MRI of Uveal Melanoma
Abstract
1. Introduction
2. Material and Methods
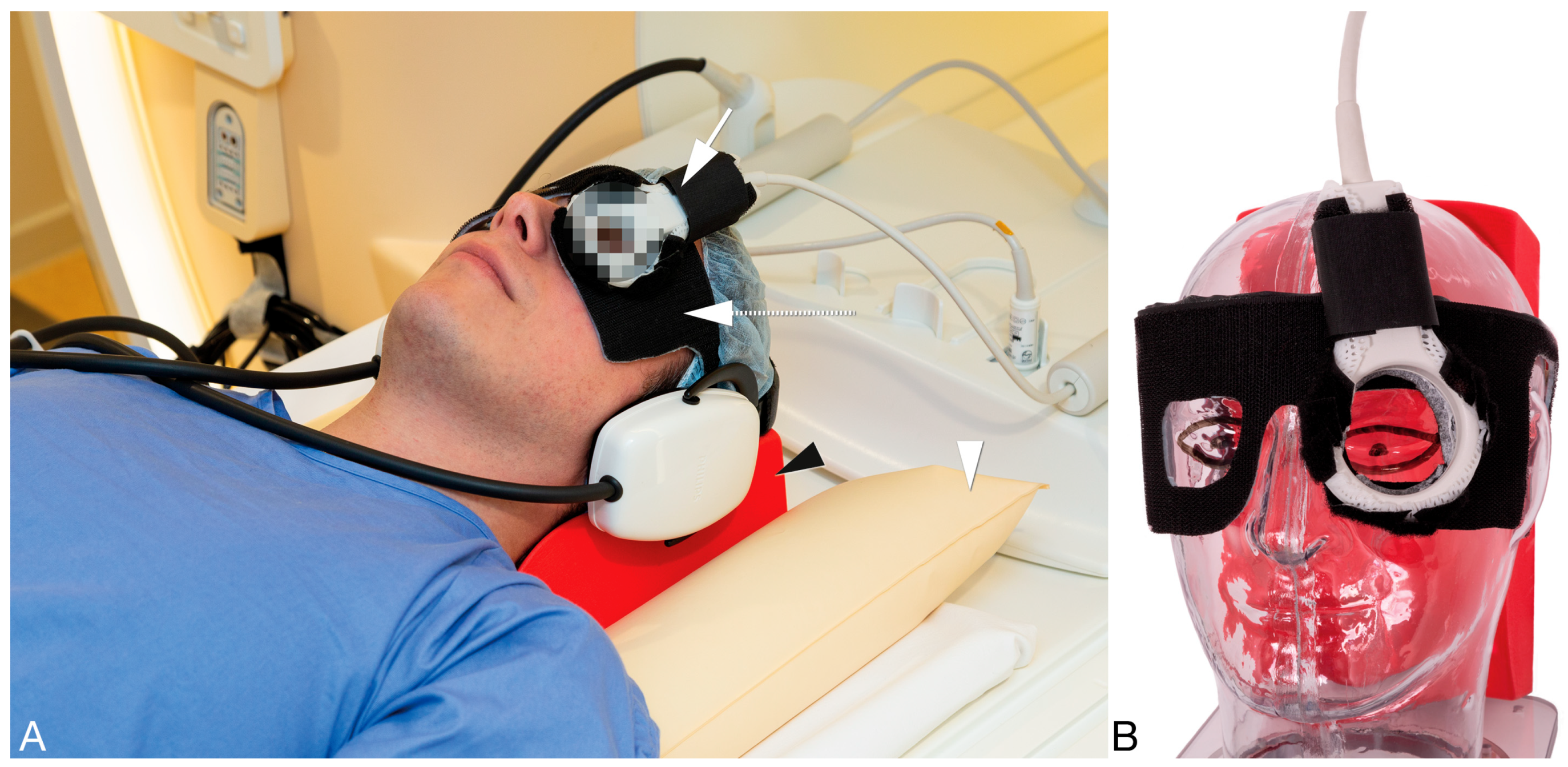
2.1. General MRI Setup
2.2. MRI Sequences
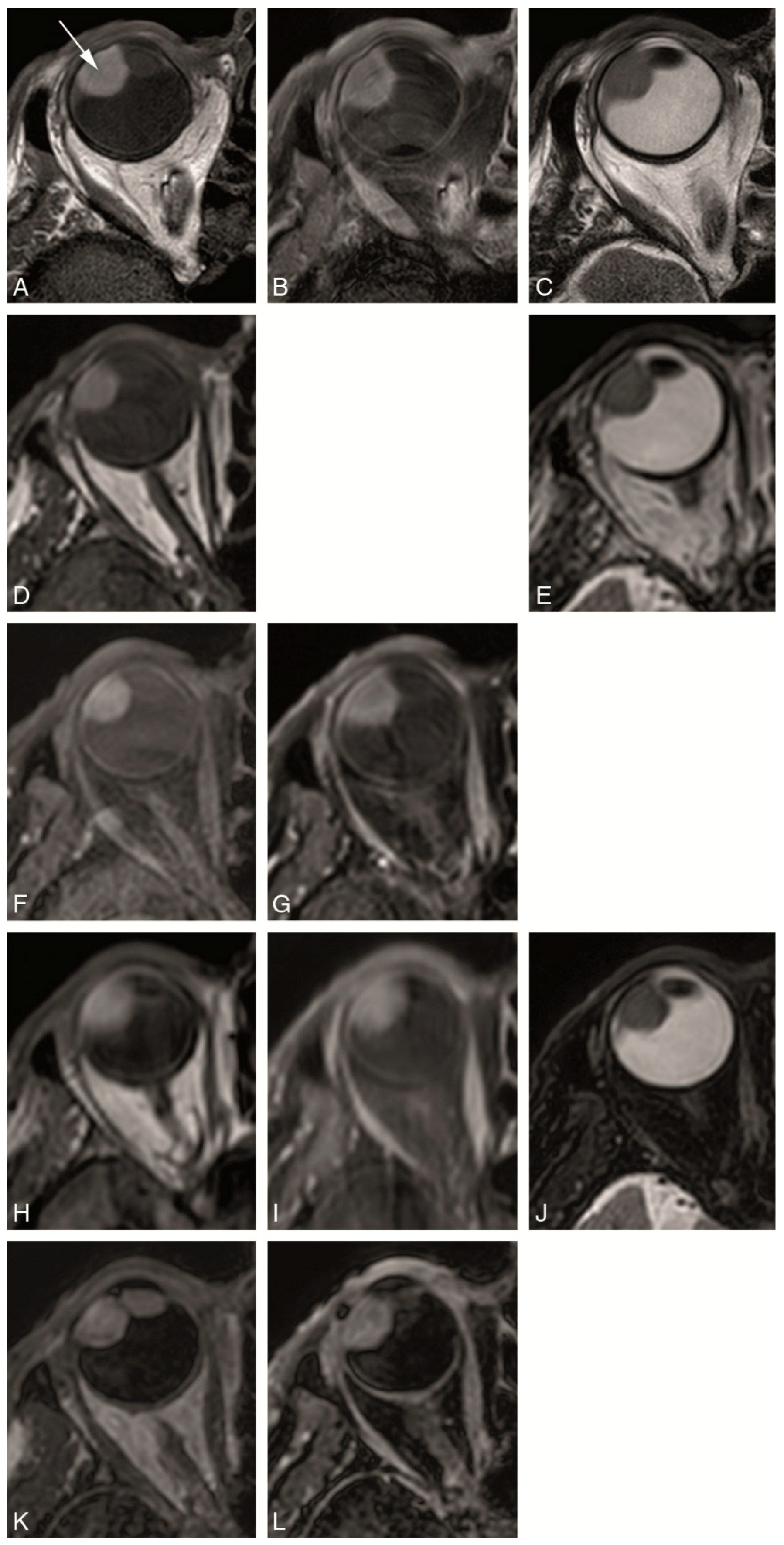
2.3. Anatomical MRI Sequences
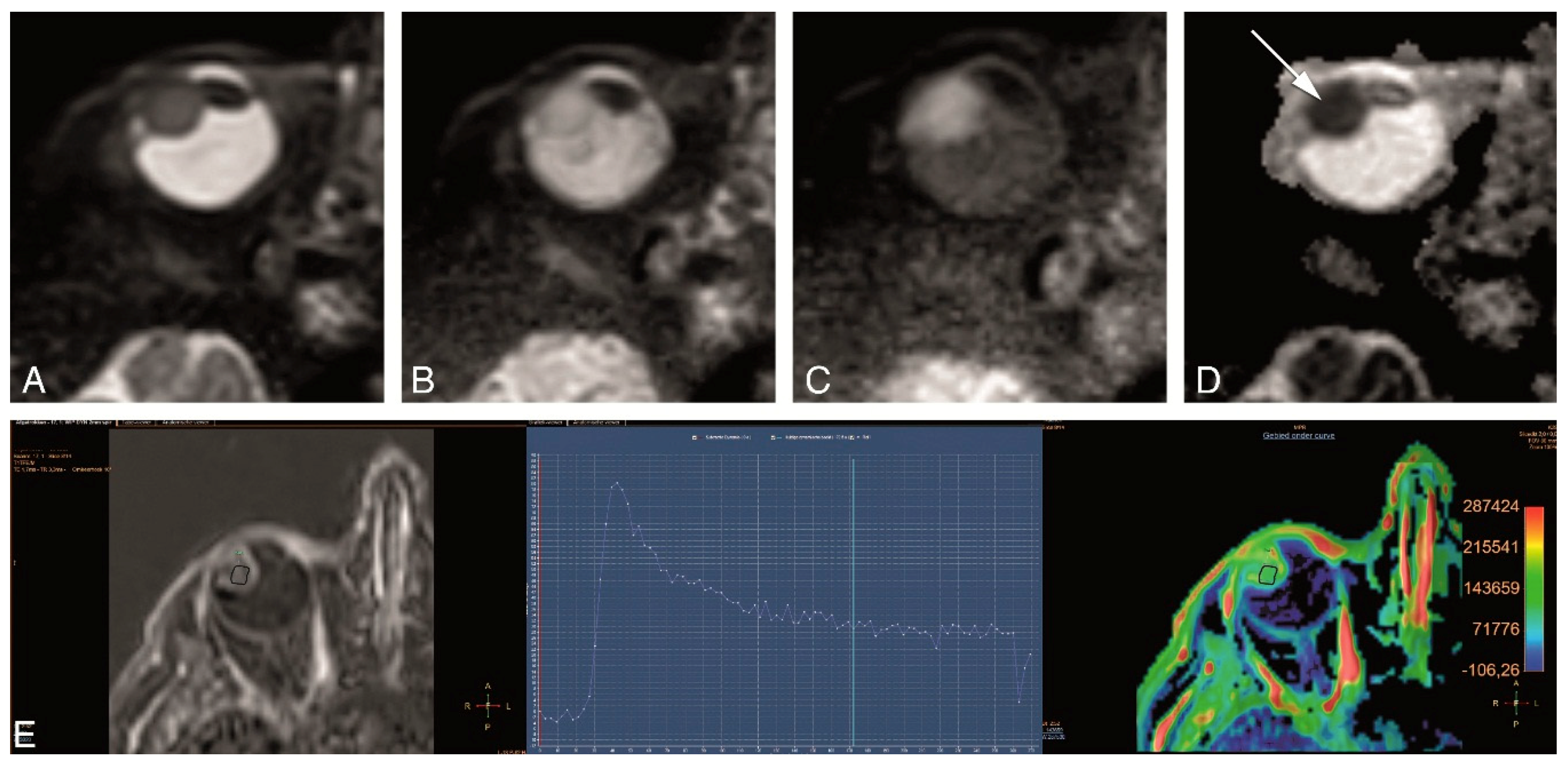
2.4. Functional MRI Sequences
2.5. Evaluation
3. Results
4. Discussion
4.1. General Technical Requirements for Ocular MRI
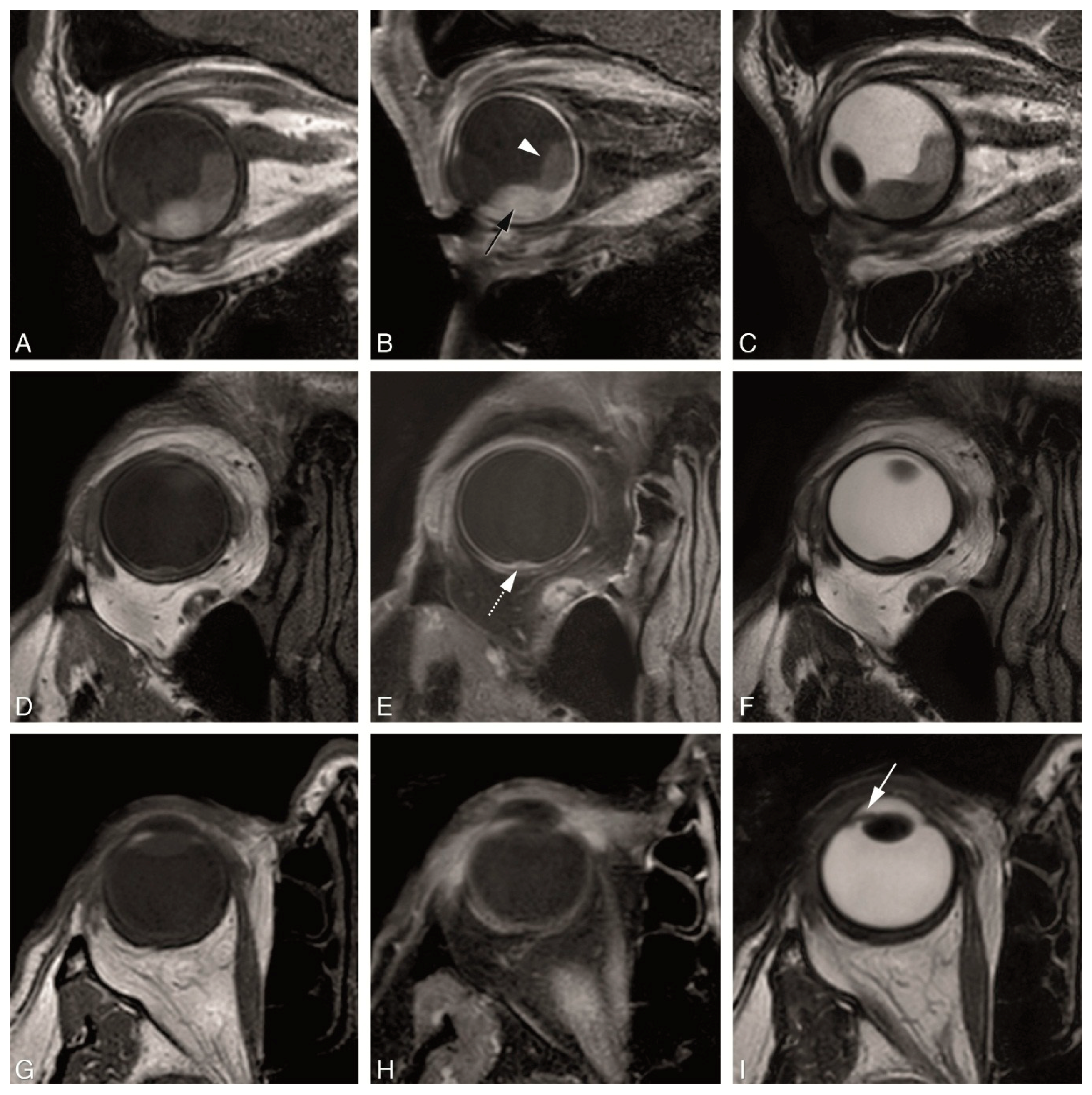
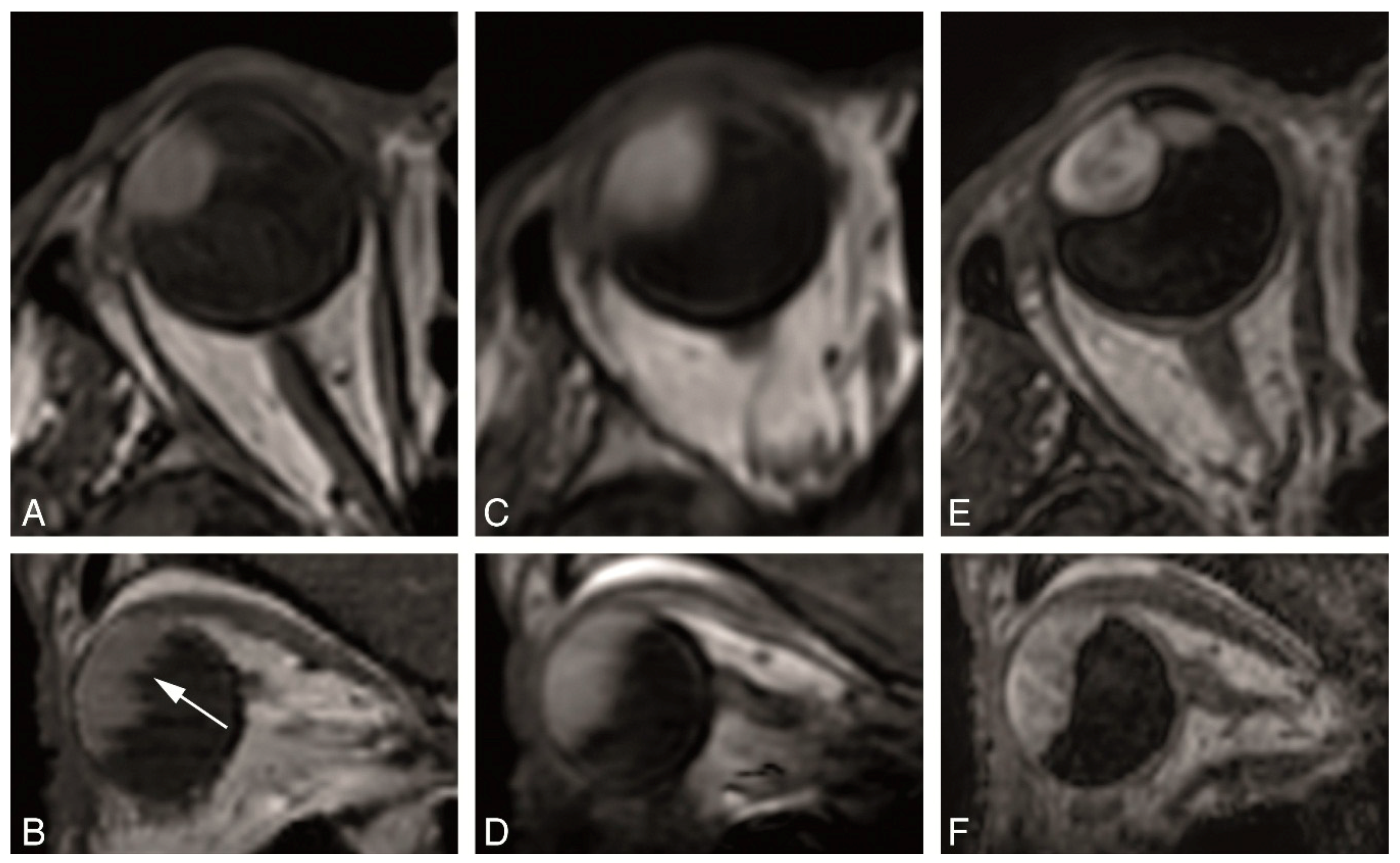
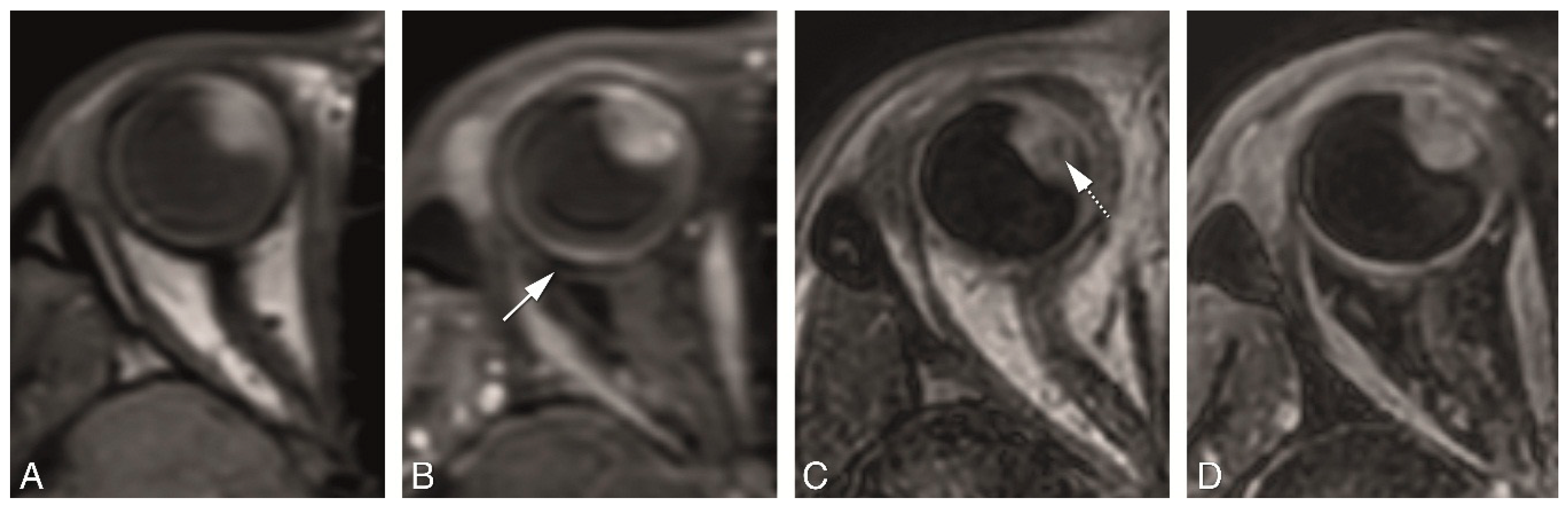
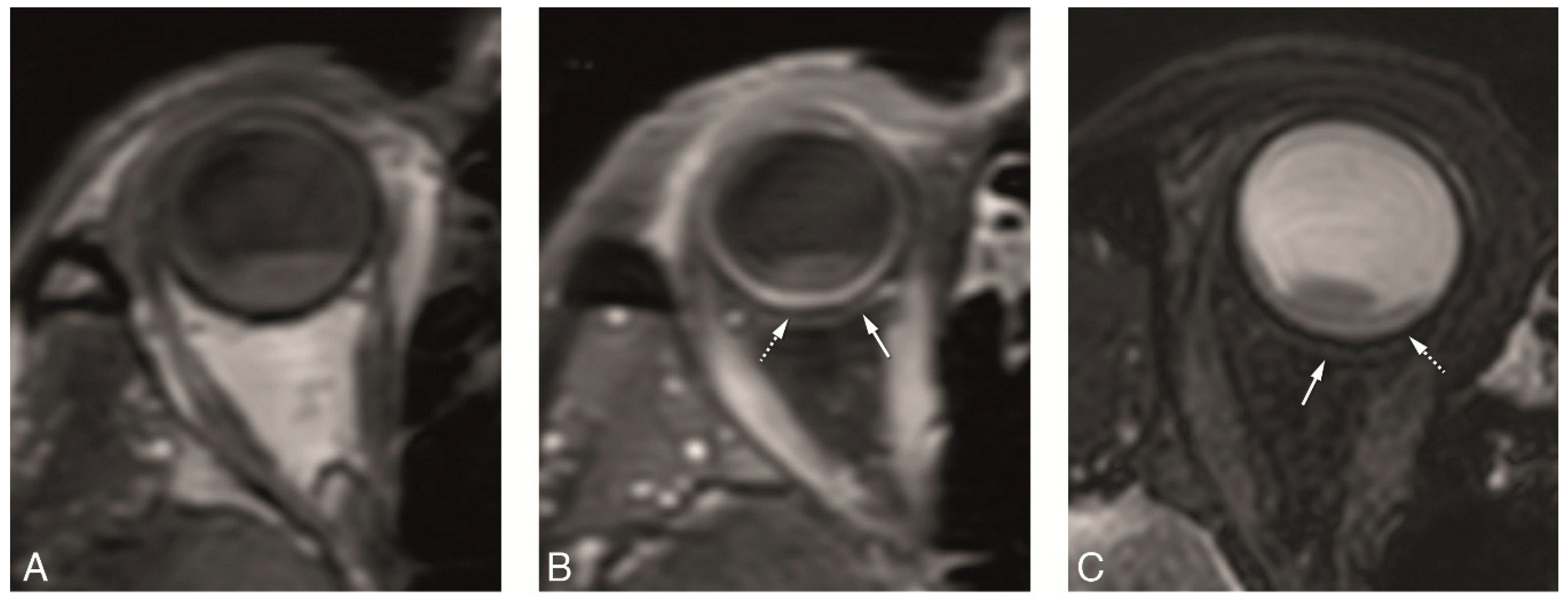
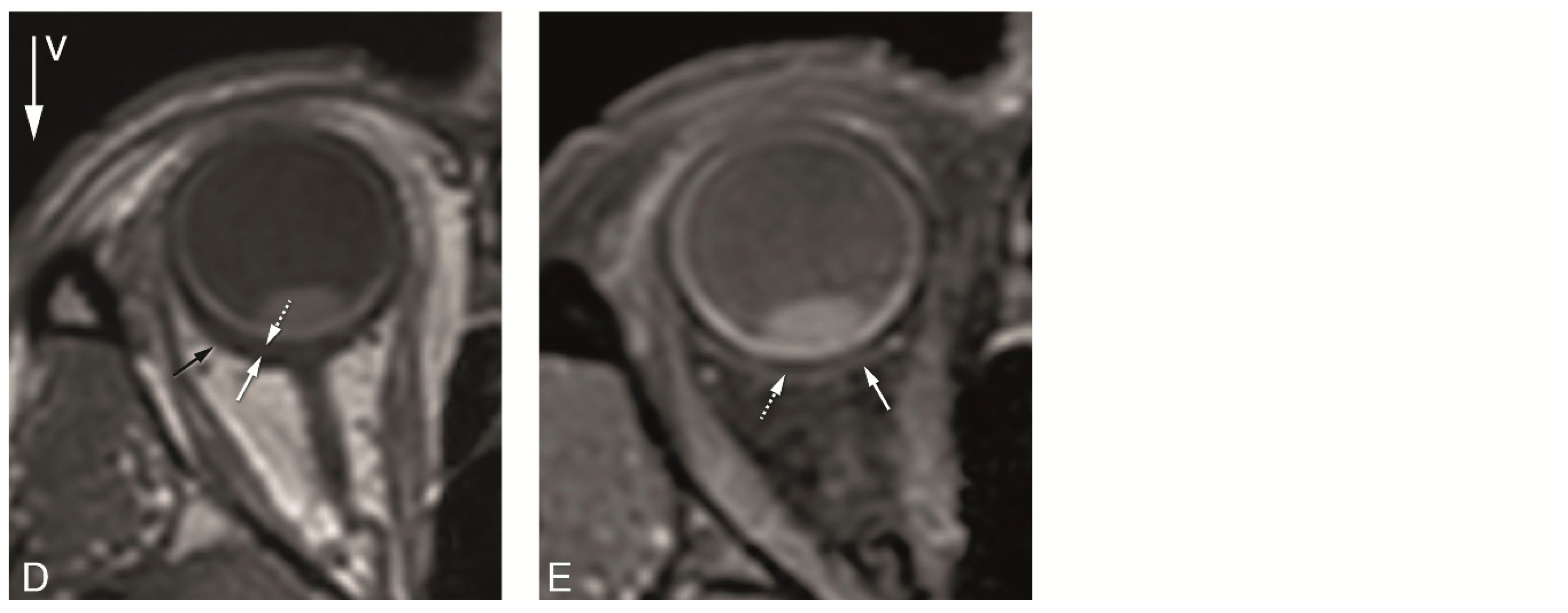
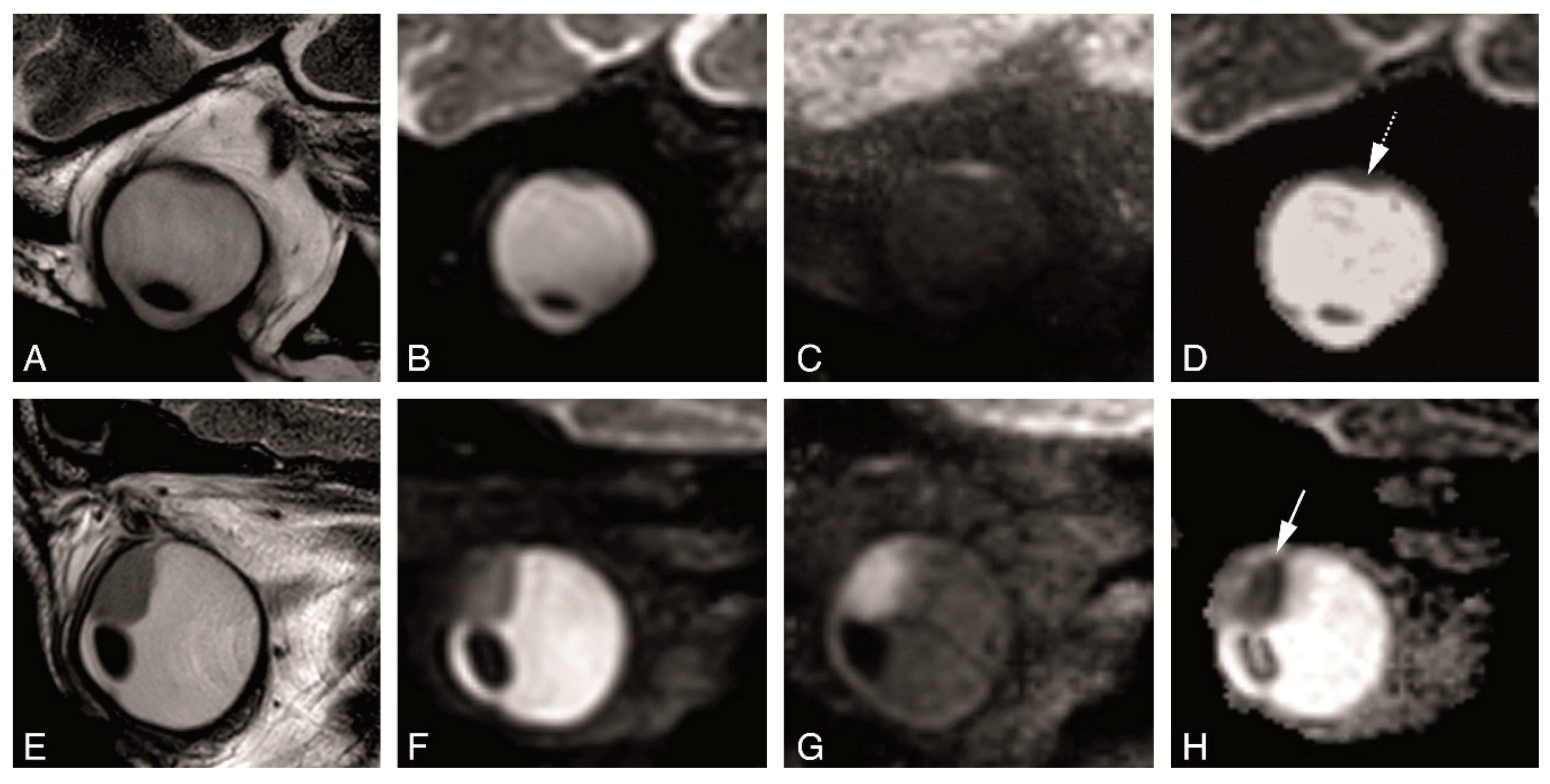
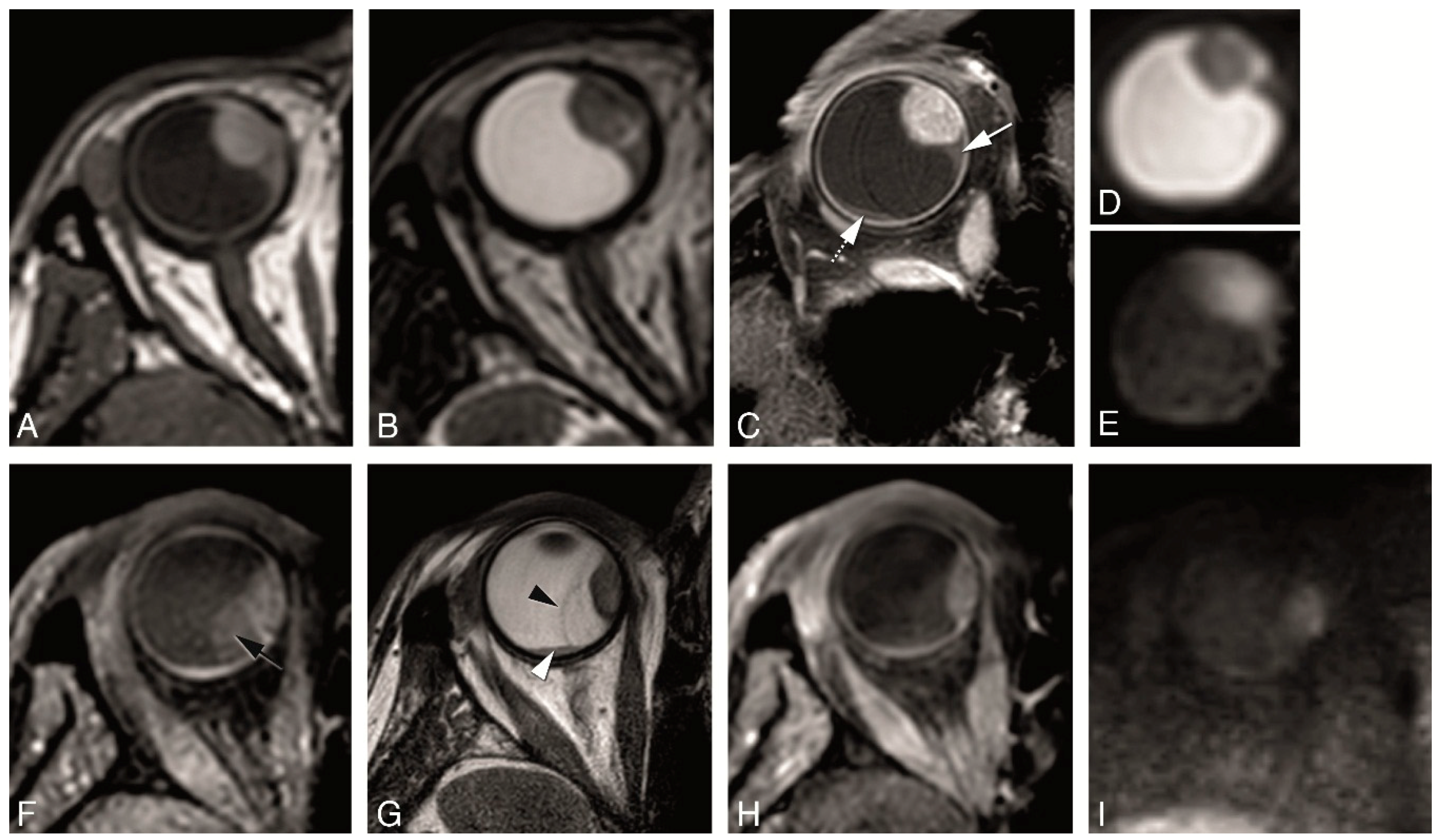
4.2. Anatomical MRI of Uveal Melanoma
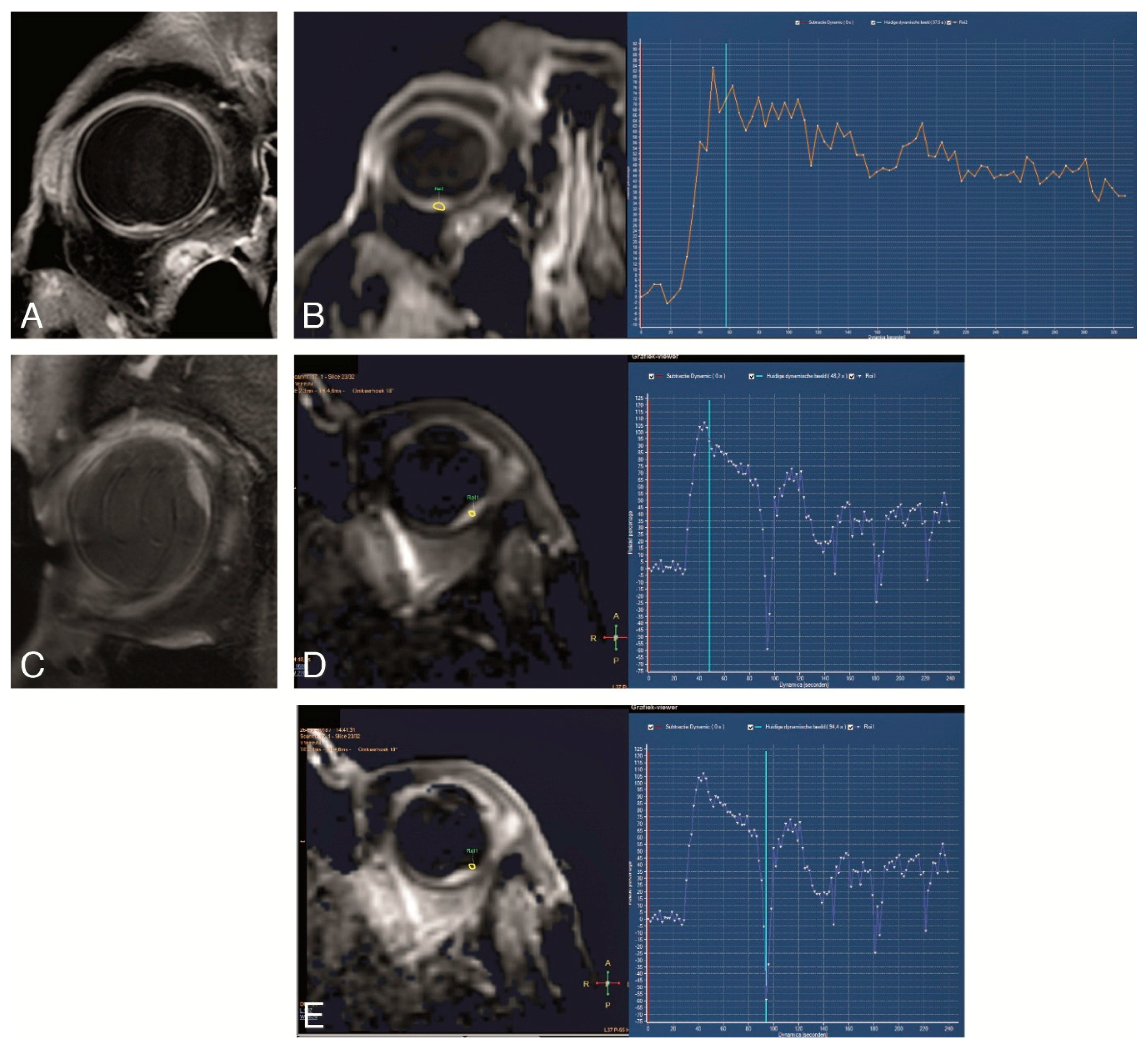
4.3. Functional MRI of Uveal Melanoma
4.4. Clinical MRI Protocol for UM
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weis, E.; Salopek, T.G.; McKinnon, J.G.; Larocque, M.P.; Temple-Oberle, C.; Cheng, T.; McWhae, J.; Sloboda, R.; Shea-Budgell, M. Management of uveal melanoma: A consensus-based provincial clinical practice guideline. Curr. Oncol. 2016, 23, e57–e64. [Google Scholar] [CrossRef]
- Dieckmann, K.; Georg, D.; Zehetmayer, M.; Bogner, J.; Georgopoulos, M.; Pötter, R. LINAC based stereotactic radiotherapy of uveal melanoma: 4 years clinical experience. Radiother. Oncol. 2003, 67, 199–206. [Google Scholar] [CrossRef]
