Improvement of Metastatic Colorectal Cancer Patient Survival: Single Institution Experience
Abstract
:1. Introduction
2. Results
2.1. Demographics
2.2. Treatment
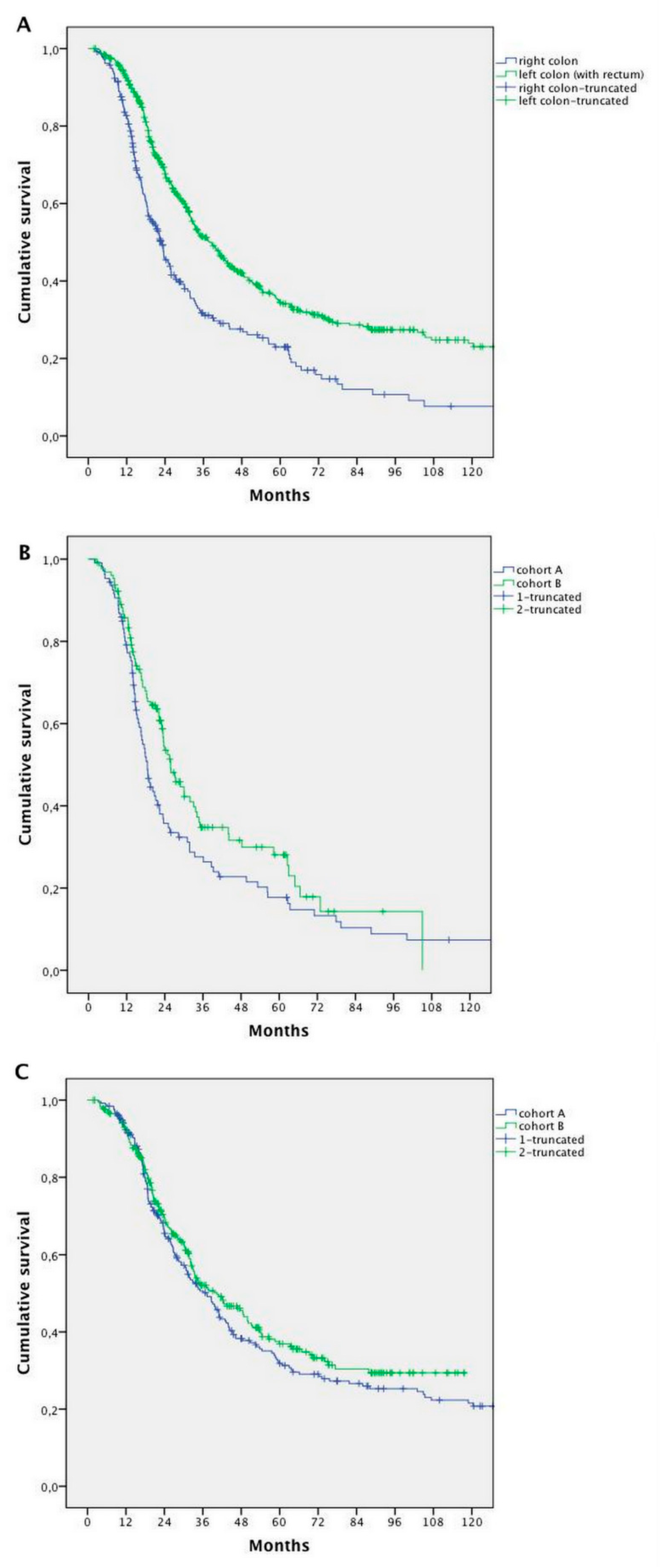
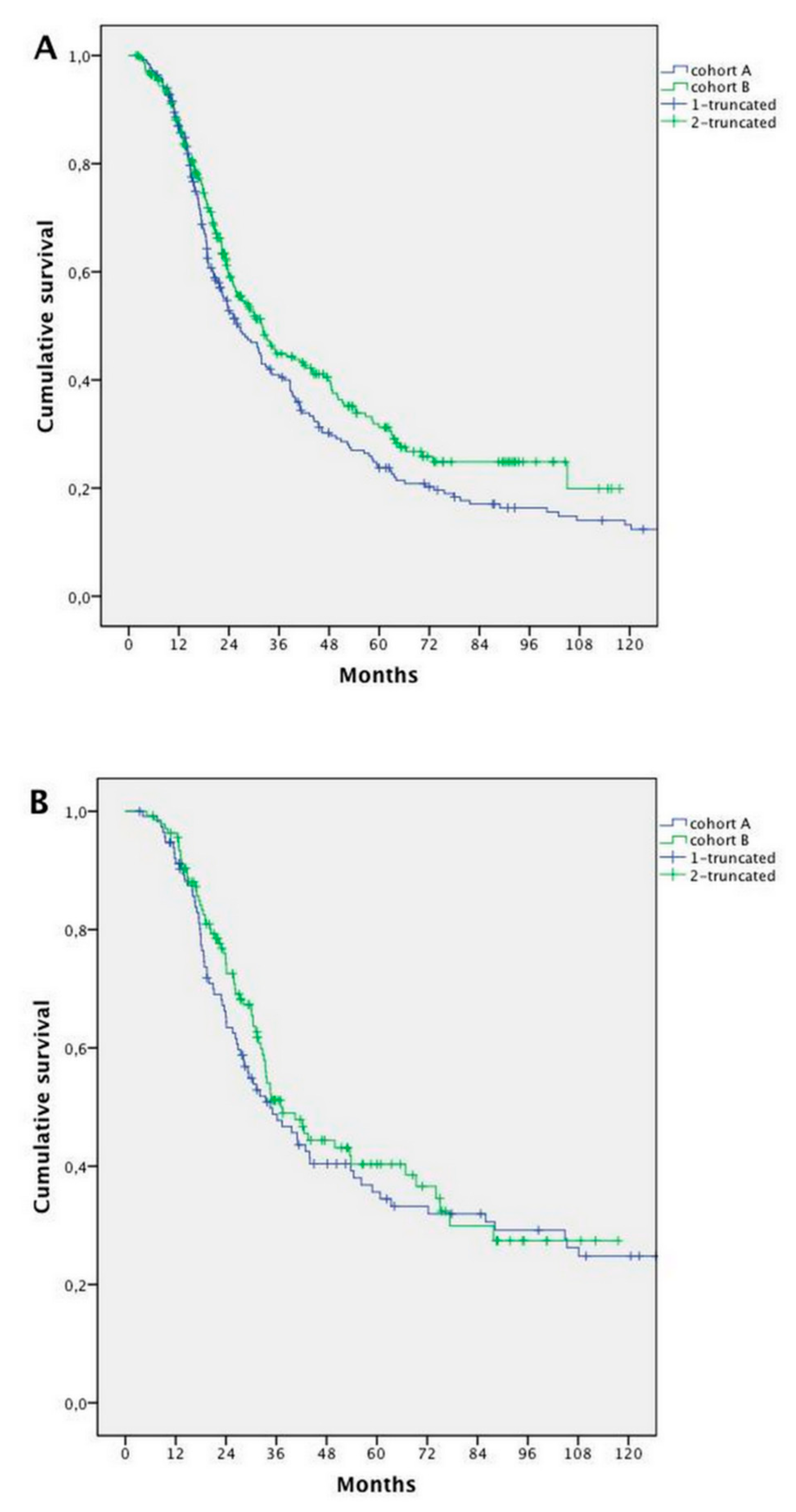
2.3. Survival Analysis
2.4. Prognostic Factors
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Statistical Analysis
4.3. Ethics Approval and Consent to Participate
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5- FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (WT) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J. Clin. Oncol 2014, 32, LBA3. [Google Scholar]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F. Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy As First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab With Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone as First-Line Treatment in Patients with Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.J.; Larson, D.W.; Grothey, A.; Vauthey, J.N.; Nagorney, D.M.; McWilliams, R.R. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Tampellini, M.; Ottone, A.; Bellini, E.; Alabiso, I.; Baratelli, C.; Bitossi, R.; Brizzi, M.P.; Ferrero, A.; Sperti, E.; Leone, F.; et al. The role of lung metastasis resection in improving outcome of colorectal cancer patients: Results from a large retrospective study. Oncologist 2012, 17, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat. Rev. 2016, 48, 42–49. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.; Rho, Y.S.; Kuruba, G.; Mamo, A.; Gilabert, M.; Kavan, T.; Panasci, L.; Melnychuk, D.; Batist, G.; Kavan, P. Clinical Practice Patterns in Chemotherapeutic Treatment Regimens for Metastatic Colorectal Cancer. Clin. Colorectal. Cancer 2016, 15, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Tomasello, G.; Borgonovo, K.; Ghidini, M.; Turati, L.; Dallera, P.; Passalacqua, R.; Sgroi, G.; Barni, S. Prognostic Survival Associated with Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 3, 211–219. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Value | ||
