Differential Activation of ERK Signaling in HPV-Related Oropharyngeal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
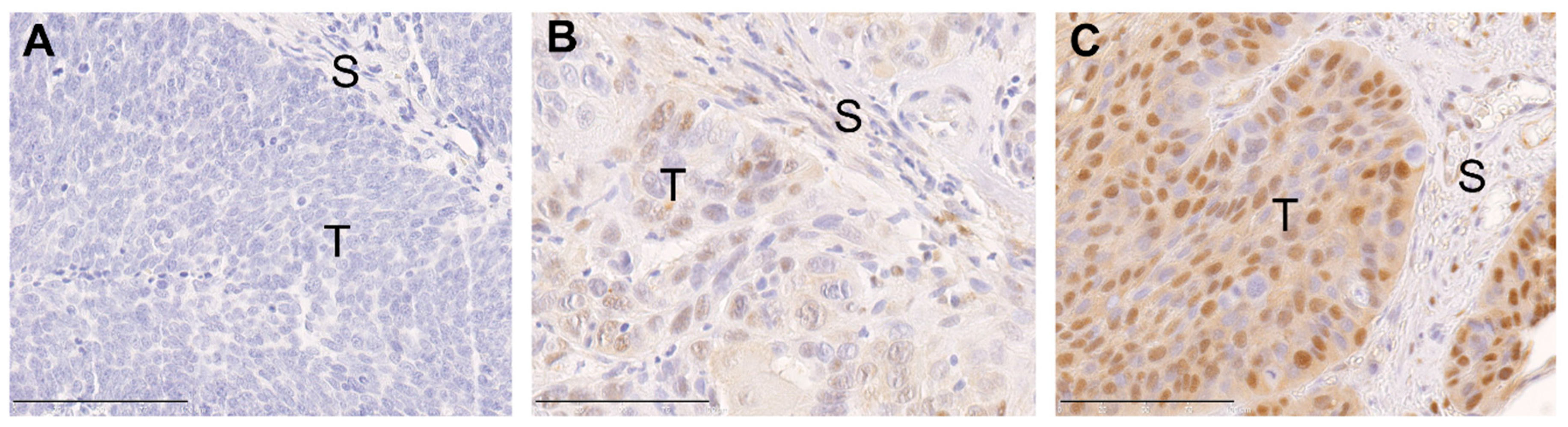
2.1. ERK1/2 Phosphorylation in Primary Tumors of Oropharyngeal Squamous Cell Carcinoma (OPSCC) Patients Is Associated with Human Papillomavirus (HPV) Status and Histopathological Grading
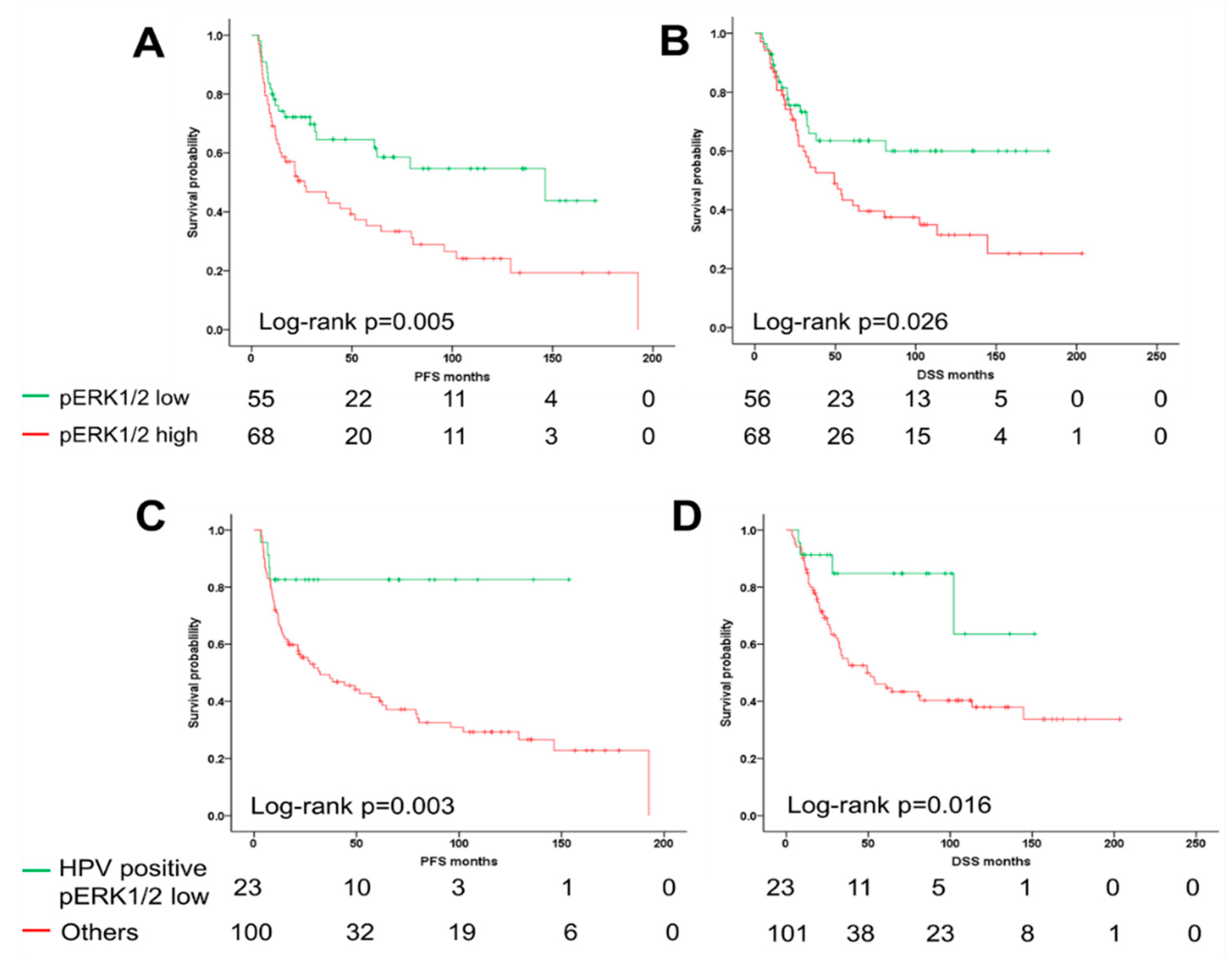
2.2. ERK1/2 Phosphorylation Is Associated with Unfavorable Clinical Outcome
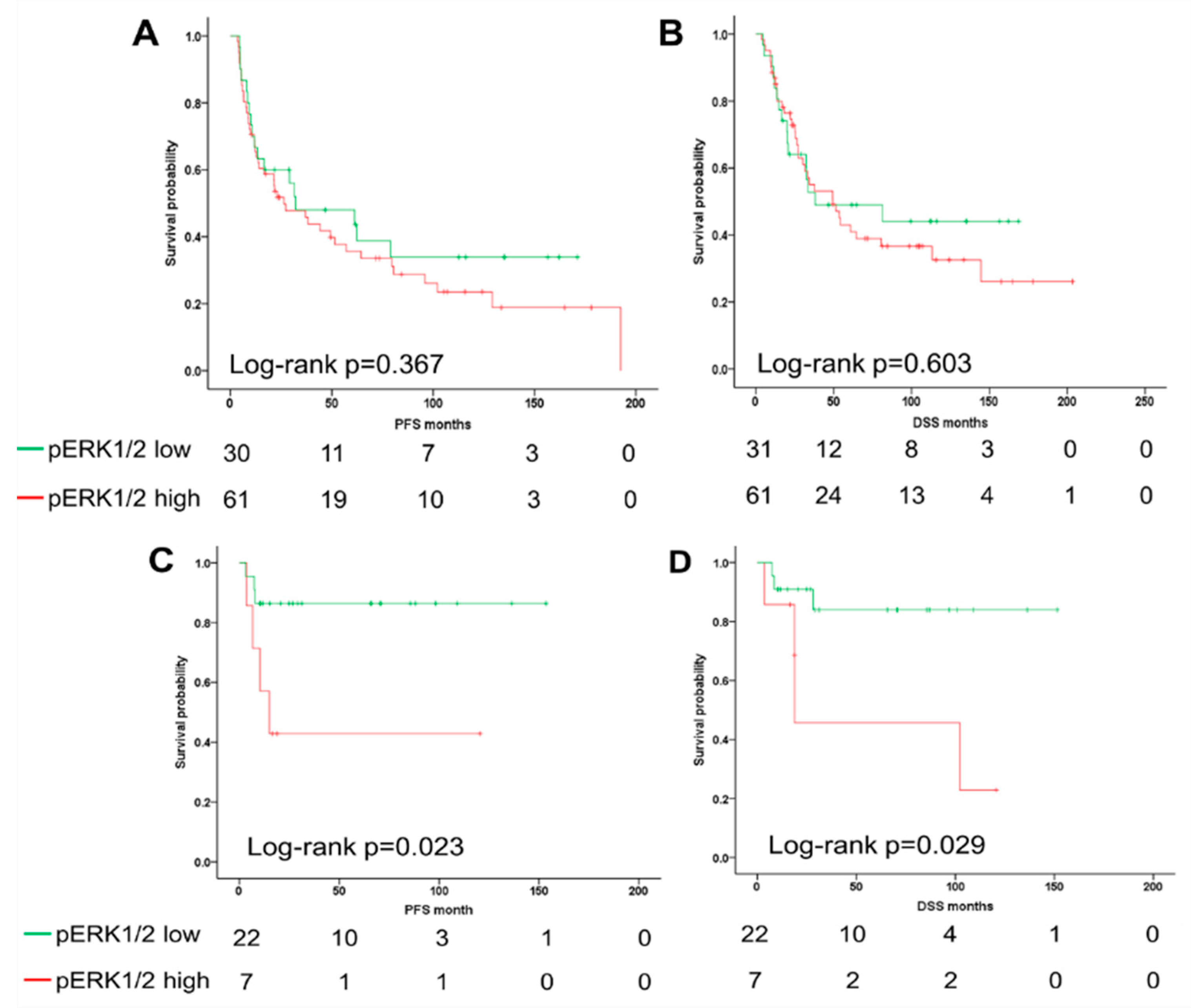
2.3. The ERK1/2 Phosphorylation Is a Prognostic Biomarker for the Survival of Oropharyngeal Squamous Cell Carcinoma (OPSCC) Patients Dependent on Human Papillomavirus (HPV) Status
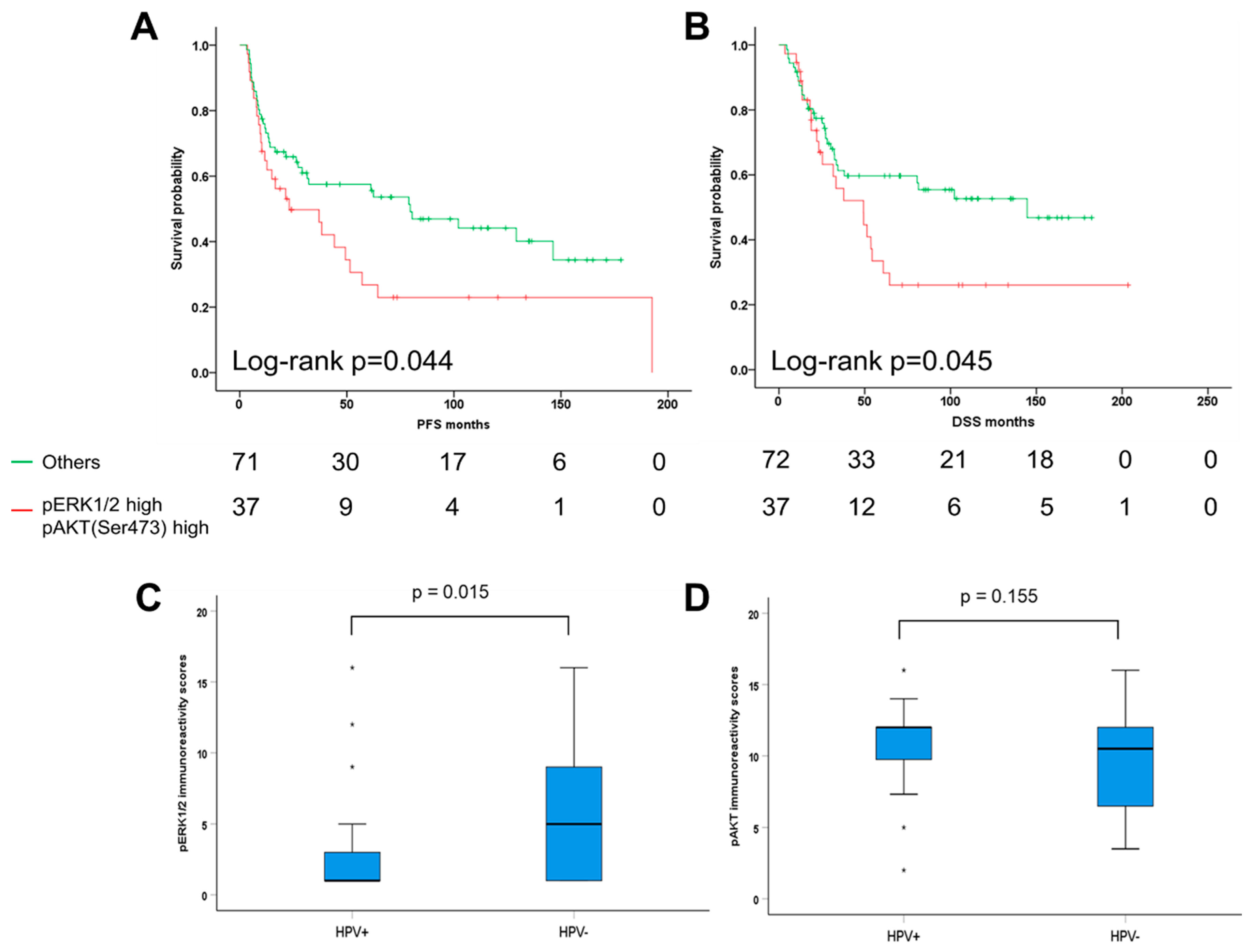
2.4. Combinatorial Analysis of ERK1/2 and AKT Phosphorylation in Oropharyngeal Squamous Cell Carcinoma (OPSCC)
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Human Papillomavirus (HPV) Genotyping and HPV16 RNA Analysis
4.3. Tissue Microarray and Immunohistochemistry
4.4. TCGA-HNSCC Dataset Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; Fernandez, L.; et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Davis, J.W.; Taylor, K.H.; Johnson, J.; Shi, Z.; Williams, R.; Atasoy, U.; Lewis, J.S., Jr.; Stack, M.S. Identification of a human papillomavirus-associated oncogenic mirna panel in human oropharyngeal squamous cell carcinoma validated by bioinformatics analysis of the cancer genome atlas. Am. J. Pathol. 2015, 185, 679–692. [Google Scholar] [CrossRef]
- Spence, T.; Bruce, J.; Yip, K.W.; Liu, F.F. Hpv associated head and neck cancer. Cancers 2016, 8, e75. [Google Scholar] [CrossRef]
- Mirghani, H.; Blanchard, P. Treatment de-escalation for hpv-driven oropharyngeal cancer: Where do we stand? Clin. Transl. Radiat. Oncol. 2018, 8, 4–11. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Sturgis, E.M.; Burtness, B.; Ridge, J.A.; Ringash, J.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (nrg oncology rtog 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (de-escalate hpv): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Sanchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Group, E.W. Prognoses and improvement for head and neck cancers diagnosed in europe in early 2000s: The eurocare-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef]