- Schueller, P.; Dogan, A.; Panke, J.E.; Micke, O.; Willich, N. Does the imaging method have an influence on the measured tumor height in ruthenium plaque therapy of uveal melanoma? Strahlenther. Onkol. 2005, 181, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.R.; Damato, B.E. Uveal melanoma: Evidence for efficacy of therapy. Int. Ophthalmol. Clin. 2015, 55, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Longo, A.; Reibaldi, M.; Russo, A.; Privitera, G.; Spatola, C.; Raffaele, L.; Salamone, V.; Farina, R.; Palmucci, S.; et al. Uveal melanoma: Quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol. Med. 2017, 122, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kamrava, M.; Sepahdari, A.R.; Leu, K.; Wang, P.-C.; Roberts, K.; Demanes, D.J.; McCannel, T.; Ellingson, B.M. Quantitative multiparametric MRI in uveal melanoma: Increased tumor permeability may predict monosomy 3. Neuroradiology 2015, 57, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Dopierala, J.; Damato, B.E.; Lake, S.L.; Taktak, A.F.G.; Coupland, S.E. Genetic heterogeneity in uveal melanoma assessed by multiplex ligation-dependent probe amplification. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4898–4905. [Google Scholar] [CrossRef] [PubMed]
- Schoenfield, L.; Pettay, J.; Tubbs, R.R.; Singh, A.D. Variation of monosomy 3 status within uveal melanoma. Arch. Pathol. Lab. Med. 2009, 133, 1219–1222. [Google Scholar]
- Beenakker, J.-W.M.; Ferreira, T.A.; Soemarwoto, K.P.; Genders, S.W.; Teeuwisse, W.M.; Webb, A.G.; Luyten, G.P.M. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. Magn. Reason. Mater. Phys. Biol. Med. 2016, 29, 571–577. [Google Scholar] [CrossRef]