|---|---|---|---|
| N | % | ||
| Primary tumour site | Right Colon | 266 | 29.6% |
| Left Colon (with rectum) | 622 | 69.1% | |
| Unknown | 11 | 1.4% | |
| Histological grade | G1–2 | 289 | 32.1% |
| G3–4 | 234 | 26% | |
| Not determined | 374 | 41.6% | |
| Chronology of metastases | Metachronous | 298 | 33.1% |
| Synchronous | 601 | 66.8% | |
| Metastatic sites at diagnosis | Liver | 605 | 67.2% |
| Lung | 216 | 24% | |
| Lymph nodes | 151 | 16.8% | |
| Peritoneum | 141 | 15.7% | |
| Ovary/Uterus | 34 | 3.8% | |
| Bone | 11 | 1.2% | |
| CNS | 3 | 0.3% | |
| Molecular Characteristics | |||
| KRAS | Wild type | 213 | 23.7% |
| Mutated | 156 | 17.3% | |
| Not determined | 530 | 58.9% | |
| NRAS | Wild type | 99 | 11% |
| Mutated | 8 | 0.9% | |
| Not determined | 792 | 88% | |
| BRAF | Wild type | 140 | 15.6% |
| Mutated | 13 | 1.4% | |
| Not determined | 746 | 82.9% | |
| PI3KCA | Wild type | 56 | 6.2% |
| Mutated | 10 | 1.1% | |
| Not determined | 833 | 92.6% | |
| MSI | MSS | 167 | 18.6% |
| MSI-H | 14 | 1.6% | |
| Not determined | 718 | 79.8% | |
| Treatment Characteristics | |||
| Chemotherapy | 899 | 100% | |
| Surgery of metastases | 456 | 50.7% | |
| Radiotherapy (palliative RT excluded) | 61 | 6.8% | |
| Locoregional treatments | 18 | 2% | |
| HIPEC/PIPAC | 11 | 1.2% | |
| Type of Surgery | Surgery of Metastases | Extra-Hepatic Surgery | Liver Surgery Alone | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Cohort A | 191 | 42% | 98 | 21.4% | 93 | 20.3% |
| Cohort B | 265 | 58% | 155 | 33.9% | 110 | 24.1% |
| Total | 456 | 50.7% | 253 | 28% | 203 | 22.6% |
| p-value | <0.009 | <0.005 | <0.91 | |||
| Surgery | Patients (N) | p-Value (Chi Square) | ||
|---|---|---|---|---|