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023. [Google Scholar] [CrossRef]
- Mroz, E.A.; Rocco, J.W. Intra-tumor heterogeneity in head and neck cancer and its clinical implications. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Squarize, C.H.; Almeida, L.O. Epigenetic modifications and head and neck cancer: Implications for tumor progression and resistance to therapy. Int. J. Mol. Sci. 2017, 18, 1506. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Chiou, V.L.; Lee, J.M.; Kohn, E.C. The mapk pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- de la Cruz-Morcillo, M.A.; Garcia-Cano, J.; Arias-Gonzalez, L.; Garcia-Gil, E.; Artacho-Cordon, F.; Rios-Arrabal, S.; Valero, M.L.; Cimas, F.J.; Serrano-Oviedo, L.; Villas, M.V.; et al. Abrogation of the p38 mapk alpha signaling pathway does not promote radioresistance but its activity is required for 5-fluorouracil-associated radiosensitivity. Cancer Lett. 2013, 335, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Affolter, A.; Drigotas, M.; Fruth, K.; Schmidtmann, I.; Brochhausen, C.; Mann, W.J.; Brieger, J. Increased radioresistance via g12s k-ras by compensatory upregulation of mapk and pi3k pathways in epithelial cancer. Head Neck 2013, 35, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Affolter, A.; Fruth, K.; Brochhausen, C.; Schmidtmann, I.; Mann, W.J.; Brieger, J. Activation of mitogen-activated protein kinase extracellular signal-related kinase in head and neck squamous cell carcinomas after irradiation as part of a rescue mechanism. Head Neck 2011, 33, 1448–1457. [Google Scholar] [CrossRef]
- Molinolo, A.A.; Amornphimoltham, P.; Squarize, C.H.; Castilho, R.M.; Patel, V.; Gutkind, J.S. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009, 45, 324–334. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of pi3k/akt/mtor signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- LoRusso, P.M. Inhibition of the pi3k/akt/mtor pathway in solid tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Hess, J.; Freier, K.; Hoffmann, J.; Freudlsperger, C. Targeting egfr-pi3k-akt-mtor signaling enhances radiosensitivity in head and neck squamous cell carcinoma. Expert. Opin. Ther. Targets 2015, 19, 795–805. [Google Scholar] [CrossRef]
