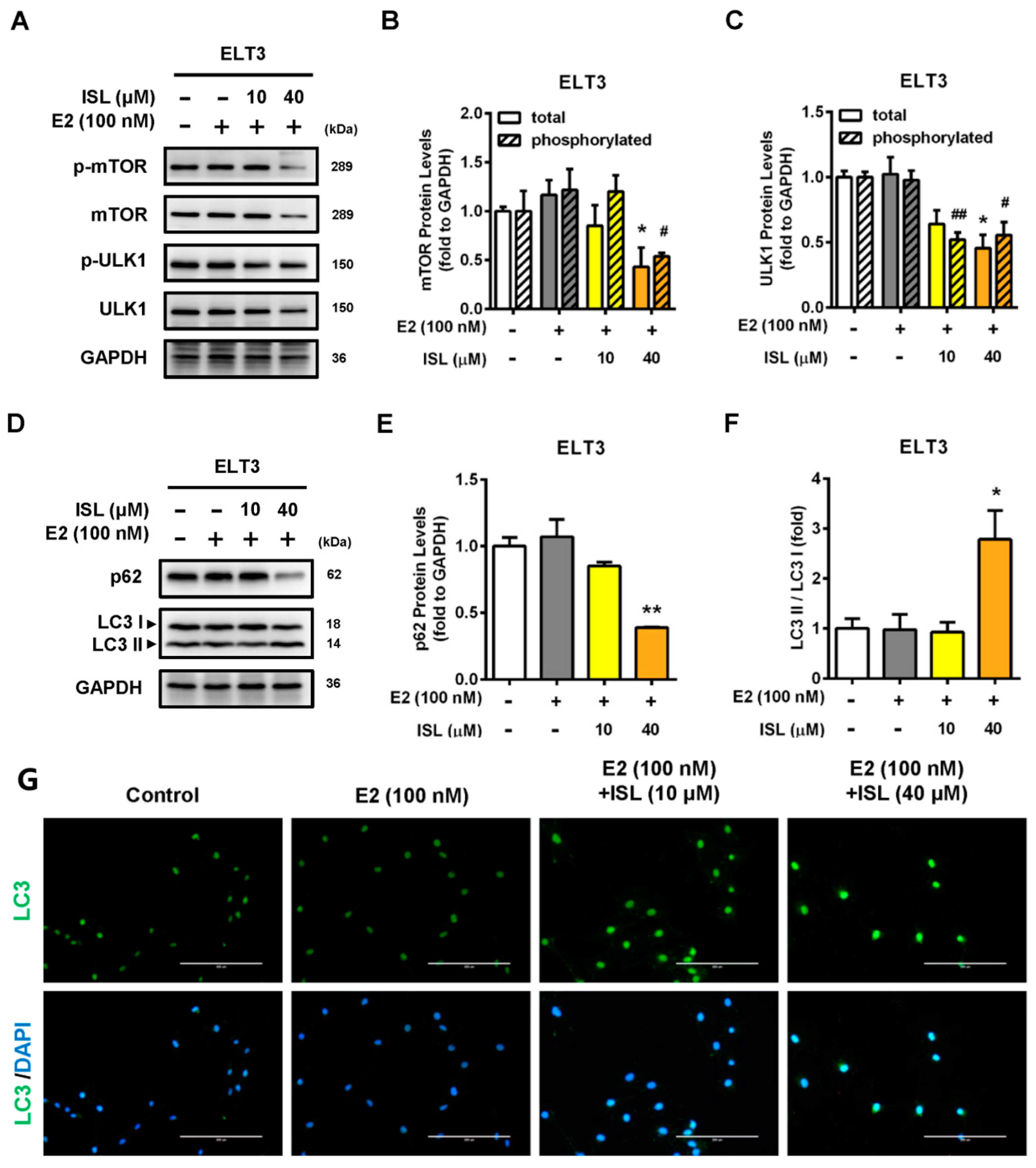
2.5. Effects of ISL Treatment on Levels of Autophagy-Associated Proteins in ELT3 Cells
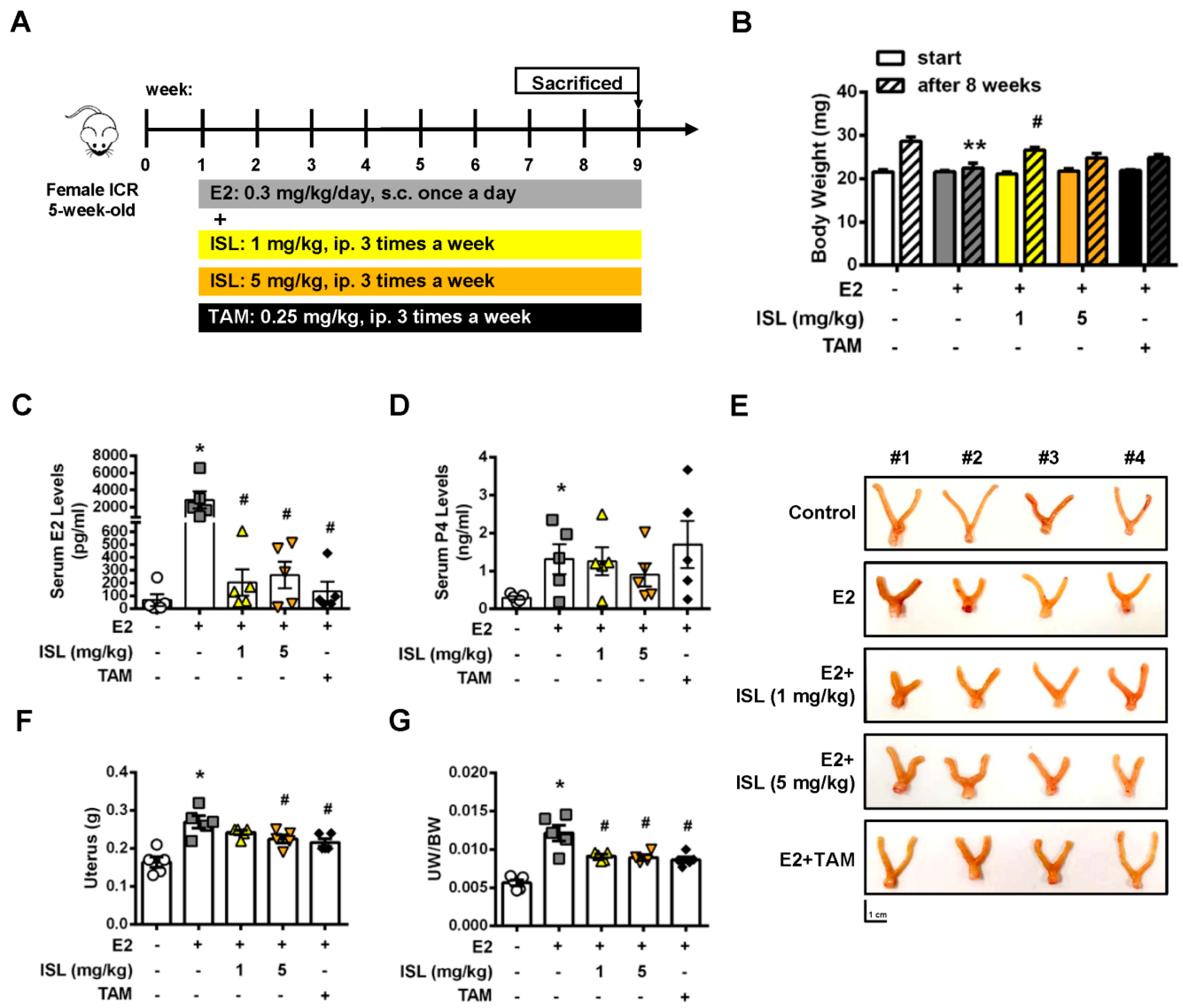
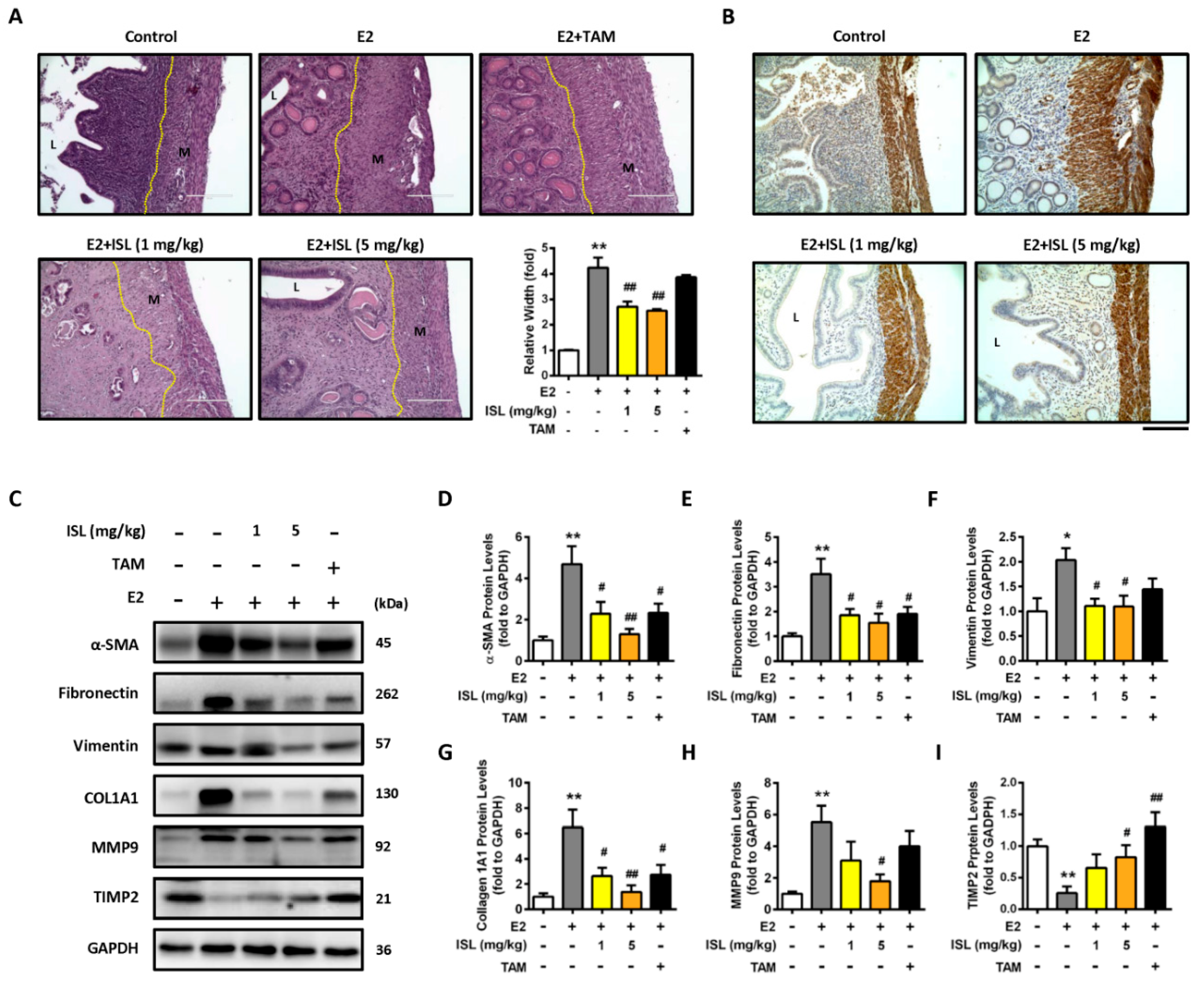
2.6. Inhibition of E2-Induced Uterine Myometrium Growth by Co-Treatment with ISL In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Cell Viability Assay
4.3. Crystal Violet Staining
4.4. Cell Counting
4.5. Flow Cytometry Analysis of Cell Cycle Distribution and Apoptosis
4.6. Hoechst 33342 Staining
4.7. Protein Preparation and Western Blot Analysis
4.8. Immunofluorescent Staining
4.9. Animal Experiments
4.10. ELISA of Serum Estradiol and Progesterone
4.11. H&E Staining
4.12. Immunohistochemistry (IHC) Staining
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.D.; Malik, M.; Britten, J.; San Pablo, A.M.; Catherino, W.H. A Comprehensive Review of the Pharmacologic Management of Uterine Leiomyoma. Biomed. Res. Int. 2018, 2018, 2414609. [Google Scholar] [CrossRef] [PubMed]
- Sparic, R.; Mirkovic, L.; Malvasi, A.; Tinelli, A. Epidemiology of Uterine Myomas: A Review. Int. J. Fertil. Steril. 2016, 9, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.K.; Schroeder, J.C.; Baird, D.D. New directions in the epidemiology of uterine fibroids. Semin. Reprod. Med. 2010, 28, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obs. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.M.; Spiegelman, D.; Barbieri, R.L.; Goldman, M.B.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obs. Gynecol. 1997, 90, 967–973. [Google Scholar] [CrossRef]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Okolo, S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract. Res. Clin. Obs. Gynaecol. 2008, 22, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.; Famuyide, A.O.; Erwin, P.J.; Stewart, E.A.; Laughlin-Tommaso, S.K. Submucosal fibroids and the relation to heavy menstrual bleeding and anemia. Am. J. Obs. Gynecol. 2014, 210, 38.e1–38.e7. [Google Scholar] [CrossRef]
- De La Cruz, M.S.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar]