| 1st Line | Cohort A | Cohort B | ||
| Surgery | NO | 235 | 241 | 0.041 |
| YES | 180 | 243 | - | |
| Sites | liver | 118 | 124 | 0.071 |
| lymph nodes | 1 | 10 | - | |
| spleen | 1 | 0 | - | |
| ovary | 7 | 14 | - | |
| lung | 13 | 27 | - | |
| pelvis | 15 | 15 | - | |
| peritoneum | 10 | 20 | - | |
| CNS | 0 | 1 | - | |
| other sites | 0 | 2 | - | |
| adrenal gland | 0 | 1 | - | |
| multiple sites | 15 | 29 | - | |
| 2nd Line | Cohort A | Cohort B | ||
| Surgery | NO | 352 | 409 | 0.896 |
| YES | 63 | 75 | - | |
| Sites | liver | 20 | 27 | 0.489 |
| lymph nodes | 3 | 2 | - | |
| spleen | 0 | 1 | - | |
| ovary | 2 | 5 | - | |
| pelvis | 8 | 4 | - | |
| peritoneum | 6 | 5 | - | |
| lung | 16 | 21 | - | |
| CNS | 4 | 1 | - | |
| other sites | 1 | 1 | - | |
| adrenal gland | 0 | 1 | - | |
| multiple sites | 3 | 6 | - | |
| 3rd Line | ||||
| Surgery | NO | 393 | 451 | 0.344 |
| YES | 22 | 33 | - | |
| Sites | liver | 5 | 9 | 0.357 |
| lymph nodes | 0 | 1 | - | |
| ovary | 1 | 0 | - | |
| pelvis | 3 | 0 | - | |
| peritoneum | 2 | 5 | - | |
| lung | 6 | 8 | - | |
| CNS | 1 | 4 | - | |
| adrenal gland | 0 | 1 | - | |
| multiple sites | 4 | 4 | - | |
| 4th Line | ||||
| Surgery | NO | 411 | 480 | 0.827 |
| YES | 4 | 4 | - | |
| Sites | liver | 1 | 1 | 0.638 |
| pelvis | 0 | 1 | - | |
| lung | 0 | 1 | - | |
| multiple sites | 1 | 0 | - | |
| CNS | 2 | 1 | - | |
| Treatment Characteristics | 1st Line | 2nd Line | 3rd Line | 4th Line | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort A (415 pts) | Cohort B (484 pts) | Cohort A (372 pts) | Cohort B (386 pts) | Cohort A (282 pts) | Cohort B (284 pts) | Cohort A (168 pts) | Cohort B (161 pts) | ||
| N (%) a | N (%) a | N (%) a | N (%) a | N (%) a | N (%) a | N (%) a | N (%) a | ||
| Chemo therapy and targeted therapy | FOLFOXIRI | none | 3 (1.2%) | none | none | none | none | none | 1 (1.1%) |
