A Systematic Review of Phase II Targeted Therapy Clinical Trials in Anaplastic Thyroid Cancer
Abstract
1. Introduction
1.1. Clinical Presentation and Histopathology
1.2. Pathogenesis
1.3. Molecular Landscape of ATC
1.4. Treatment
2. Results
2.1. Efficacy Results of Phase II Clinical Trials
2.2. Multikinase Inhibitors
2.3. BRAF V600E Inhibitors
2.4. PI3K/mTOR Inhibitors
2.5. EGFR Inhibitors
2.6. Selective VEGR Inhibitors
2.7. Vascular-Targeting Agents
2.8. Follow-Up and Treatment Discontinuation
3. Adverse Events
4. Discussion
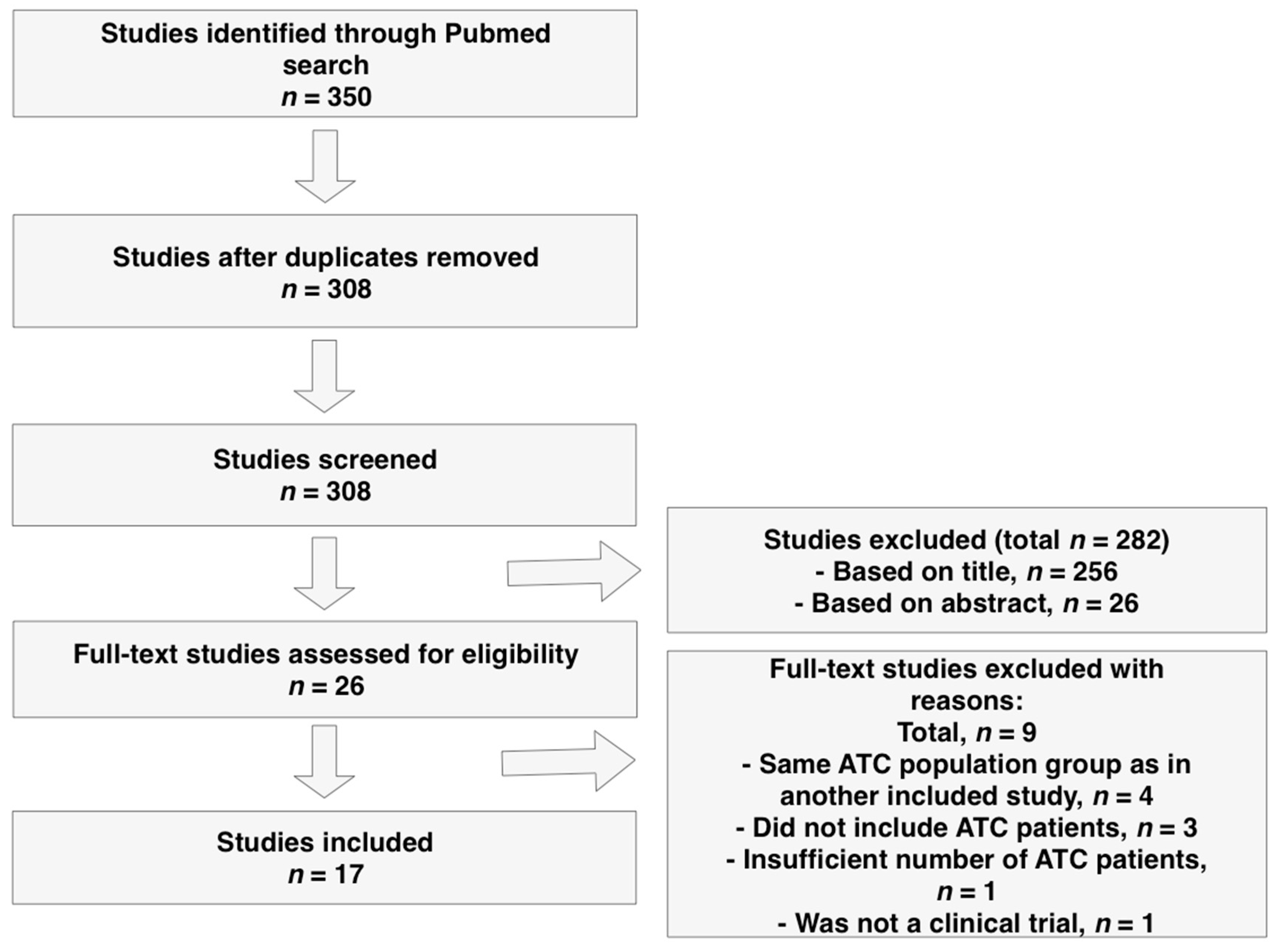
5. Materials and Methods
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Neff, R.L.; Farrar, W.B.; Kloos, R.T.; Burman, K.D. Anaplastic Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 525–538. [Google Scholar] [CrossRef]
- Smallridge, R.; Copland, J. Anaplastic Thyroid Carcinoma: Pathogenesis and Emerging Therapies. Clin. Oncol. 2010, 22, 486–497. [Google Scholar] [CrossRef]
- Nagaiah, G.; Hossain, A.; Mooney, C.J.; Parmentier, J.; Remick, S.C. Anaplastic Thyroid Cancer: A Review of Epidemiology, Pathogenesis, and Treatment. J. Oncol. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Brignardello, E.; Palestini, N.; Felicetti, F.; Castiglione, A.; Piovesan, A.; Gallo, M.; Freddi, M.; Ricardi, U.; Gasparri, G.; Ciccone, G.; et al. Early Surgery and Survival of Patients with Anaplastic Thyroid Carcinoma: Analysis of a Case Series Referred to a Single Institution Between 1999 and 2012. Thyroid 2014, 24, 1600–1606. [Google Scholar] [CrossRef]
- Prasongsook, N.; Kumar, A.; Chintakuntlawar, A.V.; Foote, R.L.; Kasperbauer, J.; Molina, J.; Garces, Y.; Ma, D.; Richardson, R.; Hay, I.; et al. Survival in Response to Multimodal Therapy in Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2017, 102, 4506–4514. [Google Scholar] [CrossRef]
- Wendler, J.; Kroiss, M.; Gast, K.; Kreissl, M.C.; Allelein, S.; Lichtenauer, U.; Blaser, R.; Spitzweg, C.; Fassnacht, M.; Schott, M.; et al. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: Results of a multicenter study in Germany. Eur. J. Endocrinol. 2016, 175, 521–529. [Google Scholar] [CrossRef]
- Ranganath, R.; Shah, M.A.; Shah, A.R. Anaplastic thyroid cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 387–391. [Google Scholar] [CrossRef]
- O’Neill, J.P.; Shaha, A.R. Anaplastic thyroid cancer. Oral Oncol. 2013, 49, 702–706. [Google Scholar] [CrossRef]
- Hardin, H.; Zhang, R.; Helein, H.; Buehler, D.; Guo, Z.; Lloyd, R.V. The evolving concept of cancer stem-like cells in thyroid cancer and other solid tumors. Lab. Investig. 2017, 97, 1142–1151. [Google Scholar] [CrossRef]
- Guo, Z.; Hardin, H.; Lloyd, R.V. Cancer stem-like cells and thyroid cancer. Endocr. Relat. Cancer 2014, 21, 285–300. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef]
- Caillou, B.; Talbot, M.; Weyemi, U.; Pioche-Durieu, C.; Al Ghuzlan, A.; Bidart, J.M.; Chouaib, S.; Schlumberger, M.; Dupuy, C. Tumor-Associated Macrophages (TAMs) Form an Interconnected Cellular Supportive Network in Anaplastic Thyroid Carcinoma. PLoS ONE 2011, 6, e22567. [Google Scholar] [CrossRef]
- Tennvall, J.; Lundell, G.; Wahlberg, P.; Bergenfelz, A.; Grimelius, L.; Akerman, M.; Skog, A.-L.H.; Wallin, G. Anaplastic thyroid carcinoma: Three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. Br. J. Cancer 2002, 86, 1848–1853. [Google Scholar] [CrossRef]