- Lemke, A.J.; Hosten, N.; Wiegel, T.; Prinz, R.D.; Richter, M.; Bechrakis, N.E.; Foerster, P.I.; Felix, R. Intraocular metastases: Differential diagnosis from uveal melanomas with high-resolution MRI using a surface coil. Eur. Radiol. 2001, 11, 2593–2601. [Google Scholar] [CrossRef]
- Jaarsma-Coes, M.G.; van Haren, G.R.; Ferreira, T.A.; Marinkovic, M.; Beenakker, J.W.M. MR-imaging enables accurate diagnosis and follow-up in uveal melanoma patients after vitrectomy. Melanoma Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.L.; Morton, S.J.; Shields, C.L. American joint committee on cancer staging of uveal melanoma. Oman J. Ophthalmol. 2013, 6, 116–118. [Google Scholar]
- De Graaf, P.; Göricke, S.; Rodjan, F.; Galluzzi, P.; Maeder, P.; Castelijns, J.A.; Brisse, H.J. Guidelines for imaging retinoblastoma: Imaging principles and MRI standardization. Pediatr. Radiol. 2012, 42, 2–14. [Google Scholar] [CrossRef]
- Vokurka, E.A.; Watson, N.A.; Watson, Y.; Thacker, N.A.; Jackson, A. Improved high resolution MR imaging for surface coils using automated intensity non-uniformity correction: Feasibility study in the orbit. J. Magn. Reson. Imaging 2001, 14, 540–546. [Google Scholar] [CrossRef]
- Berkowitz, B.; Detroit, M.; Canfield, D.; McDonald, C.; Ito, Y.; Tofts, P.; London, U.; Latif, Z.; Gross, J. Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: Eliminating blinking artifacts and demonstrating proof of concept. Magn. Reason. Med. 2001, 46, 412–416. [Google Scholar] [CrossRef]