| Oxaliplatin doublet | 152 (65.1%) | 160 (66.3%) | 60 (22.4%) | 49 (18.5%) | 40 (21.7%) | 56 (32%) | 35 (32.7%) | 22 (24.4%) | |
| Irinotecan doublet | 32 (13.6%) | 50 (20.7%) | 145 (54.3%) | 151 (56.7%) | 37 (20.1%) | 39 (22.3%) | 8 (7.5%) | 20 (22.2%) | |
| Monotherapy a | 51 (21.7%) | 27 (11.2%) | 62 (23.3%) | 66 (24.8%) | 106 (57.6%) | 71 (40.6%) | 64 (59.8%) | 38 (42.2%) | |
| p value (chi square) | <0.0001 | 0.489 | 0.001 | <0.0001 | |||||
| Anti-EGFR b | none | 24 (23.7%) c | 8 (10.2%) c | 31 (24.8%) c | 41 (59.4%) c | 52 (53.6%) c | 13 (27.1%) c | 20 (40.1%) c | |
| Anti-VEGF b | 6 (5.6%) | 69 (76.6%) | 2 (0.7%) | 55 (20.6%) | 4 (2.1%) | 21 (12%) | 6 (5.6%) | 4 (4.4%) | |
| p value (chi square) | 0.004 | <0.0001 | <0.0001 | 0.166 | |||||
| Total * | 235 (56.6%) | 241 (49.8%) | 267 (71.8%) | 266 (68.9%) | 184 (65.2%) | 175 (61.6%) | 107 (63.9%) | 90 (55.9%) | |
| Chemo therapy + surgery | FOLFOXIRI | none | 2 (0.8%) | none | none | none | none | none | none |
| Oxaliplatin doublet | 110 (67.9%) | 142 (63.6%) | 15 (38.4%) | 11 (22.9%) | 3 (27.3%) | 5 (27.7%) | none | 1 (100%) | |
| Irinotecan doublet | 14 (8.6%) | 52 (23.3%) | 20 (51.2%) | 23 (47.9%) | 3 (27.3%) | 7 (39%) | none | none | |
| Monotherapy a | 38 (23.4%) | 27 (12.1%) | 4 (10.2%) | 14 (29.1%) | 5 (45.4%) | 6 (33.3%) | 2 (100%) | none | |
| p value (chi square) | <0.0001 | 0.064 | 0.766 | 0.083 | |||||
| Anti-EGFR b | 1 (2.4%) c | 10 (9.7%) c | none | 7 (22.5%) c | 3 (42.8%) c | 6 (40.0%) c | none | none | |
| Anti-VEGF b | 1 (0.6%) | 49 (21.9%) | 1 (2.5%) | 6 (22.2%) | 1 (9%) | none | none | none | |
| p value (chi square) | <0.0001 | 0.007 | 0.422 | - | |||||
| Total * | 162 (39%) | 223 (46.1%) | 39 (10.5%) | 48 (12.4%) | 11 (3.9%) | 18 (6.3%) | 2 (1.2%) | 1 (0.6%) | |
| Surgery alone | 18 (4.3%) | 20 (4.1%) | 24 (6.4%) | 27 (9.4%) | 11 (3.9%) | 15 (5.3%) | 2 (1.2%) | 3 (1.8%) | |