- Haigh, P.I.; Ituarte, P.H.G.; Wu, H.S.; Treseler, P.A.; Posner, M.D.; Quivey, J.M.; Duh, Q.Y.; Clark, O.H. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer 2001, 91, 2335–2342. [Google Scholar] [CrossRef]
- Derbel, O.; Limem, S.; Ségura-Ferlay, C.; Lifante, J.-C.; Carrie, C.; Peix, J.-L.; Borson-Chazot, F.; Bournaud, C.; Droz, J.-P.; De La Fouchardière, C. Results of combined treatment of anaplastic thyroid carcinoma (ATC). BMC Cancer 2011, 11, 469. [Google Scholar] [CrossRef]
- Hundahl, S.A.; Cady, B.; Cunningham, M.P.; Mazzaferri, E.; McKee, R.F.; Rosai, J.; Shah, J.P.; Fremgen, A.M.; Stewart, A.K.; for the U.S. and German Thyroid Cancer Study Group. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. Cancer 2000, 89, 202–217. [Google Scholar] [CrossRef]
- Steggink, L.C.; Van Dijk, B.A.; Links, T.P.; Plukker, J.T. Survival in anaplastic thyroid cancer in relation to pre-existing goiter: A population-based study. Am. J. Surg. 2015, 209, 1013–1019. [Google Scholar] [CrossRef]
- Aldinger, K.A.; Samaan, N.A.; Ibanez, M.; Hill, C.S. Anaplastic carcinoma of the thyroid. A review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer 1978, 41, 2267–2275. [Google Scholar] [CrossRef]
- Carcangiu, M.L.; Steeper, T.; Zampi, G.; Rosai, J. Anaplastic thyroid carcinoma. A study of 70 cases. Am. J. Clin. Pathol. 1985, 83, 135–158. [Google Scholar] [CrossRef]
- Venkatesh, Y.S.S.; Ordòñez, N.G.; Schultz, P.N.; Hickey, R.C.; Goepfert, H.; Samaan, N.A. Anaplastic carcinoma of the thyroid: A clinicopathologic study of 121 cases. Cancer 1990, 66, 321–330. [Google Scholar] [CrossRef]
- Nishiyama, R.H.; Dunn, E.L.; Thompson, N.W. Anaplastic spindle-cell and giant-cell tumors of the thyroid gland. Cancer 1972, 30, 113–127. [Google Scholar] [CrossRef]
- McIver, B.; Hay, I.D.; Giuffrida, D.F.; Dvorak, C.E.; Grant, C.S.; Thompson, G.B.; Van Heerden, J.A.; Goellner, J.R. Anaplastic thyroid carcinoma: A 50-year experience at a single institution. Surgery 2001, 130, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Galera-Davidson, H.; Bibbo, M.; Dytch, H.; González-Cámpora, R.; Fernández, A.; Wied, G.; Galera-Davidson, H.; González-Cámpora, R. Nuclear DNA in anaplastic thyroid carcinoma with a differentiated component. Histopathology 1987, 11, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Gay, L.M.; Hartmaier, R.J.; Davis, S.N.; Borre, P.V.; Tan, A.-C.; Schweppe, R.E.; Fishbein, L.; Ross, J.S.; Haugen, B.R.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Wallin, G.; Bäckdahl, M.; Tallroth-Ekman, E.; Lundell, G.; Auer, G.; Löwhagen, T. Co-existent anaplastic and well differentiated thyroid carcinomas: A nuclear DNA study. Eur. J. Surg. Oncol. 1989, 15, 43–48. [Google Scholar] [PubMed]
- Xu, B.; Ghossein, R. Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr. Pathol. 2016, 27, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Chun, S.M.; Kim, D.; Kwon, H.; Jang, E.K.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jang, S.J.; Song, D.E.; et al. Genomic Alterations of Anaplastic Thyroid Carcinoma Detected by Targeted Massive Parallel Sequencing in a BRAFV600E Mutation-Prevalent Area. Thyroid 2016, 26, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, V.; Ting, S.; Herold, T.; Synoracki, S.; Latteyer, S.; Moeller, L.C.; Zwanziger, D.; Stuschke, M.; Fuehrer, D.; Schmid, K.W. NGS based identification of mutational hotspots for targeted therapy in anaplastic thyroid carcinoma. Oncotarget 2017, 8, 42613–42620. [Google Scholar] [CrossRef]