- Isaacsson Velho, P.H.; Castro, G., Jr.; Chung, C.H. Targeting the pi3k pathway in head and neck squamous cell carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2015, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Horn, D.; Freudlsperger, C.; Holzinger, D.; Kunzmann, K.; Plinkert, P.; Dyckhoff, G.; Hoffmann, J.; Freier, K.; Hess, J. Upregulation of pakt(ser473) expression in progression of hpv-positive oropharyngeal squamous cell carcinoma. Head Neck 2017, 39, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B. Current challenges and clinical investigations of epidermal growth factor receptor (egfr)- and erbb family-targeted agents in the treatment of head and neck squamous cell carcinoma (hnscc). Cancer Treat. Rev. 2014, 40, 567–577. [Google Scholar] [CrossRef]
- Holzinger, D.; Schmitt, M.; Dyckhoff, G.; Benner, A.; Pawlita, M.; Bosch, F.X. Viral rna patterns and high viral load reliably define oropharynx carcinomas with active hpv16 involvement. Cancer Res. 2012, 72, 4993–5003. [Google Scholar] [CrossRef] [PubMed]
- Syrjanen, S. Human papillomavirus (hpv) in head and neck cancer. J. Clin. Virol. 2005, 32, S59–S66. [Google Scholar] [CrossRef]
- Bussink, J.; van der Kogel, A.J.; Kaanders, J.H. Activation of the pi3-k/akt pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008, 9, 288–296. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cbio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cbioportal. Sci. Sig. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Maiello, M.R.; D’Alessio, A.; Pergameno, M.; Normanno, N. The ras/raf/mek/erk and the pi3k/akt signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert. Opin. Ther. Targets 2012, 16, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yi, H.M.; Yi, H.; Qu, J.Q.; Zhu, J.F.; Li, L.N.; Xiao, T.; Zheng, Z.; Lu, S.S.; Xiao, Z.Q. Reduced rkip enhances nasopharyngeal carcinoma radioresistance by increasing erk and akt activity. Oncotarget 2016, 7, 11463–11477. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, J.; Wang, S.; Li, M.; Wang, F.; Li, C.; Zhang, Y. Aberrant expression of extracellular signal-regulated kinase and 15-hydroxyprostaglandin dehydrogenase indicates radiation resistance and poor prognosis for patients with clival chordomas. World Neurosurg. 2018, 115, e146–e151. [Google Scholar] [CrossRef]
- Affolter, A.; Samosny, G.; Heimes, A.S.; Schneider, J.; Weichert, W.; Stenzinger, A.; Sommer, K.; Jensen, A.; Mayer, A.; Brenner, W.; et al. Multikinase inhibitors sorafenib and sunitinib as radiosensitizers in head and neck cancer cell lines. Head Neck 2017, 39, 623–632. [Google Scholar] [CrossRef]
- Adeyinka, A.; Nui, Y.; Cherlet, T.; Snell, L.; Watson, P.H.; Murphy, L.C. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin. Cancer Res. 2002, 8, 1747–1753. [Google Scholar] [PubMed]
- McClelland, R.A.; Barrow, D.; Madden, T.A.; Dutkowski, C.M.; Pamment, J.; Knowlden, J.M.; Gee, J.M.; Nicholson, R.I. Enhanced epidermal growth factor receptor signaling in mcf7 breast cancer cells after long-term culture in the presence of the pure antiestrogen ici 182,780 (faslodex). Endocrinology 2001, 142, 2776–2788. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.M.; Robertson, J.F.; Ellis, I.O.; Nicholson, R.I. Phosphorylation of erk1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int. J. Cancer 2001, 95, 247–254. [Google Scholar] [CrossRef]
- Bartholomeusz, C.; Itamochi, H.; Nitta, M.; Saya, H.; Ginsberg, M.H.; Ueno, N.T. Antitumor effect of e1a in ovarian cancer by cytoplasmic sequestration of activated erk by pea15. Oncogene 2006, 25, 79–90. [Google Scholar] [CrossRef]
- Houben, R.; Becker, J.C.; Kappel, A.; Terheyden, P.; Brocker, E.B.; Goetz, R.; Rapp, U.R. Constitutive activation of the ras-raf signaling pathway in metastatic melanoma is associated with poor prognosis. J. Carcinog. 2004, 3, 6. [Google Scholar] [CrossRef][Green Version]