| Not treated | none | none | 42 (11.2%) | 45 (11.6%) | 76 (26.9%) | 76 (26.7%) | 57 (33.9%) | 67 (41.6%) | |
| Variable | Number of Patients (A vs. B) | Median Overall Survival (Months) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Primary tumour site (Right vs. Left) | 236 vs. 542 | 22.9 vs. 38.6 | 1.774 (1.470–2.140 | <0.0001 | 0.596 (0.491–0.723) | <0.0001 |
| Histological grade (G1–G2 vs. G3–G4) | 246 vs. 209 | 34.5 vs. 33.0 | 1.058 (0.839–1.334) | 0.634 | ns | ns |
| T status (T1–T2 vs. T3–T4) | 51 vs. 554 | 43.9 vs. 38.6 | 1.293 (0.889–1.878) | 0.178 | ns | ns |
| N status (N0 vs. N+) | 191 vs. 406 | 43.0 vs. 33.3 | 1.344 (1.076–1.679) | 0.009 | ns | ns |
| Chronology of mts (Synchronous vs. Metachronous) | 536 vs. 252 | 30.0 vs. 36.2 | 0.718 (0.592–0.870) | 0.001 | 0.764 (0.628–0.929) | 0.007 |
| KRAS Mutational Status (Wild Type vs. Mutated) | 185 vs. 122 | 42.0 vs. 34.4 | 1.079 (0.793–1.469) | 0.626 | ns | ns |
| BRAF Mutational Status (Wild type vs. Mutated) | 103 vs. 10 | 49.9 vs. 102.9 | 0.696 (0.251–1.931) | 0.486 | ns | ns |
| Liver mts at diagnosis (YES vs. NO) | 530 vs. 258 | 30.7 vs. 34.5 | 1.142 (0.946–1.378) | 0.167 | ns | ns |
| Lung mts at diagnosis (YES vs. NO) | 194 vs. 594 | 30.0 vs. 32.7 | 1.089 (0.888–1.335) | 0.412 | ns | ns |
| Lymph nodes mts at diagnosis (YES vs. NO) | 141 vs. 647 | 23.3 vs. 34.2 | 1.617 (1.296–2.018) | < 0.0001 | 1.533 (1.225–1.919) | <0.0001 |
| Peritoneal mts at diagnosis (YES vs. NO) | 121 vs. 667 | 31.0 vs. 32.7 | 1.286 (1.009–1.638) | 0.048 | ns | ns |
| Ovarian mts at diagnosis (YES vs. NO) | 32 vs. 756 | 42.4 vs. 31.8 | 0.766 (0.478–1.226) | 0.267 | ns | ns |
| Bone mts at diagnosis (YES vs. NO) | 8 vs. 780 | 9.52 vs. 32.2 | 4.370 (2.165–8.820) | <0.0001 | 4.427 (2.181–8.984) | <0.0001 |
| CNS mts at diagnosis (YES vs. NO) | 3 vs. 785 | 20.3 vs. 32.1 | 2.731 (0.876–8.513) | 0.083 | ns | ns |
| Bone mts (YES vs. NO) | 80 vs. 707 | 28.3 vs. 32.2 | 1.043 (0.783–1.390) | 0.774 | ns | ns |