- Bonhomme, B.; Godbert, Y.; Perot, G.; Al Ghuzlan, A.; Bardet, S.; Belleannée, G.; Crinière, L.; Cao, C.D.; Fouilloux, G.; Guyetant, S.; et al. Molecular Pathology of Anaplastic Thyroid Carcinomas: A Retrospective Study of 144 Cases. Thyroid 2017, 27, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ci, B.; Xie, Y.; Gerber, D.E.; Beg, M.S.; Sherman, S.I.; Cabanillas, M.E.; Busaidy, N.L.; Burtness, B.A.; Heilmann, A.M.; et al. Unique mutation patterns in anaplastic thyroid cancer identified by comprehensive genomic profiling. Head Neck 2019, 41, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Latteyer, S.; Tiedje, V.; König, K.; Ting, S.; Heukamp, L.C.; Meder, L.; Schmid, K.W.; Führer, D.; Moeller, L.C. Targeted next-generation sequencing for TP53, RAS, BRAF, ALK and NF1 mutations in anaplastic thyroid cancer. Endocrine 2016, 54, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Busaidy, N.L.; Chau, N.G.; Wirth, L.J.; Barletta, J.A.; Calles, A.; Haddad, R.I.; Kraft, S.; Cabanillas, M.E.; Rabinowits, G.; et al. Genomic Correlates of Response to Everolimus in Aggressive Radioiodine-refractory Thyroid Cancer: A Phase II Study. Clin. Cancer Res. 2018, 24, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Yang, M.; Gretarsson, S.; Jansson, C.; Mylona, N.; Sydow, S.R.; Woodward, E.L.; Ekblad, L.; Wennerberg, J.; Paulsson, K. Identification of Targetable Lesions in Anaplastic Thyroid Cancer by Genome Profiling. Cancers 2019, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K.; Xing, M. Anaplastic Thyroid Cancers Harbor Novel Oncogenic Mutations of the ALK Gene. Cancer Res. 2011, 71, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
- Heldin, N.E.; Gustavsson, B.; Claesson-Welsh, L.; Hammacher, A.; Mark, J.; Heldin, C.H.; Westermark, B. Aberrant expression of receptors for platelet-derived growth factor in an anaplastic thyroid carcinoma cell line. Proc. Natl. Acad. Sci. USA 1988, 85, 9302–9306. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Fleisher, M.; Francis, G.L.; Robbins, R.J. Serum Vascular Endothelial Growth Factor Levels Are Elevated in Metastatic Differentiated Thyroid Cancer but Not Increased by Short-Term TSH Stimulation. J. Clin. Endocrinol. Metab. 2002, 87, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Fenton, C.; Patel, A.; Dinauer, C.; Robie, D.K.; Tuttle, R.M.; Francis, G.L. The Expression of Vascular Endothelial Growth Factor and the Type 1 Vascular Endothelial Growth Factor Receptor Correlate with the Size of Papillary Thyroid Carcinoma in Children and Young Adults. Thyroid 2000, 10, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Vignaud, J.-M.; Hennequin, V.; Toussaint, B.; Bresler, L.; Plenat, F.; Leclère, J.; Duprez, A.; Weryha, G. Increased Expression of the Vascular Endothelial Growth Factor Is a Pejorative Prognosis Marker in Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2001, 86, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Antona, C.; Pallares, J.; Montero-Conde, C.; Inglada-Pérez, L.; Castelblanco, E.; Landa, I.; Leskela, S.; Leandro-García, L.J.; López-Jiménez, E.; Letón, R.; et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr. Relat. Cancer 2010, 17, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.; Ivanova, K.; Ananiev, J.; Gerenova, J.; Zdraveski, A.; Stoyanov, H.; Vlaykova, T. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol. Biotechnol. Equip. 2014, 28, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Viglietto, G.; Maglione, D.; Rambaldi, M.; Cerutti, J.; Romano, A.; Trapasso, F.; Fedele, M.; Ippolito, P.; Chiappetta, G.; Botti, G. Upregulation of vascular endothelial growth factor (VEGF) and downregulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene 1995, 11, 1569–1579. [Google Scholar] [PubMed]
- Shiraiwa, K.; Matsuse, M.; Nakazawa, Y.; Ogi, T.; Suzuki, K.; Saenko, V.A.; Xu, S.; Umezawa, K.; Yamashita, S.; Tsukamoto, K.; et al. JAK/STAT3 and NF-κB Signaling Pathways Regulate Cancer Stem-Cell Properties in Anaplastic Thyroid Cancer Cells. Thyroid 2019, 29, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Vicari, L.; Colarossi, C.; Giuffrida, D.; De Maria, R.; Memeo, L. Cancer stem cells as a potential therapeutic target in thyroid carcinoma. Oncol. Lett. 2016, 12, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2012, 22, 1104–1139. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.; Zafereo, M.; Dadu, R.; Busaidy, N.L.; Hess, K.; Cote, G.J.; Williams, M.D.; William, W.N.; Sandulache, V.C.; Gross, N.D.; et al. Patterns of Treatment Failure in Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Salehian, B.; Liem, S.Y.; Mojazi Amiri, H.; Maghami, E. Clinical Trials in Management of Anaplastic Thyroid Carcinoma; Progressions and Set Backs: A Systematic Review. Int. J. Endocrinol. Metab. 2019, 17, e67759. [Google Scholar] [CrossRef]
- Kloos, R.T.; Ringel, M.D.; Knopp, M.V.; Hall, N.C.; King, M.; Stevens, R.; Liang, J.; Wakely, P.E.; Vasko, V.V.; Saji, M.; et al. Phase II Trial of Sorafenib in Metastatic Thyroid Cancer. J. Clin. Oncol. 2009, 27, 1675–1684. [Google Scholar] [CrossRef]
- Savvides, P.; Nagaiah, G.; Lavertu, P.; Fu, P.; Wright, J.J.; Chapman, R.; Wasman, J.; Dowlati, A.; Remick, S.C. Phase II Trial of Sorafenib in Patients with Advanced Anaplastic Carcinoma of the Thyroid. Thyroid 2013, 23, 600–604. [Google Scholar] [CrossRef]
- Ito, Y.; Onoda, N.; Ito, K.-I.; Sugitani, I.; Takahashi, S.; Yamaguchi, I.; Kabu, K.; Tsukada, K.; Ito, D.Y.; Onoda, D.N.; et al. Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.J.; Dunn, L.A.; Ho, A.L.; Baxi, S.S.; Ghossein, R.A.; Fury, M.G.; Haque, S.; Sima, C.S.; Cullen, G.; Fagin, J.A.; et al. Phase II Study Evaluating the Combination of Sorafenib and Temsirolimus in the Treatment of Radioactive Iodine-Refractory Thyroid Cancer. Cancer 2017, 123, 4114–4121. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Suman, V.J.; Menefee, M.E.; Smallridge, R.C.; Molina, J.R.; Maples, W.J.; Karlin, N.J.; Traynor, A.M.; Kumar, P.; Goh, B.C.; et al. A Multiinstitutional Phase 2 Trial of Pazopanib Monotherapy in Advanced Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2012, 97, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.T.; Lee, J.S.; Urba, S.; Koenig, R.J.; Sisson, J.; Giordano, T.; Worden, F.P. A Phase II Study of Imatinib in Patients with Advanced Anaplastic Thyroid Cancer. Thyroid 2010, 20, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. Lenvatinib for Anaplastic Thyroid Cancer. Front. Oncol. 