Hypoxia Selectively Impairs CAR-T Cells In Vitro
Abstract
1. Introduction
2. Results
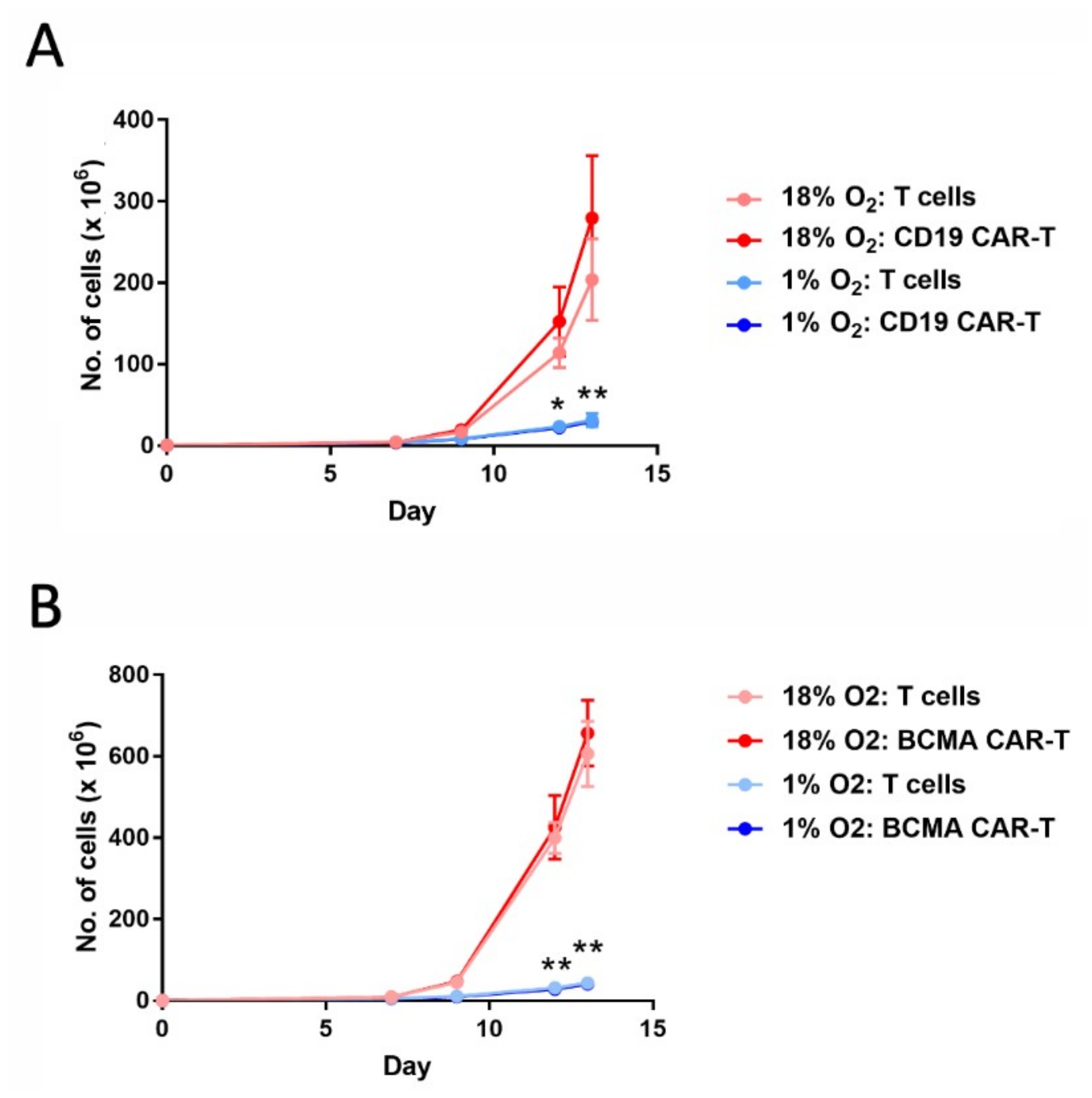
2.1. Hypoxia Greatly Decreases CAR-T Cell Expansion
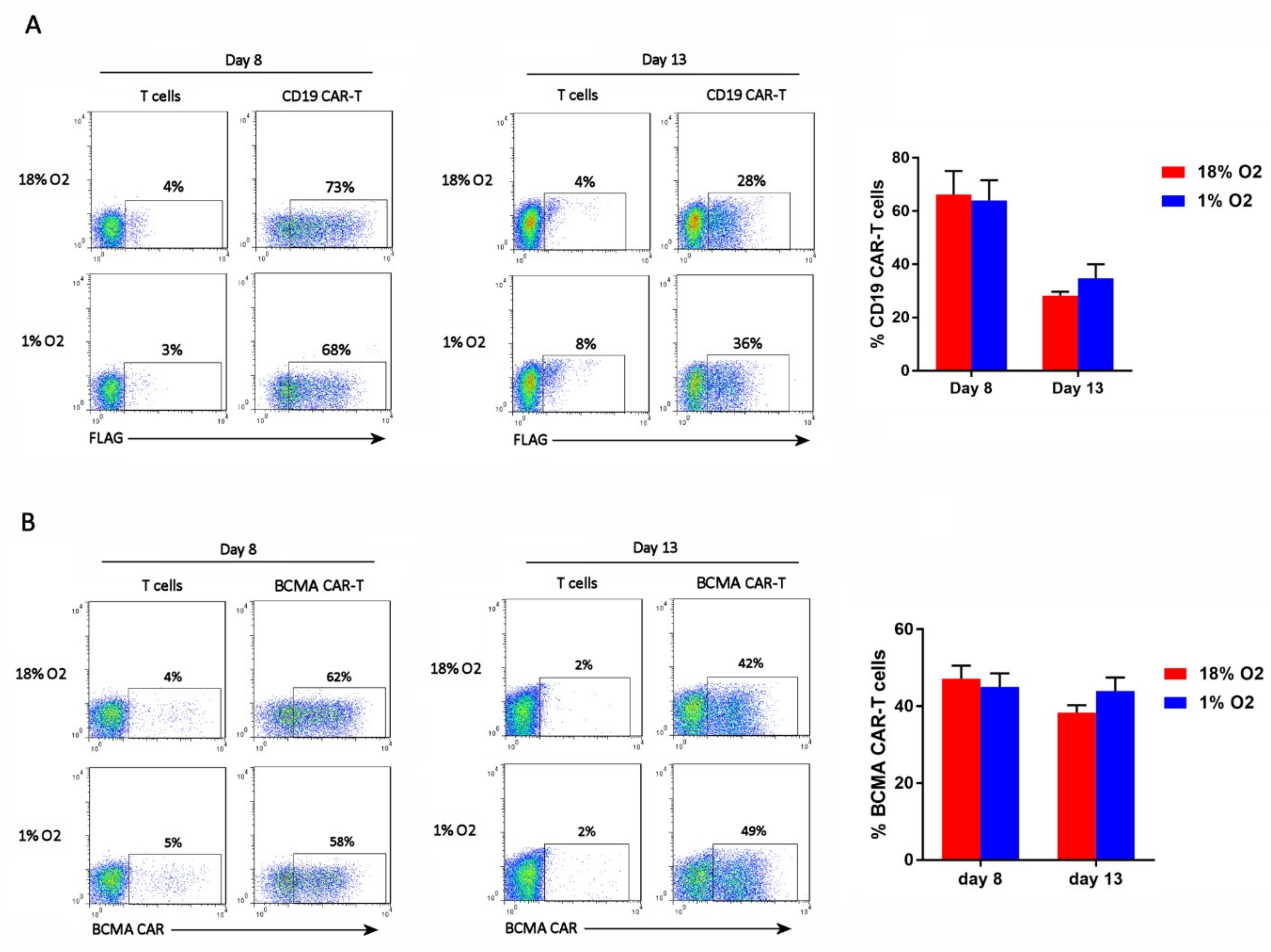
2.2. Hypoxia Does Not Affect CAR-T Cell Frequency
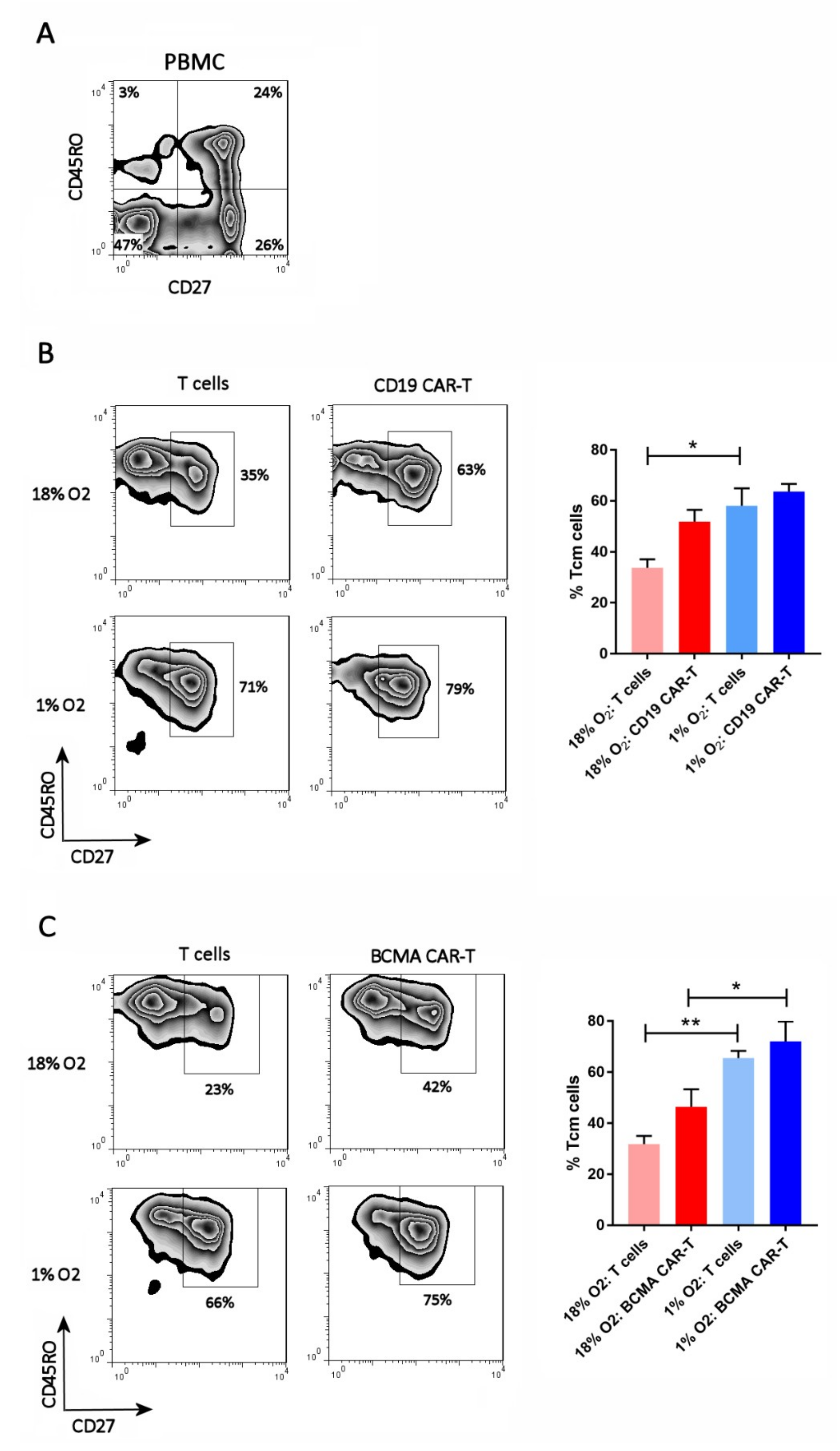
2.3. Hypoxia Inhibits CAR-T Cell Differentiation
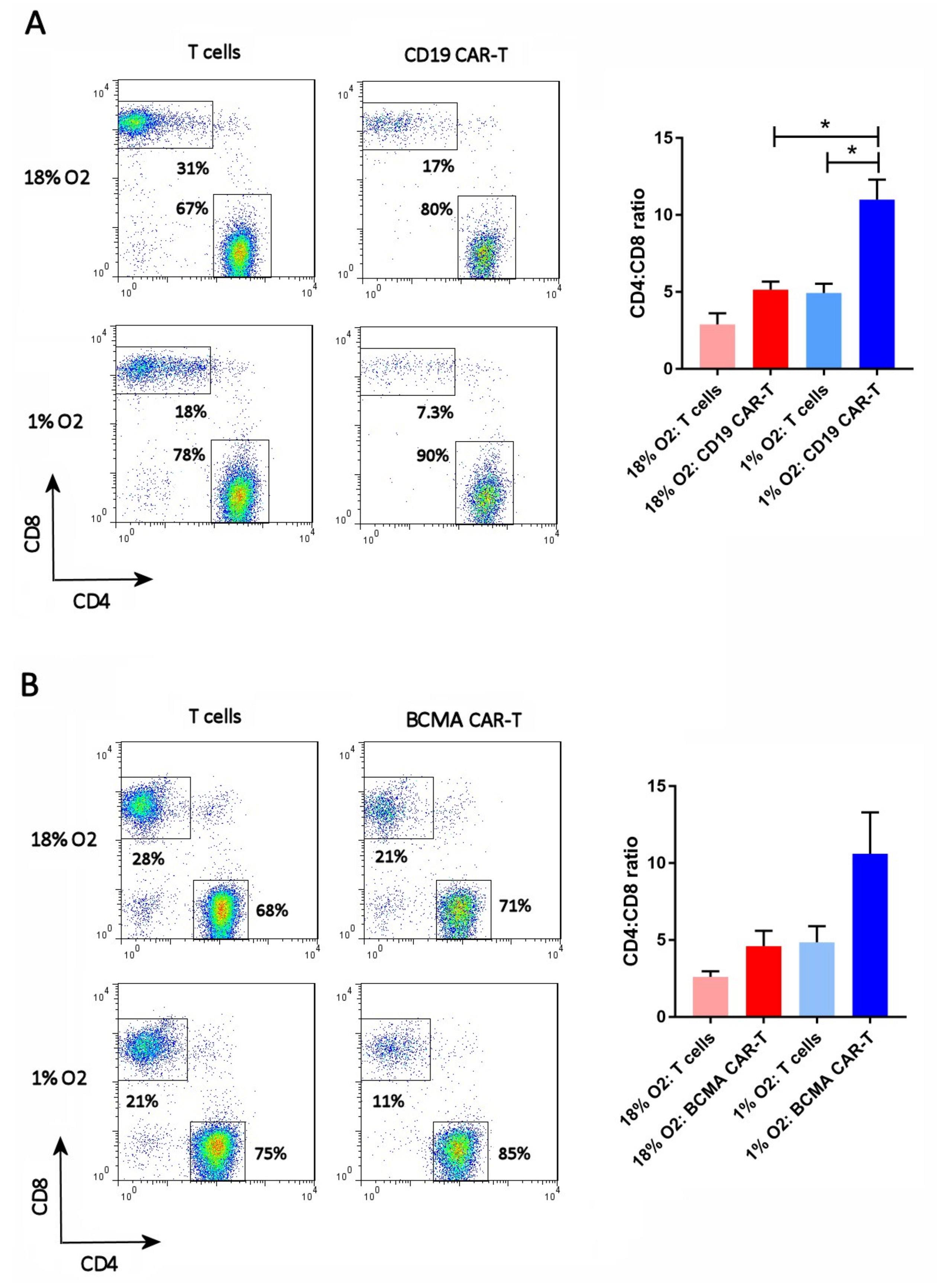
2.4. Hypoxia Increases the CAR-T Cell CD4:CD8 Ratio
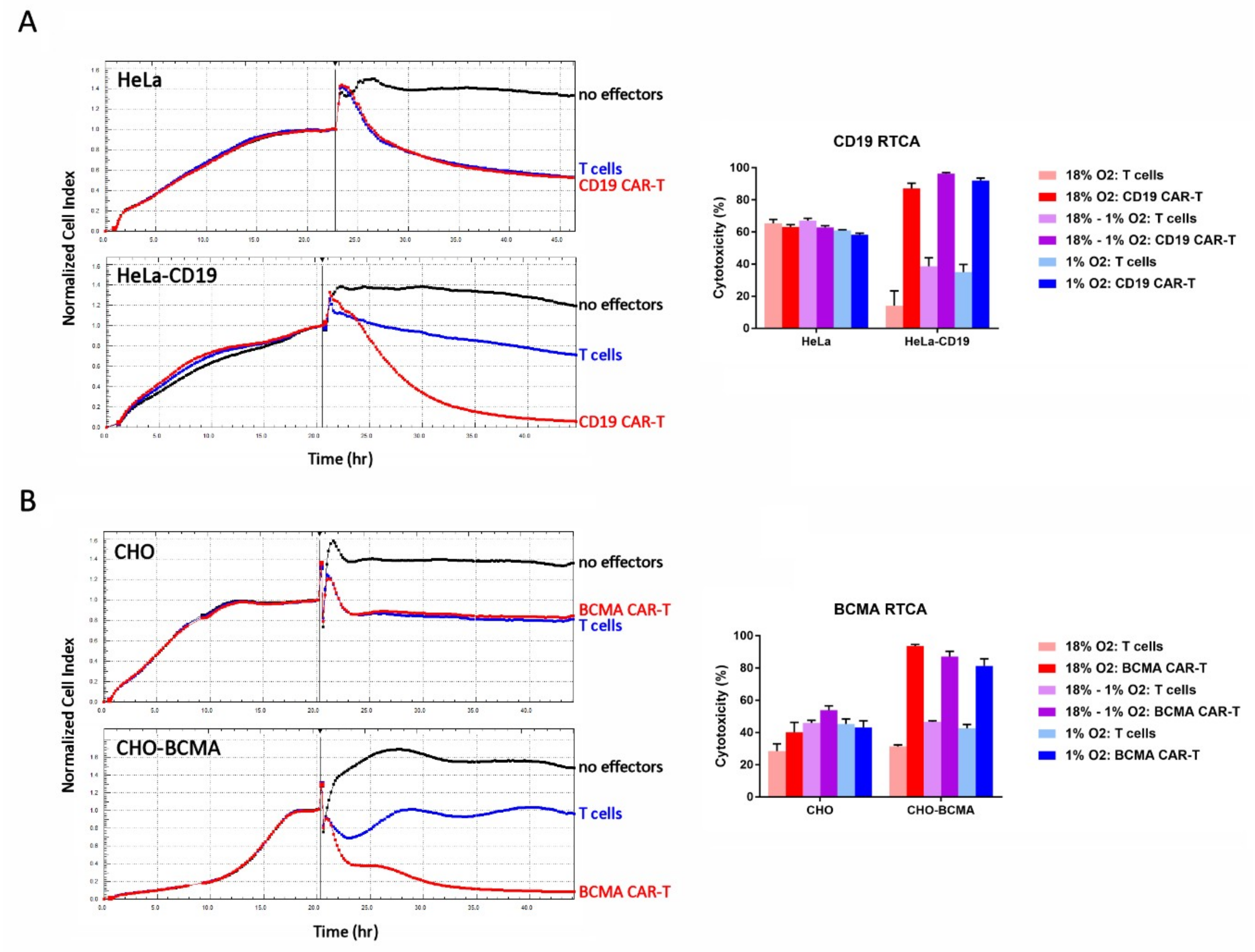
2.5. Hypoxia Does Not Affect CAR-T Cell Cytotoxicity
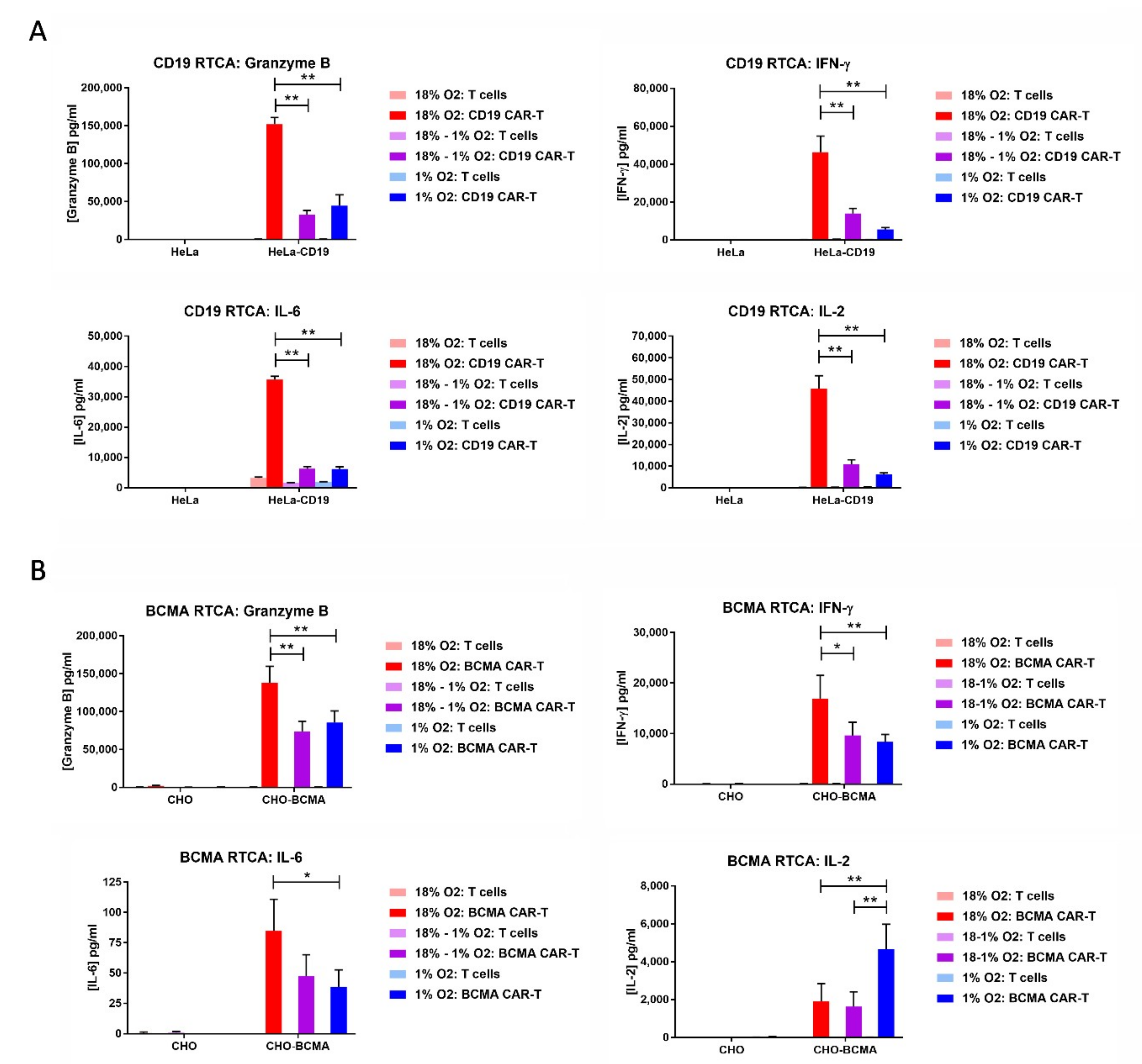
2.6. Hypoxia Decreases CAR-T Cell Granzyme B and Cytokine Production in Response to Transfected Cell Lines
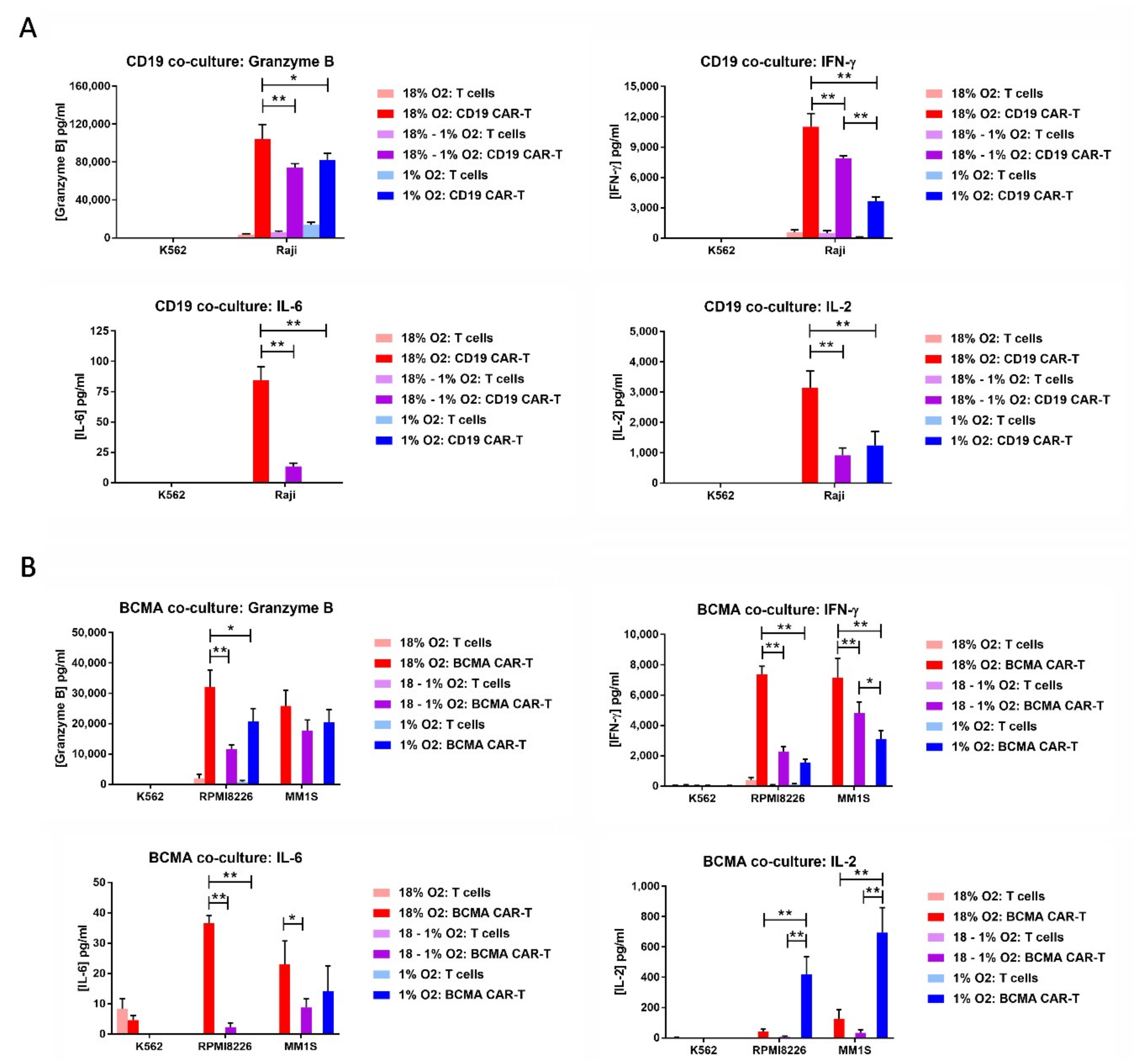
2.7. Hypoxia Decreases CAR-T Cell Granzyme B and Cytokine Production in Response to Tumor Cells
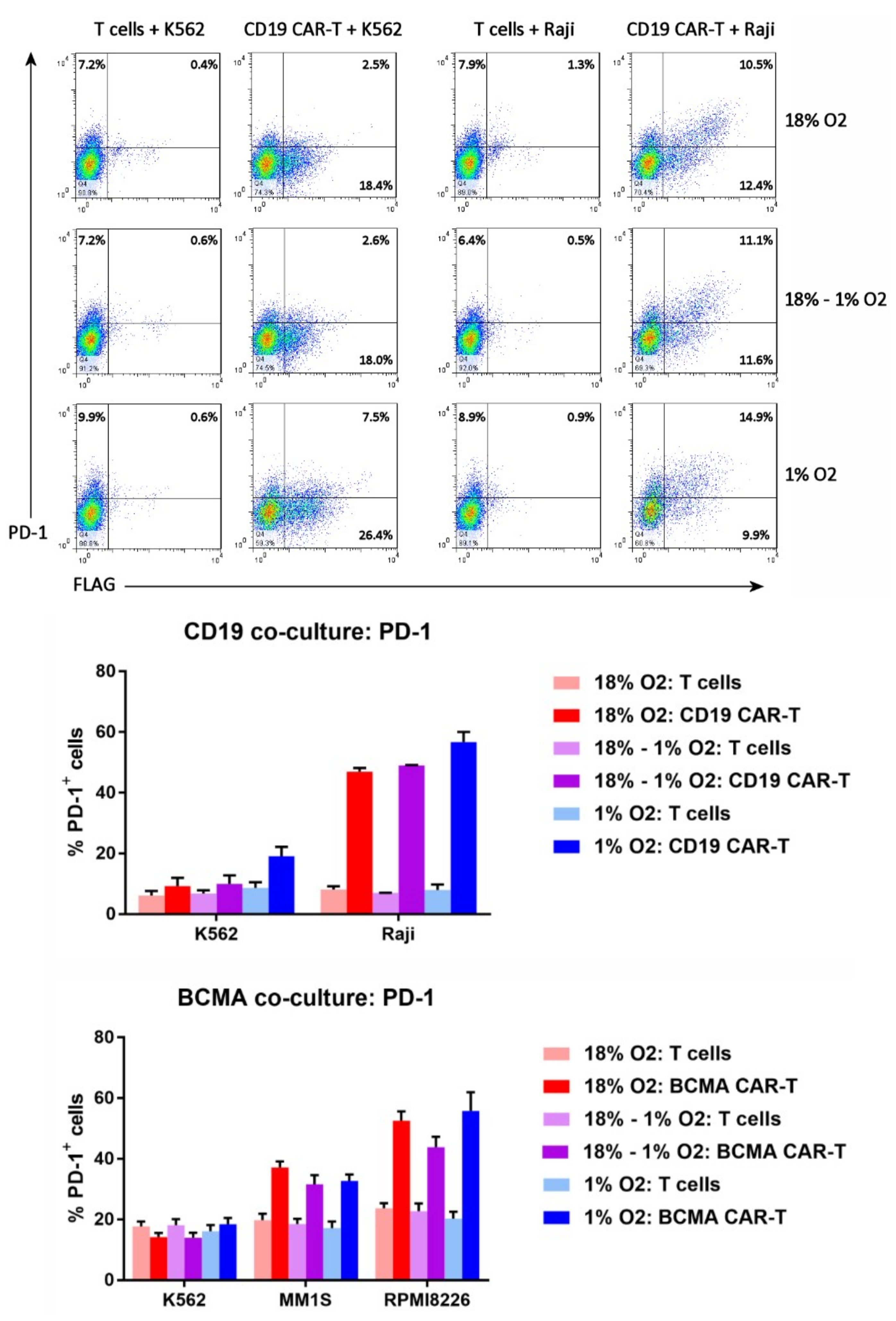
2.8. Hypoxia Does Not Affect CAR-T Cell PD-1 Upregulation
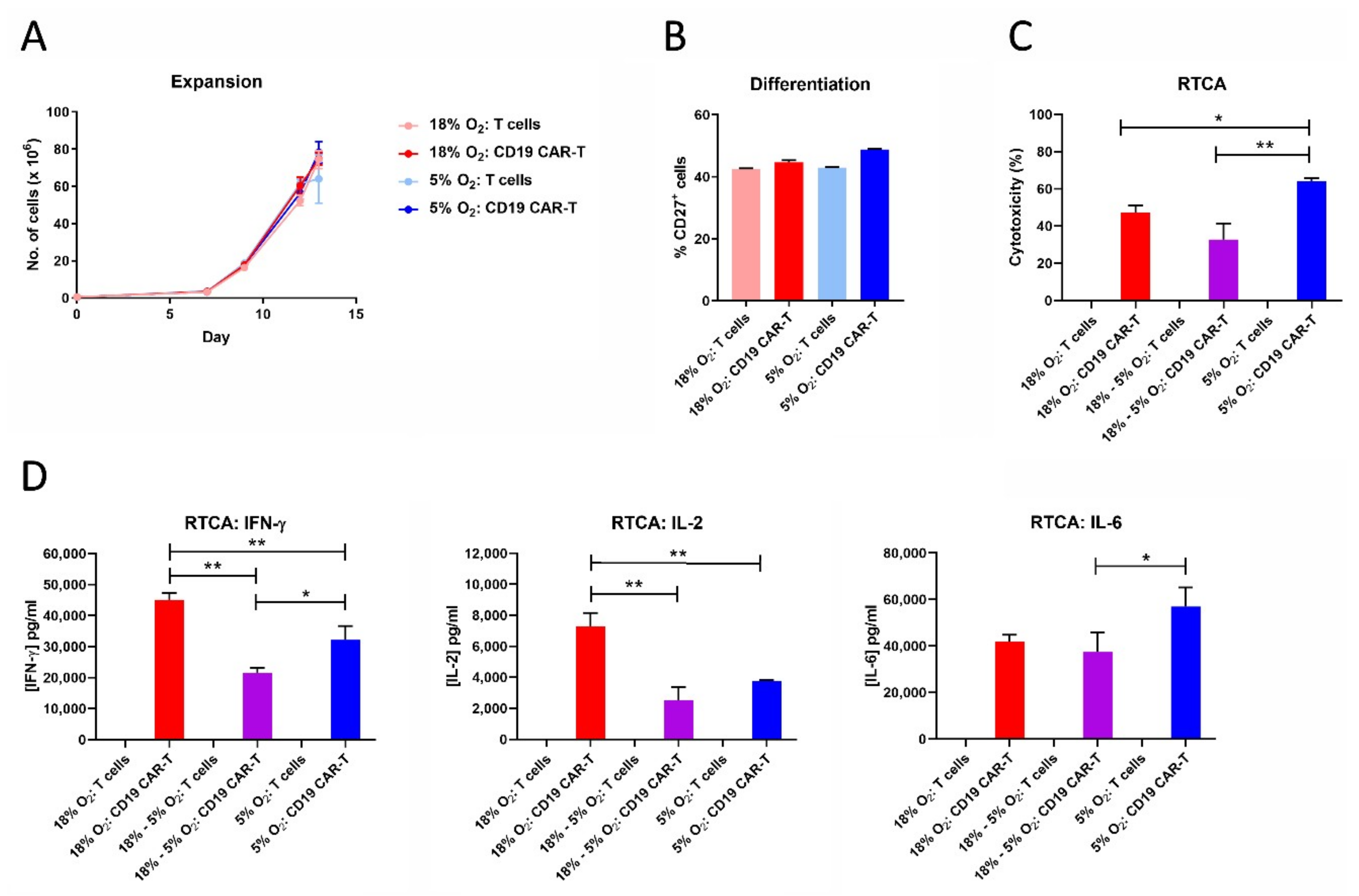
2.9. CAR-T Cell Expansion in 5% Oxygen Results in Greater Cytotoxicity and Decreased IFN-γ/IL-2 Production
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Generation of CAR-Encoding Lentivirus
4.3. Generation and Expansion of CAR-T Cells
4.4. Flow Cytometry
4.5. Cytotoxicity Assay