- Bukulmez, O.; Doody, K.J. Clinical features of myomas. Obs. Gynecol. Clin. N. Am. 2006, 33, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Sapia, F.; Rapisarda, A.M.C.; Valenti, G.; Santangelo, F.; Rossetti, D.; Chiofalo, B.; Sarpietro, G.; La Rosa, V.L.; Triolo, O.; et al. Hysteroscopic Morcellation of Submucous Myomas: A Systematic Review. Biomed. Res. Int. 2017, 2017, 6848250. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Alonso Pacheco, L.; Tinelli, A.; Haimovich, S.; Carugno, J.; Ghezzi, F.; Mazzon, I.; Bettocchi, S. Management of Asymptomatic Submucous Myomas in Women of Reproductive Age: A Consensus Statement from the Global Congress on Hysteroscopy Scientific Committee. J. Minim. Invasive Gynecol. 2019, 26, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.R.; Novetsky, A.P.; Curtin, J.P. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obs. Gynecol. 2010, 116, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.; Sizzi, O.; Soranna, L.; Cucinelli, F.; Mancuso, S.; Lanzone, A. Long-term results of laparoscopic myomectomy: Recurrence rate in comparison with abdominal myomectomy. Hum. Reprod. 2001, 16, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelkar, S.S.; Winkel, C. The direct and indirect costs of uterine fibroid tumors: A systematic review of the literature between 2000 and 2013. Am. J. Obs. Gynecol. 2015, 213, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Tropea, A.; Rossetti, D.; Carnelli, M.; Cianci, A. Management of uterine leiomyomas in pregnancy: Review of literature. Updates Surg. 2013, 65, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Padula, F.; Gulino, F.A. Management of uterine fibroids in pregnancy: Recent trends. Curr. Opin. Obs. Gynecol. 2015, 27, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Noventa, M.; Di Spiezio Sardo, A.; Saccone, G.; Gizzo, S.; Borgato, S.; Vitale, S.G.; Lagana, A.S.; Nardelli, G.B.; Litta, P.S.; et al. Uterine fibroid size modifications during pregnancy and puerperium: Evidence from the first systematic review of literature. Arch. Gynecol. Obs. 2018, 297, 823–835. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, H.; Lee, Y.; Lee, Y.-I. Phytochemical and Pharmacological Role of Liquiritigenin and Isoliquiritigenin from Radix Glycyrrhizae in Human Health and Disease Models. Front. Aging Neurosci. 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A Review: The Pharmacology of Isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Denzer, I.; Munch, G.; Pischetsrieder, M.; Friedland, K. S-allyl-L-cysteine and isoliquiritigenin improve mitochondrial function in cellular models of oxidative and nitrosative stress. Food Chem. 2016, 194, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Santos, J.; Grenier, D. Comparative evaluation of two structurally related flavonoids, isoliquiritigenin and liquiritigenin, for their oral infection therapeutic potential. J. Nat. Prod. 2011, 74, 1862–1867. [Google Scholar] [CrossRef]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, P.; Zhang, X.; Ma, Y.; Li, W.; Chen, J.M.; Guo, H.M.; Bucala, R.; Zhuang, J.; Li, J. Natural antioxidant-isoliquiritigenin ameliorates contractile dysfunction of hypoxic cardiomyocytes via AMPK signaling pathway. Mediat. Inflamm. 2013, 2013, 390890. [Google Scholar] [CrossRef]
- Liao, M.; Zhao, Y.; Huang, L.; Cheng, B.; Huang, K. Isoliquiritigenin and liquiritin from Glycyrrhiza uralensis inhibit α-synuclein amyloid formation. RSC Adv. 2016, 6, 86640–86649. [Google Scholar] [CrossRef]
- Hwang, C.K.; Chun, H.S. Isoliquiritigenin isolated from licorice Glycyrrhiza uralensis prevents 6-hydroxydopamine-induced apoptosis in dopaminergic neurons. Biosci. Biotechnol. Biochem. 2012, 76, 536–543. [Google Scholar] [CrossRef]
- Link, P.; Wetterauer, B.; Fu, Y.; Wink, M. Extracts of Glycyrrhiza uralensis and isoliquiritigenin counteract amyloid-beta toxicity in Caenorhabditis elegans. Planta Med. 2015, 81, 357–362. [Google Scholar] [CrossRef]