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Ravaud, A.; De La Fouchardière, C.; Caron, P.; Doussau, A.; Cao, C.D.; Asselineau, J.; Rodien, P.; Pouessel, D.; Nicolli-Sire, P.; Klein, M.; et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: Mature data from the THYSU study. Eur. J. Cancer 2017, 76, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients with Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef]
- Lim, S.M.; Chang, H.; Yoon, M.J.; Hong, Y.K.; Kim, H.; Chung, W.Y.; Park, C.S.; Nam, K.H.; Kang, S.W.; Kim, M.K.; et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann. Oncol. 2013, 24, 3089–3094. [Google Scholar] [CrossRef]
- Schneider, T.C.; de Wit, D.; Links, T.P.; van Erp, N.P.; van der Hoeven, J.J.; Gelderblom, H.; Roozen, I.C.; Bos, M.; Corver, W.E.; van Wezel, T.; et al. Everolimus in Patients With Advanced Follicular-Derived Thyroid Cancer: Results of a Phase II Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 102, 698–707. [Google Scholar] [CrossRef]
- Pennell, N.A.; Daniels, G.H.; Haddad, R.I.; Ross, D.S.; Evans, T.; Wirth, L.J.; Fidias, P.H.; Temel, J.S.; Gurubhagavatula, S.; Heist, R.S.; et al. A Phase II Study of Gefitinib in Patients with Advanced Thyroid Cancer. Thyroid 2008, 18, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Tortorici, M.; Kim, S.; Ingrosso, A.; Pithavala, Y.K.; Bycott, P. A Phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: Long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemother. Pharmacol. 2014, 74, 1261–1270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mooney, C.J.; Nagaiah, G.; Fu, P.; Wasman, J.K.; Cooney, M.M.; Savvides, P.S.; Bokar, J.A.; Dowlati, A.; Wang, D.; Agarwala, S.S.; et al. A Phase II Trial of Fosbretabulin in Advanced Anaplastic Thyroid Carcinoma and Correlation of Baseline Serum-Soluble Intracellular Adhesion Molecule-1 with Outcome. Thyroid 2009, 19, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sosa, J.A.; Elisei, R.; Jarzab, B.; Balkissoon, J.; Lu, S.-P.; Bal, C.; Marur, S.; Gramza, A.; Ben Yosef, R.; Gitlitz, B.; et al. Randomized Safety and Efficacy Study of Fosbretabulin with Paclitaxel/Carboplatin Against Anaplastic Thyroid Carcinoma. Thyroid 2014, 24, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Habra, M.A.; Zafereo, M.; Gross, N.; Hess, K.R.; Gule-Monroe, M.; Williams, M.D.; et al. Real-World Experience with Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid 2018, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Peddi, P.F.; Glaspy, J.A.; Rosove, M.H. BRAF V600E Inhibition in Anaplastic Thyroid Cancer. N. Engl. J. Med. 2013, 368, 684–685. [Google Scholar]
- Marten, K.A.; Gudena, V.K. Use of vemurafenib in anaplastic thyroid carcinoma: A case report. Cancer Biol. Ther. 2015, 16, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef]
- Godbert, Y.; De Figueiredo, B.H.; Bonichon, F.; Chibon, F.; Hostein, I.; Perot, G.; Dupin, C.; Daubech, A.; Belleannée, G.; Gros, A.; et al. Remarkable Response to Crizotinib in Woman with Anaplastic Lymphoma Kinase-Rearranged Anaplastic Thyroid Carcinoma. J. Clin. Oncol. 2015, 33, 84–87. [Google Scholar] [CrossRef]