- Schmitz, K.J.; Wohlschlaeger, J.; Lang, H.; Sotiropoulos, G.C.; Malago, M.; Steveling, K.; Reis, H.; Cicinnati, V.R.; Schmid, K.W.; Baba, H.A. Activation of the erk and akt signalling pathway predicts poor prognosis in hepatocellular carcinoma and erk activation in cancer tissue is associated with hepatitis c virus infection. J. Hepatol. 2008, 48, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.H.; Yu, J.; Shang, L.; Zhu, Y.Q.; Hao, J.J.; Cai, Y.; Xu, X.; Zhang, Y.; Wang, M.R. Kiaa1522 overexpression promotes tumorigenicity and metastasis of esophageal cancer cells through potentiating the erk activity. OncoTargets Ther. 2017, 10, 3743–3754. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Xu, Y.; Wang, Y.H.; Sun, H.X.; Sun, Y.F.; He, Y.F.; Zhu, Q.F.; Hu, B.; Zhang, X.; Xia, J.L.; et al. Hoxb7 promotes tumor progression via bfgf-induced activation of mapk/erk pathway and indicated poor prognosis in hepatocellular carcinoma. Oncotarget 2017, 8, 47121–47135. [Google Scholar] [CrossRef] [PubMed]
- Hew, K.E.; Miller, P.C.; El-Ashry, D.; Sun, J.; Besser, A.H.; Ince, T.A.; Gu, M.; Wei, Z.; Zhang, G.; Brafford, P.; et al. Mapk activation predicts poor outcome and the mek inhibitor, selumetinib, reverses antiestrogen resistance in er-positive high-grade serous ovarian cancer. Clin. Cancer Res. 2016, 22, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B.; Xu, W.; Zhao, H.; Chen, D.D.; Ringash, J.; Hope, A.; Razak, A.; Gilbert, R.; Irish, J.; et al. Temporal nodal regression and regional control after primary radiation therapy for n2-n3 head-and-neck cancer stratified by hpv status. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 1078–1085. [Google Scholar] [CrossRef]
- Mirghani, H.; Amen, F.; Tao, Y.; Deutsch, E.; Levy, A. Increased radiosensitivity of hpv-positive head and neck cancers: Molecular basis and therapeutic perspectives. Cancer Treat. Rev. 2015, 41, 844–852. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef]
- Krigsfeld, G.S.; Chung, C.H. Novel targets in head and neck cancer: Should we be optimistic? Clin. Cancer Res. 2015, 21, 495–497. [Google Scholar] [CrossRef]