4.6. Cytokine and PD-1 Induction Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maus, M.V.; June, C.H. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin. Cancer Res. 2016, 22, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Feins, S.; Kong, W.; Williams, E.F.; Milone, M.C.; Fraietta, J.A. An introduction to chimeric antigen receptor (CAR) T cell immunotherapy for human cancer. Am. J. Hematol. 2019, 94, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Abken, H. Adoptive therapy with CAR redirected T cells: The challenges in targeting solid tumors. Immunotherapy 2015, 7, 535–544. [Google Scholar] [CrossRef]
- Beatty, G.L.; Moon, E.K. Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology 2014, 3, e970027. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Calderon, H.; Posey, A.D.J.; Maus, M.V. Engineering and design of chimeric antigen receptors. Mol. Ther. Methods Clin. Dev. 2019, 12, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Eshhar, Z.; Waks, T.; Gross, G. The emergence of T-bodies/CAR T cells. Cancer J. 2014, 20, 123–126. [Google Scholar] [CrossRef]
- Becker, A.; Stadler, P.; Krause, U.; Utzig, D.; Hansgen, G.; Lautenschlager, C.; Rath, F.W.; Molls, M.; Dunst, J. Association between elevated serum VEGF and polarographically measured tumor hypoxia in head and neck carcinomas. Strahlenther. Onkol. 2001, 177, 182–188. [Google Scholar] [CrossRef]