- Masago, K.; Miura, M.; Toyama, Y.; Togashi, Y.; Mishima, M. Good clinical response to erlotinib in a patient with anaplastic thyroid carcinoma harboring an epidermal growth factor somatic mutation, L858R, in exon 21. J. Clin. Oncol. 2011, 29, e465–e467. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, W.K.; Kang, C.W.; Ku, C.R.; Cho, Y.H.; Lee, E.J. A selective cyclin-dependent kinase 4, 6 dual inhibitor, Ribociclib (LEE011) inhibits cell proliferation and induces apoptosis in aggressive thyroid cancer. Cancer Lett. 2018, 417, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-F.; Lin, J.-D.; Hsueh, C.; Chou, T.-C.; Wong, R.J. A cyclin-dependent kinase inhibitor, dinaciclib in preclinical treatment models of thyroid cancer. PLoS ONE 2017, 12, e0172315. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ventura, S.; Pojo, M.; Matias, A.T.; Moura, M.M.; Marques, I.J.; Leite, V.; Cavaco, B.M. The efficacy of HRAS and CDK4/6 inhibitors in anaplastic thyroid cancer cell lines. J. Endocrinol. Investig. 2019, 42, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Tulla, K.; Maker, A.V.; Burman, K.D.; Prabhakar, B.S. Therapeutic advances in anaplastic thyroid cancer: A current perspective. Mol. Cancer 2018, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Poli, R.; Pugliese, M.; Fortunati, N.; Boccuzzi, G. Emerging molecular therapies of advanced thyroid cancer. Mol. Asp. Med. 2010, 31, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Bastman, J.J.; Serracino, H.S.; Zhu, Y.; Koenig, M.R.; Mateescu, V.; Sams, S.B.; Davies, K.D.; Raeburn, C.D.; McIntyre, R.C.; Haugen, B.R.; et al. Tumor-Infiltrating T Cells and the PD-1 Checkpoint Pathway in Advanced Differentiated and Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, A.V.; Rumilla, K.M.; Smith, C.Y.; Jenkins, S.M.; Foote, R.L.; Kasperbauer, J.L.; Morris, J.C.; Ryder, M.; Alsidawi, S.; Hilger, C.; et al. Expression of PD-1 and PD-L1 in Anaplastic Thyroid Cancer Patients Treated with Multimodal Therapy: Results From a Retrospective Study. J. Clin. Endocrinol. Metab. 2017, 102, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.; Ghossein, R.A.; Ricarte-Filho, J.C.M.; Knauf, J.A.; Fagin, J.A. Increased density of tumor associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr. Relat. Cancer 2008, 15, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair deficient/microsatellite instability–high colorectal cancer (CheckMate 142): Results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, R.; Schneider, B.; Radovich, M.; Babu, S.; Kiel, P.J. Exceptional Response with Immunotherapy in a Patient with Anaplastic Thyroid Cancer. Oncologist 2017, 22, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, G.B.; Grosu, H.; et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef] [PubMed]
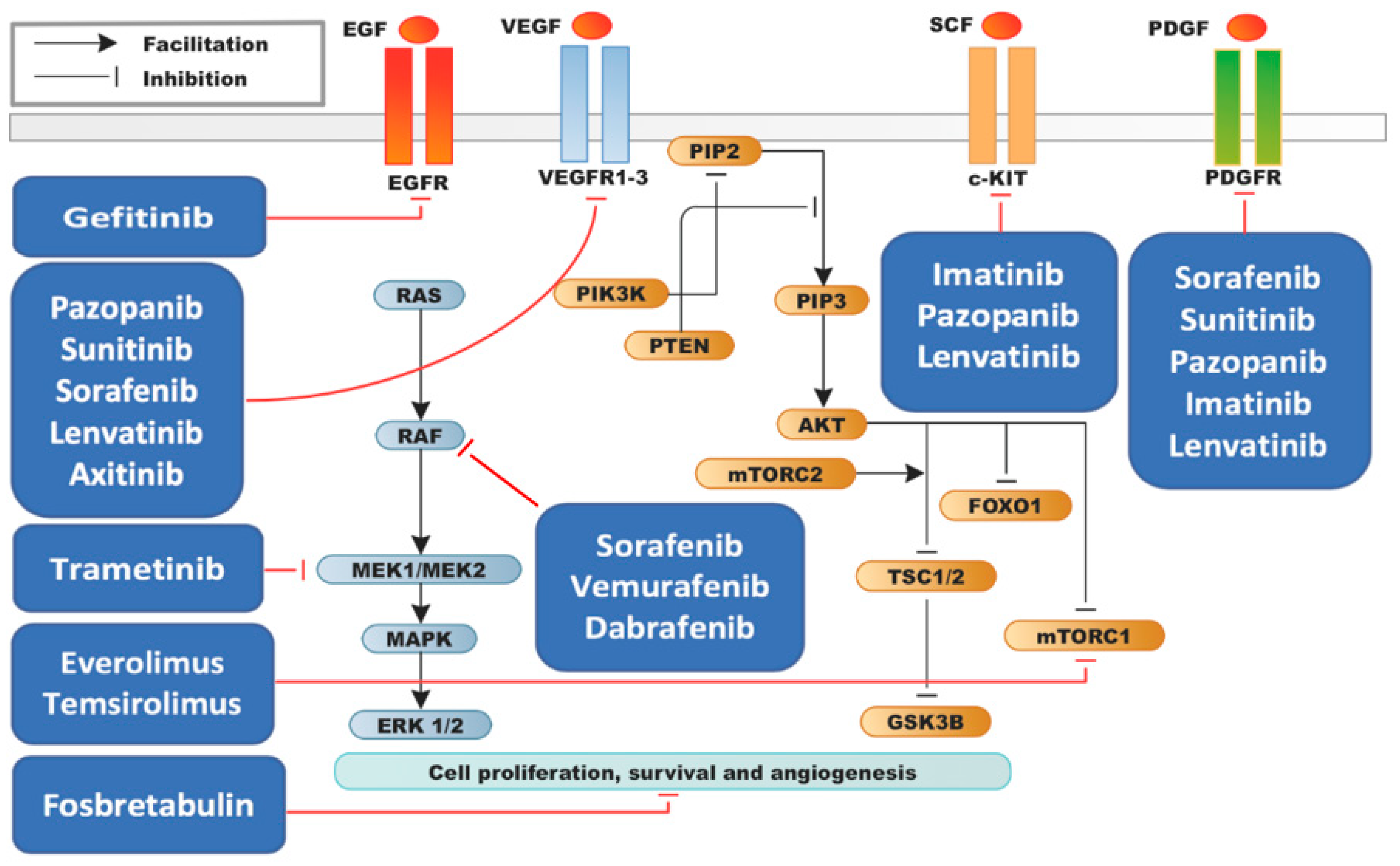
| Alteration | Latteyer [33] | Landa [24] | Pozdeyev [25] | Jeon [28] | Kunstman [29] | Hanna [34] | Tiedje [30] | Bonhomme * [31] | Khan [32] | Ravi [35] | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 30) | (n = 33) | (n = 196) | (n = 11) | (n = 22) | (n = 7) | (n = 118) | (n = 144) | (n = 90) | (n = 14) | ||
| TERT promoter mutation | NA | 73% | 65% | NA | NA | NA | 73% | 54% | 32% | 36% | 32–73% |
| RAS-RAF-MAPK pathway | |||||||||||
| BRAF | 7% | 45% | 41% | 91% | 27% | 29% | 11% | 14% | 34% | 18% | 7–91% |