- Aguilar-Martinez, E.; Morrisroe, C.; Sharrocks, A.D. The ubiquitin ligase ube3a dampens erk pathway signalling in hpv e6 transformed hela cells. PloS ONE 2015, 10, e0119366. [Google Scholar] [CrossRef][Green Version]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zouhair, A.; Azria, D.; Ozsahin, M. The epidermal growth factor receptor (egfr) in head and neck cancer: Its role and treatment implications. Radiat. Oncol. 2006, 1, 11. [Google Scholar] [CrossRef]
- Kalyankrishna, S.; Grandis, J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- Hong, A.; Dobbins, T.; Lee, C.S.; Jones, D.; Jackson, E.; Clark, J.; Armstrong, B.; Harnett, G.; Milross, C.; O’Brien, C.; et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur. J. Cancer 2010, 46, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Cordell, K.G.; Lee, J.S.; Worden, F.P.; Prince, M.E.; Tran, H.H.; Wolf, G.T.; Urba, S.G.; Chepeha, D.B.; Teknos, T.N.; et al. Egfr, p16, hpv titer, bcl-xl and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J. Clin. Oncol. 2008, 26, 3128–3137. [Google Scholar] [CrossRef] [PubMed]
- Al-Swiahb, J.N.; Huang, C.C.; Fang, F.M.; Chuang, H.C.; Huang, H.Y.; Luo, S.D.; Chen, C.H.; Chen, C.M.; Chien, C.Y. Prognostic impact of p16, p53, epidermal growth factor receptor, and human papillomavirus in oropharyngeal cancer in a betel nut-chewing area. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 502–508. [Google Scholar] [CrossRef]
- Freier, K.; Joos, S.; Flechtenmacher, C.; Devens, F.; Benner, A.; Bosch, F.X.; Lichter, P.; Hofele, C. Tissue microarray analysis reveals site-specific prevalence of oncogene amplifications in head and neck squamous cell carcinoma. Cancer Res. 2003, 63, 1179–1182. [Google Scholar] [PubMed]
- Bayo, P.; Jou, A.; Stenzinger, A.; Shao, C.; Gross, M.; Jensen, A.; Grabe, N.; Mende, C.H.; Rados, P.V.; Debus, J.; et al. Loss of sox2 expression induces cell motility via vimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. Mol. Oncol. 2015, 9, 1704–1719. [Google Scholar] [CrossRef]