- Dunst, J.; Stadler, P.; Becker, A.; Kuhnt, T.; Lautenschlager, C.; Molls, M.; Haensgen, G. Tumor hypoxia and systemic levels of vascular endothelial growth factor (VEGF) in head and neck cancers. Strahlenther. Onkol. 2001, 177, 469–473. [Google Scholar] [CrossRef]
- Bollinger, T.; Gies, S.; Naujoks, J.; Feldhoff, L.; Bollinger, A.; Solbach, W.; Rupp, J. HIF-1alpha- and hypoxia-dependent immune responses in human CD4+CD25high T cells and T helper 17 cells. J. Leukoc. Biol. 2014, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gabrilovich, D.I. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014, 143, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Combe, P.; Gey, A.; Granier, C.; Roussel, H.; De Guillebon, E.; Oudard, S.; Tartour, E. PD-1 and PDL-1 expression in cancer: Significance and prognostic value. Med. Sci. (Paris) 2013, 29, 570–572. [Google Scholar] [CrossRef][Green Version]
- Denis, H.; Davoine, C.; Bermudez, E.; Grosjean, G.; Schwager, M.; Ifrah, N.; Dahan, M.; Negellen, S. Specific immunotherapies in the treatment of cancers. Bull. Cancer 2019, 106, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, M.; Garcia-Ruiz, J.C.; Berra, E. The hypoxia signalling pathway in haematological malignancies. Oncotarget 2017, 8, 36832–36844. [Google Scholar] [CrossRef]