- Ma, J.; Fu, N.Y.; Pang, D.B.; Wu, W.Y.; Xu, A.L. Apoptosis induced by isoliquiritigenin in human gastric cancer MGC-803 cells. Planta Med. 2001, 67, 754–757. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lim, D.Y.; Choi, H.J.; Jung, J.I.; Chung, W.Y.; Park, J.H. Induction of cell cycle arrest in prostate cancer cells by the dietary compound isoliquiritigenin. J. Med. Food 2009, 12, 8–14. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, M.H.; Lim, D.Y.; Kim, J.E.; Singh, P.; Lee, S.Y.; Jeong, C.H.; Lim, T.G.; Chen, H.; Chi, Y.I.; et al. Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J. Biol. Chem. 2014, 289, 35839–35848. [Google Scholar] [CrossRef]
- Lee, Y.M.; Jeong, G.S.; Lim, H.D.; An, R.B.; Kim, Y.C.; Kim, E.C. Isoliquiritigenin 2′-methyl ether induces growth inhibition and apoptosis in oral cancer cells via heme oxygenase-1. Toxicol. Vitr. 2010, 24, 776–782. [Google Scholar] [CrossRef]
- Hsia, S.M.; Yu, C.C.; Shih, Y.H.; Chen, M.Y.; Wang, T.H.; Huang, Y.T.; Shieh, T.M. Isoliquiritigenin as a cause of DNA damage and inhibitor of ataxia-telangiectasia mutated expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinoma. Head Neck 2016, 38, E360–E371. [Google Scholar] [CrossRef]
- Wang, K.L.; Hsia, S.M.; Chan, C.J.; Chang, F.Y.; Huang, C.Y.; Bau, D.T.; Wang, P.S. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin. Ther. Targets 2013, 17, 337–349. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, T.C.; Shieh, T.M.; Wu, C.H.; Lin, L.C.; Hsia, S.M. Isoliquiritigenin Induces Autophagy and Inhibits Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2017, 18, 2025. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, H.Y.; Wang, C.W.; Shieh, T.M.; Huang, T.C.; Lin, L.C.; Wang, K.L.; Hsia, S.M. Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget 2016, 7, 73432–73447. [Google Scholar] [CrossRef]
- Lin, L.-C.; Wu, C.-H.; Shieh, T.-M.; Chen, H.-Y.; Huang, T.-C.; Hsia, S.-M. The licorice dietary component isoliquiritigenin chemosensitizes human uterine sarcoma cells to doxorubicin and inhibits cell growth by inducing apoptosis and autophagy via inhibition of m-TOR signaling. J. Funct. Foods 2017, 33, 332–344. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vergara, D.; Favilli, A.; La Rosa, V.L.; Tinelli, A.; Gerli, S.; Noventa, M.; Vitagliano, A.; Triolo, O.; Rapisarda, A.M.C.; et al. Epigenetic and genetic landscape of uterine leiomyomas: A current view over a common gynecological disease. Arch. Gynecol. Obstet. 2017, 296, 855–867. [Google Scholar] [CrossRef]