- Santarelli, A.; Mascitti, M.; Rubini, C.; Bambini, F.; Giannatempo, G.; Lo Russo, L.; Sartini, D.; Emanuelli, M.; Procaccini, M.; Lo Muzio, L. Nuclear survivin as a prognostic factor in squamous-cell carcinoma of the oral cavity. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 566–570. [Google Scholar] [CrossRef]
- Freudlsperger, C.; Horn, D.; Weissfuss, S.; Weichert, W.; Weber, K.J.; Saure, D.; Sharma, S.; Dyckhoff, G.; Grabe, N.; Plinkert, P.; et al. Phosphorylation of akt(ser473) serves as an independent prognostic marker for radiosensitivity in advanced head and neck squamous cell carcinoma. Int. J. Cancer 2015, 136, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
| Clinicopathological Features | pERK1/2 low | pERK1/2 high | p-Value |
|---|---|---|---|
| Age(years) | 0.060 | ||
| <58 | 21 | 37 | |
| ≥58 | 35 | 31 | |
| Gender | 0.677 | ||
| Male | 43 | 50 | |
| Female | 13 | 18 | |
| TNM status | |||
| T1/T2 | 28 | 31 | 0.680 |
| T3/T4 | 28 | 36 | |
| missing | 1 | ||
| N0 | 10 | 19 | 0.172 |
| N+ | 46 | 48 | |
| missing | 1 | ||
| Pathological Grade | 0.039 | ||
| G1/G2 | 27 | 37 | |
| G3 | 28 | 17 | |
| missing | 1 | 14 | |
| Tobacco | 0.293 | ||
| Never/former | 17 | 15 | |
| Current | 39 | 53 | |
| Alcohol | 0.371 | ||
| Never/former | 11 | 18 | |
| Current | 45 | 50 | |
| HPV | <0.001 | ||
| HPV- | 31 | 61 | |
| HPV+ | 22 | 7 | |
| missing | 3 | ||
| Therapy | 0.231 | ||
| RT | 51 | 57 | |
| Non-RT | 5 | 11 |
| Factors | PFS | DSS | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age(years) | 0.925 (0.576–1.484) | 0.747 | 1.037 (0.621–1.732) | 0.890 |
| ≥58 vs. <58 | ||||
| Gender | 1.494 (0.816–2.737) | 0.193 | 1.797 (0.909–3.549) | 0.092 |
| male vs. female | ||||
| T status | 2.000 (1.210–3.308) | 0.007 | 2.321 (1.328–4.054) | 0.003 |
| T3–4 vs. T1–2 | ||||
| N status | 1.433 (0.793–2.588) | 0.233 | 2.274 (1.115–4.636) | 0.024 |
| N+ vs. N0 | ||||
| pathological Grade | 0.842 (0.483–1.467) | 0.543 | 0.928 (0.507–1.698) | 0.808 |
| G3 vs. G1–2 | ||||
| Tobacco | 3.094 (1.575–6.077) | 0.001 | 2.781 (1.318–5.866) | 0.007 |
| Current vs. Never/former | ||||
| Alcohol | 1.305 (0.712–2.392) | 0.389 | 1.347 (0.699–2.594) | 0.373 |
| Current vs. Never/former | ||||