- Berahovich, R.; Xu, S.; Zhou, H.; Harto, H.; Xu, Q.; Garcia, A.; Liu, F.; Golubovskaya, V.M.; Wu, L. FLAG-tagged CD19-specific CAR-T cells eliminate CD19-bearing solid tumor cells in vitro and in vivo. Front. Biosci. (Landmark Ed.) 2017, 22, 1644–1654. [Google Scholar]
- Davila, M.L.; Bouhassira, D.C.; Park, J.H.; Curran, K.J.; Smith, E.L.; Pegram, H.J.; Brentjens, R. Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. Int. J. Hematol. 2014, 99, 361–371. [Google Scholar] [CrossRef]
- Berahovich, R.; Zhou, H.; Xu, S.; Wei, Y.; Guan, J.; Guan, J.; Harto, H.; Fu, S.; Yang, K.; Zhu, S.; et al. CAR-T cells based on novel BCMA monoclonal antibody block multiple myeloma cell growth. Cancers (Basel) 2018, 10, 323. [Google Scholar] [CrossRef]
- Caldwell, C.C.; Kojima, H.; Lukashev, D.; Armstrong, J.; Farber, M.; Apasov, S.G.; Sitkovsky, M.V. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 2001, 167, 6140–6149. [Google Scholar] [CrossRef]
- Atkuri, K.R.; Herzenberg, L.A.; Herzenberg, L.A. Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc. Natl. Acad. Sci. USA 2005, 102, 3756–3759. [Google Scholar] [CrossRef]