- Yang, Q.; Mas, A.; Diamond, M.P.; Al-Hendy, A. The Mechanism and Function of Epigenetics in Uterine Leiomyoma Development. Reprod. Sci. 2016, 23, 163–175. [Google Scholar] [CrossRef]
- Segars, J.H.; Parrott, E.C.; Nagel, J.D.; Guo, X.C.; Gao, X.; Birnbaum, L.S.; Pinn, V.W.; Dixon, D. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: Comprehensive review, conference summary and future recommendations. Hum. Reprod. Update 2014, 20, 309–333. [Google Scholar] [CrossRef]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update 2004, 10, 207–220. [Google Scholar] [CrossRef]
- Ishikawa, H.; Reierstad, S.; Demura, M.; Rademaker, A.W.; Kasai, T.; Inoue, M.; Usui, H.; Shozu, M.; Bulun, S.E. High aromatase expression in uterine leiomyoma tissues of African-American women. J. Clin. Endocrinol. Metab. 2009, 94, 1752–1756. [Google Scholar] [CrossRef]
- Shozu, M.; Sumitani, H.; Segawa, T.; Yang, H.J.; Murakami, K.; Kasai, T.; Inoue, M. Overexpression of aromatase P450 in leiomyoma tissue is driven primarily through promoter I.4 of the aromatase P450 gene (CYP19). J. Clin. Endocrinol. Metab. 2002, 87, 2540–2548. [Google Scholar] [CrossRef][Green Version]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef]
- Marquez, R.T.; Xu, L. Bcl-2:Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar]
- Decuypere, J.-P.; Parys, J.B.; Bultynck, G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells 2012, 1, 284–312. [Google Scholar] [CrossRef]
- Walker, C.L.; Stewart, E.A. Uterine fibroids: The elephant in the room. Science 2005, 308, 1589–1592. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Bioactivity and potential health benefits of licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; Liu, C.; Wu, Y.; Liu, Y.; Cai, Y. Inhibitory effect of liquiritigenin on migration via downregulation proMMP-2 and PI3K/Akt signaling pathway in human lung adenocarcinoma A549 cells. Nutr. Cancer 2012, 64, 627–634. [Google Scholar] [CrossRef]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen Receptors and Signaling in Fibroids: Role in Pathobiology and Therapeutic Implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef]
- Benassayag, C.; Leroy, M.J.; Rigourd, V.; Robert, B.; Honore, J.C.; Mignot, T.M.; Vacher-Lavenu, M.C.; Chapron, C.; Ferre, F. Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: Pregnancy and leiomyoma. Am. J. Physiol. 1999, 276, E1112–E1118. [Google Scholar] [CrossRef]
- Brandon, D.D.; Bethea, C.L.; Strawn, E.Y.; Novy, M.J.; Burry, K.A.; Harrington, M.S.; Erickson, T.E.; Warner, C.; Keenan, E.J.; Clinton, G.M. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am. J. Obs. Gynecol. 1993, 169, 78–85. [Google Scholar] [CrossRef]
- Andersen, J.; DyReyes, V.M.; Barbieri, R.L.; Coachman, D.M.; Miksicek, R.J. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J. Soc. Gynecol. Investig. 1995, 2, 542–551. [Google Scholar] [CrossRef]
- Maggiolini, M.; Statti, G.; Vivacqua, A.; Gabriele, S.; Rago, V.; Loizzo, M.; Menichini, F.; Amdo, S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 82, 315–322. [Google Scholar] [CrossRef]
- Blake, R.E. Leiomyomata uteri: Hormonal and molecular determinants of growth. J. Natl. Med. Assoc. 2007, 99, 1170–1184. [Google Scholar]
- Templeman, C.; Marshall, S.F.; Clarke, C.A.; Henderson, K.D.; Largent, J.; Neuhausen, S.; Reynolds, P.; Ursin, G.; Bernstein, L. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil. Steril. 2009, 92, 1436–1446. [Google Scholar] [CrossRef]
- Park, I.; Park, K.K.; Park, J.H.; Chung, W.Y. Isoliquiritigenin induces G2 and M phase arrest by inducing DNA damage and by inhibiting the metaphase/anaphase transition. Cancer Lett. 2009, 277, 174–181. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Kim, D.C.; Ramachandran, S.; Baek, S.H.; Kwon, S.H.; Kwon, K.Y.; Cha, S.D.; Bae, I.; Cho, C.H. Induction of growth inhibition and apoptosis in human uterine leiomyoma cells by isoliquiritigenin. Reprod. Sci. 2008, 15, 552–558. [Google Scholar] [CrossRef]