| CNS mts (YES vs. NO) | 41 vs. 746 | 30.7 vs. 32.1 | 1.137 (0.776–1.664) | 0.510 | ns | ns |
| Prognostic Factors | Cohort A | Cohort B | p-Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Right Colon | 107 | 29.3% | 129 | 30.5% | 0.766 |
| Left Colon | 252 | 69.0% | 290 | 68.6% | |
| Synchronous mts | 250 | 68.5% | 286 | 67.6% | 0.791 |
| Metachronous mts | 115 | 31.5% | 137 | 32.4% | |
| Lymph nodes mts | 59 | 16.2% | 82 | 19.4% | 0.239 |
| Bone mts | 3 | 0.8% | 5 | 1.2% | 0.615 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenocchio, E.; Colombi, F.; Calella, M.G.; Filippi, R.; Depetris, I.; Chilà, G.; Lombardi, P.; Marino, D.; Cagnazzo, C.; Ferraris, R.; et al. Improvement of Metastatic Colorectal Cancer Patient Survival: Single Institution Experience. Cancers 2019, 11, 369. https://doi.org/10.3390/cancers11030369
Fenocchio E, Colombi F, Calella MG, Filippi R, Depetris I, Chilà G, Lombardi P, Marino D, Cagnazzo C, Ferraris R, et al. Improvement of Metastatic Colorectal Cancer Patient Survival: Single Institution Experience. Cancers. 2019; 11(3):369. https://doi.org/10.3390/cancers11030369
Chicago/Turabian StyleFenocchio, Elisabetta, Federica Colombi, Maria Grazia Calella, Roberto Filippi, Ilaria Depetris, Giovanna Chilà, Pasquale Lombardi, Donatella Marino, Celeste Cagnazzo, Renato Ferraris, and et al. 2019. "Improvement of Metastatic Colorectal Cancer Patient Survival: Single Institution Experience" Cancers 11, no. 3: 369. https://doi.org/10.3390/cancers11030369
APA StyleFenocchio, E., Colombi, F., Calella, M. G., Filippi, R., Depetris, I., Chilà, G., Lombardi, P., Marino, D., Cagnazzo, C., Ferraris, R., Vaira, M., Aglietta, M., & Leone, F. (2019). Improvement of Metastatic Colorectal Cancer Patient Survival: Single Institution Experience. Cancers, 11(3), 369. https://doi.org/10.3390/cancers11030369