- Larbi, A.; Zelba, H.; Goldeck, D.; Pawelec, G. Induction of HIF-1alpha and the glycolytic pathway alters apoptotic and differentiation profiles of activated human T cells. J. Leukoc. Biol. 2010, 87, 265–273. [Google Scholar] [CrossRef]
- Ikejiri, A.; Nagai, S.; Goda, N.; Kurebayashi, Y.; Osada-Oka, M.; Takubo, K.; Suda, T.; Koyasu, S. Dynamic regulation of Th17 differentiation by oxygen concentrations. Int. Immunol. 2012, 24, 137–146. [Google Scholar] [CrossRef]
- Westendorf, A.M.; Skibbe, K.; Adamczyk, A.; Buer, J.; Geffers, R.; Hansen, W.; Pastille, E.; Jendrossek, V. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting Treg activity. Cell. Physiol. Biochem. 2017, 41, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Vuillefroy de Silly, R.; Ducimetiere, L.; Yacoub Maroun, C.; Dietrich, P.Y.; Derouazi, M.; Walker, P.R. Phenotypic switch of CD8(+) T cells reactivated under hypoxia toward IL-10 secreting, poorly proliferative effector cells. Eur. J. Immunol. 2015, 45, 2263–2275. [Google Scholar] [CrossRef]
- Robbins, J.R.; Lee, S.M.; Filipovich, A.H.; Szigligeti, P.; Neumeier, L.; Petrovic, M.; Conforti, L. Hypoxia modulates early events in t cell receptor-mediated activation in human t lymphocytes via kv1.3 channels. J. Physiol. 2005, 564, 131–143. [Google Scholar] [CrossRef]