- Beenakker, J.W.M.; van Rijn, G.A.; Luyten, G.P.M.; Webb, A.G. High-resolution MRI of uveal melanoma using a microcoil phased array at 7 T. NMR Biomed. 2013, 26, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Van Vaals, J.J.; Brummer, M.E.; Dixon, W.T.; Tuithof, H.H.; Engels, H.; Nelson, R.C.; Gerety, B.M.; Chezmar, J.L.; Den Boer, J.A. “Keyhole” method for accelerating imaging of contrast agent uptake. J. Magn. Reson. Imaging 1993, 3, 671–675. [Google Scholar] [CrossRef]
- Tudorica, L.A.; Oh, K.Y.; Roy, N.; Kettler, M.D.; Chen, Y.; Hemmingson, S.L.; Afzal, A.; Grinstead, J.W.; Laub, G.; Li, X.; et al. A feasible high spatiotemporal resolution breast DCE-MRI protocol for clinical settings. Magn. Reason. Imaging 2012, 30, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.A.; Jakob, P.M.; Heidemann, R.M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reason. Med. 2002, 47, 1202–1210. [Google Scholar] [CrossRef]
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. SENSE: Sensitivity encoding for fast MRI. Magn. Reason. Med. 1999, 42, 952–962. [Google Scholar] [CrossRef]
- Lemke, A.-J.; Alai-Omid, M.; Hengst, S.A.; Kazi, I.; Felix, R. Eye imaging with a 3.0-T MRI using a surface coi—A study on volunteers and initial patients with uveal melanoma. Eur. Radiol. 