| RAS | 23% | 24% | 27% | 9% | 27% | 14% | 20% | 43% | 26% | 18% | 9–43% |
| PI3K-AKT-mTOR pathway | |||||||||||
| PIK3CA | NA | 18% | 14% | 18% | 8% | 0% | 12% | 6% | 12% | 18% | 0–18% |
| PTEN ¶ | NA | 15% | 11% | 9% | 0% | 0% | 0% | 9% | 13% | 18% | 0–18% |
| SWI/SNF complex | NA | 36% | 18% | NA | 9% | 14% | NA | 1% | 6% | 27% | 1–36% |
| Histone methyl-transferases | NA | 24% | 19% | 18% | 5% | 0% | NA | NA | 3% | 54% | 0–54% |
| Mismatch repair | NA | 12% | 4% | NA | 27% | 0% | NA | 0% | 2% | 36% | 0–36% |
| Additional genes | |||||||||||
| TP53 | 60% | 73% | 65% | 73% | 27% | 43% | 55% | 54% | 66% | 55% | 27–73% |
| ATM | NA | 9% | 4% | 0% | 0% | 57% | NA | 4% | NA | 27% | 0–57% |
| EIF1AX | NA | 9% | NA | 0% | 14% | 0% | NA | N/A | NA | 0% | 0–14% |
| NF1 | 37% | 9% | 9% | 0% | 9% | 14% | NA | NA | 12% | 9% | 0–37% |
| NF2 | NA | 6% | 12% | 27% | 5% | 0% | NA | NA | 14% | 18% | 0–27% |
| RB1 | NA | 9% | 7% | 0% | 0% | 0% | 0.8% | 1% | 6% | 18% | 0–18% |
| TSHR | NA | 6% | 3% | 0% | 5% | 0% | NA | NA | NA | 9% | 0–9% |
| STK11 | NA | 6% | 0% | 0% | 0% | 0% | NA | 1% | NA | 9% | 0–9% |
| ALK † | 20% | 0% | 0% | 0% | 0% | 0% | 0% | 1% | 2% | 0% | 0–20% |
| CCNE1 # | NA | NA | 4% | NA | NA | NA | NA | NA | 4% | 29% | 4–29% |
| Trial | Tested Agent | Design | No. of ATC Patients | PR | CR | SD | PD | PFS | OS |
|---|---|---|---|---|---|---|---|---|---|
| Kloos [49] | Sorafenib | Single arm | 4 (out of 56) | 0% | 0% | 25% | 75% | NA | NA |
| Savvides [50] | Sorafenib | Single arm | 20 | 10% | 0% | 25% | NA | 1.9 months (95% CI: 1.3–3.6 months) | 3.9 months (95% CI: 2.2–7.1 months) |
| Ito [51] | Sorafenib | Single arm | 10 (out of 18) | NA | NA | 40% | 60% | 2.8 months (95% CI: 0.7–5.6) | 5 months (95% CI: 0.7–5.7) |
| Sherman [52] | Sorafenib/ Temsirolimus | Single arm | 2 (out of 36) | 50% | 0% | 0% | 50% | NA | NA |
| Bible [53] | Pazopanib | Single arm | 15 | 0% | 0% | 0% | 100% | 2 months | 3.7 months |
| Ha [54] | Imatinib | Single arm | 8 (11 total, but 8 evaluable) | 25% | 0% | 50% | NA | 6 months: 36% (95% CI: 9–65) 12 months: 24.2% (95% CI: 3.8–54.1) 18 months: 12.1% (95% CI: 0.7–41.1) | 6 months: 45% (95% CI: 16–70) |
| Tahara [55] | Lenvatinib | Single arm | 17 (out of 51) | 24% | 71% | 6% | 7.4 months (95% CI: 1.7–12.9) | 10.6 months (95% CI: 3.8–19.8) | |
| Ravaud [56] | Sunitinib | Single arm | 4 (out of 71) | 0% | 0% | 25% | NA | 9.8 months (95% CI: 7.8–11.9) | NA |
| Hyman [57] | Vemurafenib | Single arm | 7 (out of 122) | 14% | 14% | 0% | 57% | NA | NA |
| Subbiah [58] | Dabrafenib + Trametinib | Single arm | 16 (out of 100) | 63% | 6% | 19% | 6% | 79% * | 80% * |
| Lim [59] | Everolimus | Single arm | 6 (out of 38) | 0% | 0% | NA | NA | 2.5 months (95% CI: 4.8–16.0) | NA |
| Schneider [60] | Everolimus | Single arm | 7 (out of 35) | 0% | 0% | 0% | 100% | 3.7 months | 4.7 months |
| Hanna [34] | Everolimus | Single arm | 7 (out of 50) | 14% | 0% | 29% | 43% | 2.2 months (95% CI: 1–17.9) | 4.6 months (95% CI: 1–29.9) |
| Pennell [61] | Gefitinib | Single arm | 5 (out of 27) | 0% | 0% | 20% | NA | NA | NA |
| Cohen [62] | Axitinib | Single arm | 2 (out of 60) | 50% | 0% | 0% | 50% | NA | NA |
| Mooney [63] | Fosbretabulin | Single arm | 26 | 0% | 0% | 27% | 58% | NA | Median OS: 4.7 months 6 months: 34% 12 months: 23% |