- Gaber, T.; Tran, C.L.; Schellmann, S.; Hahne, M.; Strehl, C.; Hoff, P.; Radbruch, A.; Burmester, G.R.; Buttgereit, F. Pathophysiological hypoxia affects the redox state and il-2 signalling of human cd4+ t cells and concomitantly impairs survival and proliferation. Eur. J. Immunol. 2013, 43, 1588–1597. [Google Scholar] [CrossRef]
- Hubbi, M.E.; Luo, W.; Baek, J.H.; Semenza, G.L. Mcm proteins are negative regulators of hypoxia-inducible factor 1. Mol. Cell 2011, 42, 700–712. [Google Scholar] [CrossRef]
- Berger, C.; Jensen, M.C.; Lansdorp, P.M.; Gough, M.; Elliott, C.; Riddell, S.R. Adoptive transfer of effector cd8+ t cells derived from central memory cells establishes persistent t cell memory in primates. J. Clin. Investig. 2008, 118, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Negishi, Y.; Shimizu, M.; Takahashi, M.; Ichikawa, M.; Takahashi, H. Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol. Lett. 2015, 167, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Hombach, A.A.; Abken, H. Most do, but some do not: CD4(+)CD25(-) T cells, but not CD4(+)CD25(+) Treg cells, are cytolytic when redirected by a chimeric antigen receptor (CAR). Cancers (Basel) 2017, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hanafi, L.A.; Berger, C.; Hudecek, M.; Pender, B.; Robinson, E.; Hawkins, R.; Chaney, C.; Cherian, S.; Chen, X.; et al. Immunotherapy of non-hodgkin’s lymphoma with a defined ratio of cd8+ and cd4+ cd19-specific chimeric antigen receptor-modified t cells. Sci. Transl. Med. 2016, 8, 355ra116. [Google Scholar] [CrossRef]