2006, 16, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Minja, F.J.; Crum, A.; Burrowes, D. Ocular anatomy and cross-sectional imaging of the eye. Semin. Ultrasound CT MRI 2011, 32, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.J.; Hosten, N.; Bornfeld, N.; Bechrakis, N.E.; Schüler, A.; Richter, M.; Stroszczynski, C.; Felix, R. Uveal melanoma: Correlation of histopathologic and radiologic findings by using thin-section MR imaging with a surface coil. Radiology 1999, 210, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kuai, X.-P.; Chen, X.-S.; Tao, X.-F. Assessment of dynamic contrast-enhanced magnetic resonance imaging in the differentiation of malignant from benign orbital masses. Eur. J. Radiol. 2013, 82, 1506–1511. [Google Scholar] [CrossRef]
- Jiang, X.; Asbach, P.; Willerding, G.; Dulce, M.; Xu, K.; Taupitz, M.; Hamm, B.; Erb-Eigner, K. Dynamic contrast-enhanced MRI of ocular melanoma. Melanoma Res. 2015, 25, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Sepahdari, A.R.; Politi, L.S.; Aakalu, V.K.; Kim, H.J.; Razek, A.A.K.A. Diffusion-weighted imaging of orbital masses: Multi-institutional data support a 2-ADC threshold model to categorize lesions as benign, malignant, or indeterminate. Am. J. Neuroradiol. 2014, 35, 170–175. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Saraiva, P.; Genders, S.W.; Buchem, M.V.; Luyten, G.P.M.; Beenakker, J.-W. CT and MR imaging of orbital inflammation. Neuroradiology 2018, 60, 1253–1266. [Google Scholar] [CrossRef]
- Buerk, B.M.; Pulido, J.S.; Chiong, I.; Folberg, R.; Edward, D.P.; Duffy, M.T.; Thulborn, K.R. Vascular perfusion of choroidal melanoma by 3.0 tesla magnetic resonance imaging. Trans. Am. Ophthalmol. Soc. 2004, 102, 209. [Google Scholar]

