Cordycepin Suppresses Endothelial Cell Proliferation, Migration, Angiogenesis, and Tumor Growth by Regulating Focal Adhesion Kinase and p53
Abstract
:1. Introduction
2. Results
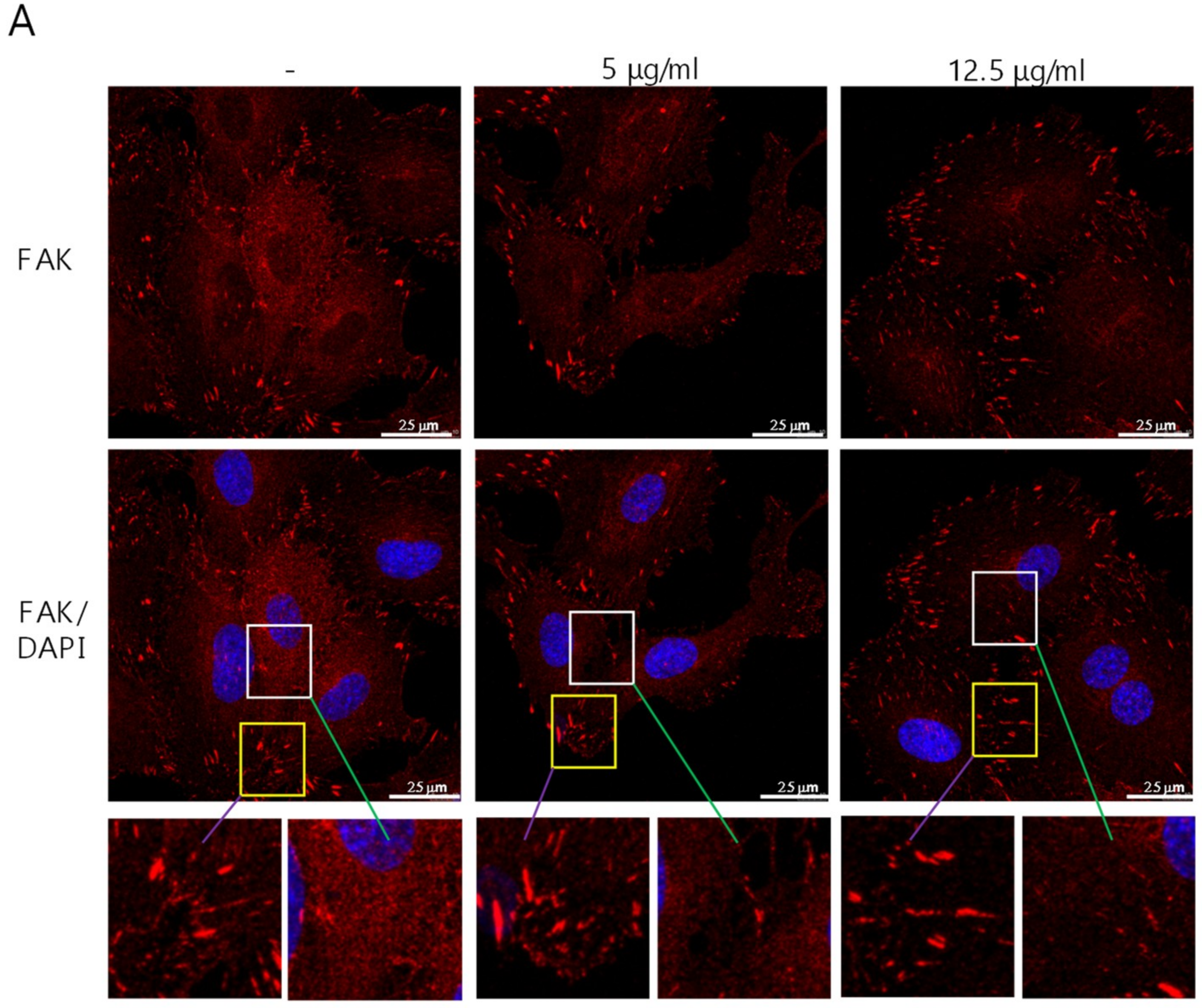
2.1. Suppression of FAK Expression in ECs by Cordycepin
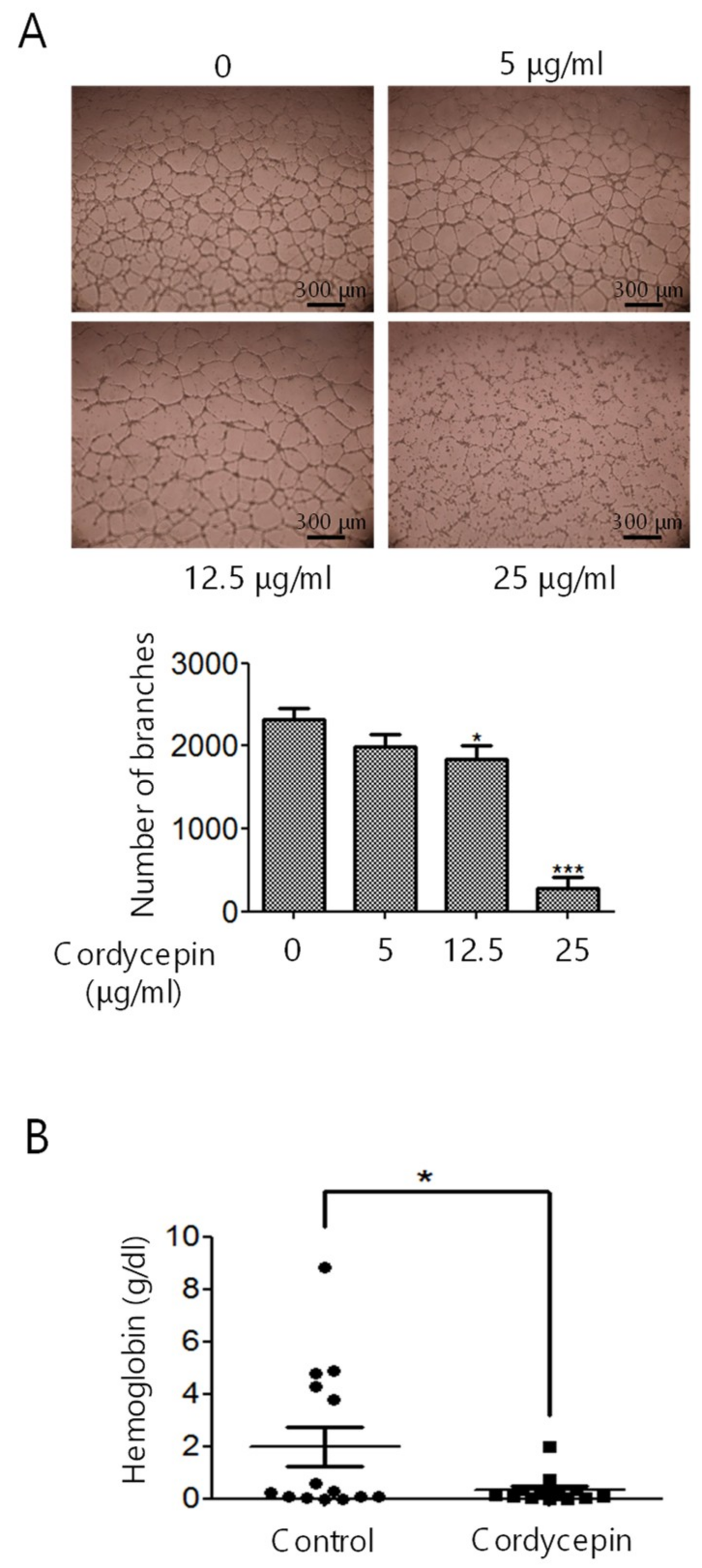
2.2. Inhibition of EC Migration, Proliferation, Tube Formation, and In Vivo Angiogenesis by Cordycepin
2.3. Suppression of the Expression of FAK in ECs by Cordycepin
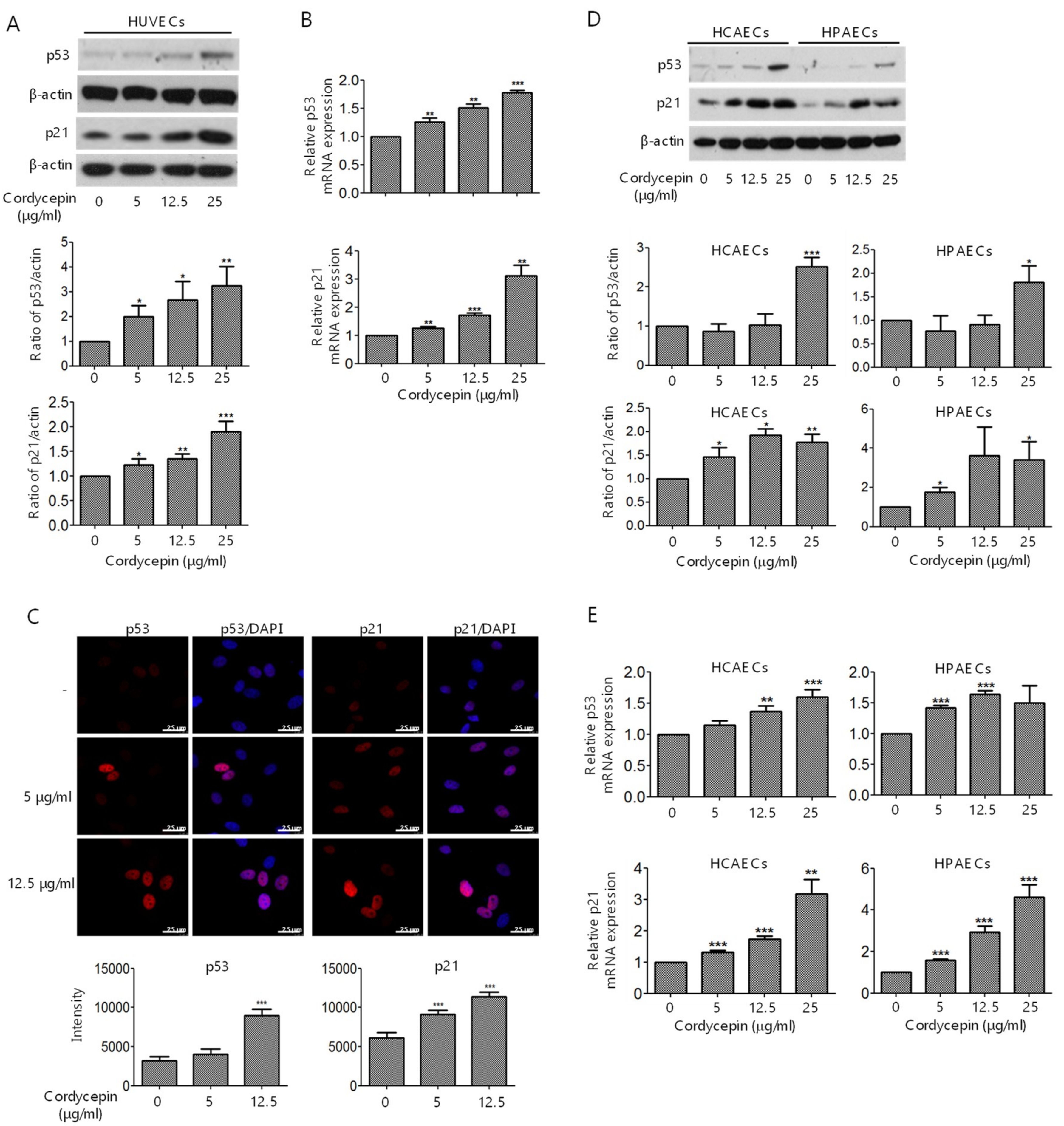
2.4. Induction of p53 and p21 Expression in ECs by Cordycepin
2.5. Inhibition of the Proliferation and Tumor Growth of HCC Cells by Cordycepin
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Western Blot Analysis
4.3. qPCR Analysis
4.4. Cell Migration and Wound Healing Analysis
4.5. Cell Proliferation Analysis
4.6. Flow Cytometry Analysis
4.7. Tube Formation