| Sosa [64] | Fosbretabulin + Carboplatin/ Paclitaxel (FCP) vs. Carboplatin/ Paclitaxel (CP) | RCT | 80 | FCP = 20% CP = 16% | 0% | FCP = 40% CP = 44% | FCP = 49% CP = 52% | FCP = 3.3 months (95% CI: 2.3–5.6) CP = 3.1 months (95% CI: 2.7–5.4) | Median OS: FCP = 5.2 months (95% CI: 3.1–9.0) CP = 4.0 months (95% CI: 2.8–6.2) 1-year OS: FCP = 26% CP = 9% |
| Trial | No. of Patients | Grade of TrAE/AE | Any Grade | DR | TD | ||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | |||||
| Kloos [49] | 56 (4 ATC) | VM | NA | NA (but few) | 2%, likely unrelated to treatment | NA | 52% | NA | |
| Savvides [50] | 20 | VM | NA | 5% | 0% | NA | NA | NA | |
| Ito [51]∆ | 18 (10 ATC) | NA | NA | NA | NA | 17%, unrelated to treatment | NA | 78% | 67% (only 17% permanent) |
| Sherman [52] | 36 (2 ATC) | NA | NA | 64% grade 3 or greater | 3% | NA | 61% | 14% (an additional 17% discontinued only sorafenib) | |
| Bible [53] | 15 | >70% | >10% | 6.7%, possibly related | NA | 27% | 11% | ||
| Ha [54]∆ | 11 | >70% | >25% | 0% | 0% | NA | 27% | 0% | |
| Tahara [55]∆ | 51 (17 ATC) | VM | 77% | 6% | 8%, unrelated to treatment | 100% | 88% | 65% | |
| Ravaud [56]∆ | 71 (4 ATC) | NA | >25% | >1% | >7% | >80% | NA | NA | |
| Hyman [57]∆ | 122 (7 ATC) | NA | 43% (for ATC cohort) | NA | 86% (for ATC cohort) | NA | NA | ||
| Subbiah [58]∆ | 100 (16 ATC) | NA | 50% (for ATC cohort) | NA | 94% (for ATC cohort) | 30% (entire cohort, n = 100) | 8% (entire cohort, n = 100) | ||
| Lim [59]∆ | 38 (6 ATC) | >84% | >15% | 2% | 2%, unrelated to treatment | NA | NA | 2% | |
| Schneider [60]∆ | 35 (7 ATC) | Safety data not provided for ATC patients. | |||||||
| Hanna [34] | 50 (7 ATC) | 50% | 42% | 2% | 0% | 94% | 62% | NA | |
| Pennell [61]∆ | 27 (5 ATC) | NA | NA | 11% | 0% | 0% | NA | NA | 7 % |
| Cohen [62] | 60 (2 ATC) | NA | NA | 35% | NA | 93% | 42% | 7% | |
| Mooney [63]∆ | 26 | VM | NA | 35% | 4% | 0% | NA | NA | 4 % |
| Sosa [64] | 80 | FCP = 27.5% CP = 29.2% | FCP = 25.5% CP = 29.2% | FCP = 37.3% CP = 16.7% | 1%, possibly related to treatment | NA | NA | 1% | |
| Search String | Filters Used | Search Results |
|---|---|---|
| 1. MESH term: “Thyroid Neoplasms/drug therapy” | Clinical trials | 269 |
| 2. “Anaplastic thyroid cancer” | Clinical trials | 20 |
| 3. “Anaplastic thyroid carcinoma” | Clinical trials | 21 |
| 4. “Anaplastic thyroid cancer” OR ”Anaplastic thyroid carcinoma”) AND (”Phase 2” OR ”Phase II” OR ”Phase 3” OR ”Phase III”) | None | 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ljubas, J.; Ovesen, T.; Rusan, M. A Systematic Review of Phase II Targeted Therapy Clinical Trials in Anaplastic Thyroid Cancer. Cancers 2019, 11, 943. https://doi.org/10.3390/cancers11070943
Ljubas J, Ovesen T, Rusan M. A Systematic Review of Phase II Targeted Therapy Clinical Trials in Anaplastic Thyroid Cancer. Cancers. 2019; 11(7):943. https://doi.org/10.3390/cancers11070943
Chicago/Turabian StyleLjubas, Josip, Therese Ovesen, and Maria Rusan. 2019. "A Systematic Review of Phase II Targeted Therapy Clinical Trials in Anaplastic Thyroid Cancer" Cancers 11, no. 7: 943. https://doi.org/10.3390/cancers11070943
APA StyleLjubas, J., Ovesen, T., & Rusan, M. (2019). A Systematic Review of Phase II Targeted Therapy Clinical Trials in Anaplastic Thyroid Cancer. Cancers, 11(7), 943. https://doi.org/10.3390/cancers11070943