| Patients | Sex | Eye | Treatment | Histology | Classification (AJCC) |
|---|---|---|---|---|---|
| 1 | Female | OD | PBT | T3b | |
| 2 | Male | OD | Enucleation | Melanoma | T2b |
| 3 | Male | OD | Brachytherapy | T1a | |
| 4 | Male | OD | Brachytherapy | T1a | |
| 5 | Male | OS | Brachytherapy | T2a | |
| 6 | Female | OD | Enucleation | Melanoma | T4b |
| 7 | Male | OD | Brachytherapy | T3a | |
| 8 | Male | OD | Enucleation | Melanoma | T4a |
| 9 | Male | OD | Enucleation | Melanoma | T3a |
| Purpose | Scan Name | Voxel Size (mm3) | FOV (mm3) | Oversampling (mm) | Echo Train Length | TE(ms)/TR(ms)/Flip or ref. Angle (deg) | Fat Supr. | Avg. | Scan Time (mm:ss) | Additional Parameters |
|---|---|---|---|---|---|---|---|---|---|---|
| 3D measurements | MS TSE 1 mm T1 | 0.9 × 0.9 × 1.0 | 80 × 80 × 40 | 70 mm | 8 | 8.0/718/180 | - | 1 | 02:35 | |
| MS TSE 1 mm T1 SPIR | 0.9 × 0.9 × 1.0 | 80 × 80 × 40 | 60 mm | 6 | 8/636/180 | SPIR | 1 | 02:27 | ||
| MS TSE 1 mm T2 | 0.9 × 0.9 × 1.0 | 80 × 80 × 40 | 20 mm | 17 | 90/4436/120 | - | 2 | 02:04 | ||
| 3D TSE T1 | 1.0 × 1.0 × 1.0 | 80 × 80 × 40 | 40 mm | 14 | 9.4/350/180 | - | 1 | 03:23 | ||
| 3D TSE T1 SPIR | 1.0 × 1.1 × 1.0 | 80 × 80 × 40 | 45 mm | 14 | 9.4/350/180 | SPIR | 1 | 03:23 | ||
| 3D TSE T2 SPIR | 0.8 × 0.8 × 0.8 | 50 × 81 × 40 | 4 REST slabs | 117 | 293/2500/35 | SPIR | 2 | 03:35 | ||