| HPV status | 0.357 (0.163–0.782) | 0.010 | 0.452 (0.205–0.996) | 0.049 |
| Driven vs. Non-driven | ||||
| Therapy | 1.578 (0.722–3.451) | 0.253 | 2.507 (0.908–6.922) | 0.076 |
| RT vs. Non-RT | ||||
| Phospho-ERK1/2 | 2.042 (1.229–3.392) | 0.006 | 1.844 (1.068–3.185) | 0.028 |
| High vs. Low | ||||
| Factors | PFS | DSS | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| T status | 1.751 (1.028–2.984) | 0.039 | 1.740 (0.969–3.122) | 0.064 |
| T3–4 vs. T1–2 | ||||
| N status | 1.656 (0.881–3.114) | 0.117 | 2.637 (1.248–5.570) | 0.011 |
| N+ vs. N0 | ||||
| Tobacco | 2.684 (1.350–5.334) | 0.005 | 2.589 (1.213–5.529) | 0.014 |
| Current vs. Never/former | ||||
| HPV status | 0.535 (0.232–1.236) | 0.143 | 0.615 (0.265–1.430) | 0.259 |
| Driven vs. Non-driven | ||||
| Phospho-ERK1/2 | 1.746 (1.009–3.023) | 0.046 | 1.653 (0.923–2.958) | 0.091 |
| High vs. Low | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, C.; Muller, M.; Flechtenmacher, C.; Holzinger, D.; Dyckhoff, G.; Bulut, O.C.; Horn, D.; Plinkert, P.; Hess, J.; Affolter, A. Differential Activation of ERK Signaling in HPV-Related Oropharyngeal Squamous Cell Carcinoma. Cancers 2019, 11, 584. https://doi.org/10.3390/cancers11040584
Rong C, Muller M, Flechtenmacher C, Holzinger D, Dyckhoff G, Bulut OC, Horn D, Plinkert P, Hess J, Affolter A. Differential Activation of ERK Signaling in HPV-Related Oropharyngeal Squamous Cell Carcinoma. Cancers. 2019; 11(4):584. https://doi.org/10.3390/cancers11040584
Chicago/Turabian StyleRong, Chao, Marie Muller, Christa Flechtenmacher, Dana Holzinger, Gerhard Dyckhoff, Olcay Cem Bulut, Dominik Horn, Peter Plinkert, Jochen Hess, and Annette Affolter. 2019. "Differential Activation of ERK Signaling in HPV-Related Oropharyngeal Squamous Cell Carcinoma" Cancers 11, no. 4: 584. https://doi.org/10.3390/cancers11040584
APA StyleRong, C., Muller, M., Flechtenmacher, C., Holzinger, D., Dyckhoff, G., Bulut, O. C., Horn, D., Plinkert, P., Hess, J., & Affolter, A. (2019). Differential Activation of ERK Signaling in HPV-Related Oropharyngeal Squamous Cell Carcinoma. Cancers, 11(4), 584. https://doi.org/10.3390/cancers11040584