- Chen, G.; Hu, X.; Zhang, W.; Xu, N.; Wang, F.Q.; Jia, J.; Zhang, W.F.; Sun, Z.J.; Zhao, Y.F. Mammalian target of rapamycin regulates isoliquiritigenin-induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis 2012, 17, 90–101. [Google Scholar] [CrossRef]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, T.C.; Lin, L.C.; Shieh, T.M.; Wu, C.H.; Wang, K.L.; Hong, Y.H.; Hsia, S.M. Fucoidan Inhibits the Proliferation of Leiomyoma Cells and Decreases Extracellular Matrix-Associated Protein Expression. Cell. Physiol. Biochem. 2018, 49, 1970–1986. [Google Scholar] [CrossRef]
- Bogusiewicz, M.; Stryjecka-Zimmer, M.; Postawski, K.; Jakimiuk, A.J.; Rechberger, T. Activity of matrix metalloproteinase-2 and-9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol. Endocrinol. 2007, 23, 541–546. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-H.; Kung, H.-L.; Chen, H.-Y.; Huang, K.-C.; Hsia, S.-M. Isoliquiritigenin Suppresses E2-Induced Uterine Leiomyoma Growth through the Modulation of Cell Death Program and the Repression of ECM Accumulation. Cancers 2019, 11, 1131. https://doi.org/10.3390/cancers11081131
Lin P-H, Kung H-L, Chen H-Y, Huang K-C, Hsia S-M. Isoliquiritigenin Suppresses E2-Induced Uterine Leiomyoma Growth through the Modulation of Cell Death Program and the Repression of ECM Accumulation. Cancers. 2019; 11(8):1131. https://doi.org/10.3390/cancers11081131
Chicago/Turabian StyleLin, Po-Han, Hsiang-Lin Kung, Hsin-Yuan Chen, Ko-Chieh Huang, and Shih-Min Hsia. 2019. "Isoliquiritigenin Suppresses E2-Induced Uterine Leiomyoma Growth through the Modulation of Cell Death Program and the Repression of ECM Accumulation" Cancers 11, no. 8: 1131. https://doi.org/10.3390/cancers11081131
APA StyleLin, P.-H., Kung, H.-L., Chen, H.-Y., Huang, K.-C., & Hsia, S.-M. (2019). Isoliquiritigenin Suppresses E2-Induced Uterine Leiomyoma Growth through the Modulation of Cell Death Program and the Repression of ECM Accumulation. Cancers, 11(8), 1131. https://doi.org/10.3390/cancers11081131