| 3D TFE T1 | 0.8 × 0.8 × 0.8 | 80 × 80 × 40 | 4 REST slabs | 100 | 2.5/5/10 | - | 1 | 03:21 | Tinv: 1000 ms | |
| MS TSE 1 mm T1 SPIR Gd | 0.9 × 0.9 × 1.0 | 80 × 80 × 40 | 60 mm | 6 | 8/636/180 | SPIR | 1 | 02:27 | ||
| 3D TSE T1 SPIR Gd | 1.0 × 1.1 × 1.0 | 80 × 80 × 40 | 45 mm | 14 | 9.4/350/180 | SPIR | 1 | 03:23 | ||
| 3D TFE T1 PROSET Gd | 0.8 × 0.8 × 0.8 | 80 × 80 × 40 | 4 REST slabs | 100 | 4.8/8.4/10 | Proset 1331 | 1 | 03:22 | ||
| Tumor origin & extension | MS TSE 2 mm T1 | 0.5 × 0.5 × 2.0 | 100 × 100 × 24 | - | 6 | 8/718/180 | - | 1 | 01:16 | |
| MS TSE 2 mm T1 SPIR Gd | 0.5 × 0.5 × 2.0 | 100 × 100 × 24 | - | 6 | 80/764/180 | SPIR | 1 | 01:16 | ||
| MS TSE 2 mm T2 | 0.4 × 0.4 × 2.0 | 100 × 100 × 24 | - | 17 | 90/1331/120 | - | 2 | 01:25 | ||
| Functional scans | DWI (TSE) | 1.25 × 1.4 × 2.4 | 100 × 100 × 24 | 24 mm | single shot | 64/5759/50 | SPIR | 5 | 03:21 | B = 0, 400, 800 s/mm2 |
| DCE | 1.25 × 1.5 × 1.5 | 80 × 80 × 32 | 2.3/4.5/5 | Proset 11 | 1 | 04:20 | 2 sec/dynamic |
| Evaluated Parameters | Isotropic Sequences | 2D Sequences Perpendicular to the Tumor—MS 2 mm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2-Weighted | T1-Weighted Before Contrast | T1-Weighted After Contrast | ||||||||||
| MS TSE | 3D TSE SPIR | MS TSE | MS TSE SPIR | 3D TSE | 3D TFE | MS TSE SPIR | 3D TSE SPIR | 3D TFE PROSET | MS T2 | MS T1 | MS T1 SPIR Gd | |
| In-plane image quality | ||||||||||||
| General image quality | ++ | + | + | + | +− | +− | + | +− | + | + | + | ++ |