- Wang, D.; Aguilar, B.; Starr, R.; Alizadeh, D.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; Forman, S.J.; Brown, C.E. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight 2018, 3, 99048. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific car t cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 130, 126397. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zi, Z.; Jin, Y.; Li, G.; Shao, K.; Cai, Q.; Ma, X.; Wei, F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol. Immunother. 2019, 68, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Morello, A.; Tano, Z.; Adusumilli, P.S. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology 2017, 6, e1273302. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Siddiqi, T.; Chavez, J.C.; Hosing, C.M.; Ghobadi, A.; Budde, L.E.; Bot, A.; Rossi, J.M.; et al. Phase 1 results of zuma-1: A multicenter study of kte-c19 anti-cd19 car t cell therapy in refractory aggressive lymphoma. Mol. Ther. 2017, 25, 285–295. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large b-cell lymphoma (zuma-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Xu, Q.; Harto, H.; Berahovich, R.; Xu, S.; Zhou, H.; Golubovskaya, V.; Wu, L. Generation of CAR-T cells for cancer immunotherapy. Methods Mol. Biol. 2019, 1884, 349–360. [Google Scholar]
- Golubovskaya, V.; Berahovich, R.; Zhou, H.; Xu, S.; Harto, H.; Li, L.; Chao, C.C.; Mao, M.M.; Wu, L. CD47-CAR-T cells effectively kill target cancer cells and block pancreatic tumor growth. Cancers (Basel) 2017, 9, 139. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berahovich, R.; Liu, X.; Zhou, H.; Tsadik, E.; Xu, S.; Golubovskaya, V.; Wu, L. Hypoxia Selectively Impairs CAR-T Cells In Vitro. Cancers 2019, 11, 602. https://doi.org/10.3390/cancers11050602
Berahovich R, Liu X, Zhou H, Tsadik E, Xu S, Golubovskaya V, Wu L. Hypoxia Selectively Impairs CAR-T Cells In Vitro. Cancers. 2019; 11(5):602. https://doi.org/10.3390/cancers11050602
Chicago/Turabian StyleBerahovich, Robert, Xianghong Liu, Hua Zhou, Elias Tsadik, Shirley Xu, Vita Golubovskaya, and Lijun Wu. 2019. "Hypoxia Selectively Impairs CAR-T Cells In Vitro" Cancers 11, no. 5: 602. https://doi.org/10.3390/cancers11050602
APA StyleBerahovich, R., Liu, X., Zhou, H., Tsadik, E., Xu, S., Golubovskaya, V., & Wu, L. (2019). Hypoxia Selectively Impairs CAR-T Cells In Vitro. Cancers, 11(5), 602. https://doi.org/10.3390/cancers11050602