| Contrast | + | ++ | +− | + | +− | + | ++ | + | ++ | ++ | + | ++ |
| Outer limit of sclera | ++ | + | + | + | + | − | + | + | − | ++ | ++ | + |
| Outer limits of tumor | +− | +− | + | + | +− | − | ++ | + | − | + | + | + |
| Differentiate tumor vs RD | ++ | ++ | + | ++ | ||||||||
| 3D analysis | ||||||||||||
| Geometrical accuracy | ||||||||||||
| Good of complete eye | 75% | 78% | 44% | 44% | 77% | 100% | 50% | 88% | 100% | |||
| Small local artefacts | 25% | 22% | 33% | 55% | 11% | 0% | 25% | 13% | 0% | |||
| Not usable | 0% | 0% | 22% | 0% | 11% | 0% | 25% | 0% | 0% | |||
| Outer limit of sclera | ++ | + | +− | +− | + | − | +− | + | − | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, T.A.; Grech Fonk, L.; Jaarsma-Coes, M.G.; van Haren, G.G.R.; Marinkovic, M.; Beenakker, J.-W.M. MRI of Uveal Melanoma. Cancers 2019, 11, 377. https://doi.org/10.3390/cancers11030377
Ferreira TA, Grech Fonk L, Jaarsma-Coes MG, van Haren GGR, Marinkovic M, Beenakker J-WM. MRI of Uveal Melanoma. Cancers. 2019; 11(3):377. https://doi.org/10.3390/cancers11030377
Chicago/Turabian StyleFerreira, Teresa A., Lorna Grech Fonk, Myriam G. Jaarsma-Coes, Guido G. R. van Haren, Marina Marinkovic, and Jan-Willem M. Beenakker. 2019. "MRI of Uveal Melanoma" Cancers 11, no. 3: 377. https://doi.org/10.3390/cancers11030377
APA StyleFerreira, T. A., Grech Fonk, L., Jaarsma-Coes, M. G., van Haren, G. G. R., Marinkovic, M., & Beenakker, J.-W. M. (2019). MRI of Uveal Melanoma. Cancers, 11(3), 377. https://doi.org/10.3390/cancers11030377