4.8. Immunofluorescence Confocal Microscopy
4.9. In Vivo Matrigel Plug Angiogenesis Assay and Tumor Xenograft Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ECs | endothelial cells |
| EMT | epithelial–mesenchymal transition |
| FAK | focal adhesion kinase |
| p-FAK | FAK phosphorylation at Tyr397 |
| HCAECs | human coronary artery endothelial cells |
| HCC | hepatocellular carcinoma |
| HPAECs | human pulmonary artery endothelial cells |
| HUVECs | human umbilical vein endothelial cells |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Q-PCR | quantitative real-time PCR. |
References
- Folkman, J. Seminars in medicine of the beth israel hospital, boston. Clinical applications of research on angiogenesis. N. Engl. J. Med. 1995, 333, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- McLean, G.W.; Carragher, N.O.; Avizienyte, E.; Evans, J.; Brunton, V.G.; Frame, M.C. The role of focal-adhesion kinase in cancer—A new therapeutic opportunity. Nat. Rev. Cancer 2005, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.A.; Brugge, J.S. Integrins and signal transduction pathways: The road taken. Science 1995, 268, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. Fak integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Furuta, Y.; Kanazawa, S.; Takeda, N.; Sobue, K.; Nakatsuji, N.; Nomura, S.; Fujimoto, J.; Okada, M.; Yamamoto, T. Reduced cell motility and enhanced focal adhesion contact formation in cells from fak-deficient mice. Nature 1995, 377, 539–544. [Google Scholar] [PubMed]
- Shen, T.L.; Park, A.Y.; Alcaraz, A.; Peng, X.; Jang, I.; Koni, P.; Flavell, R.A.; Gu, H.; Guan, J.L. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 2005, 169, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Braren, R.; Hu, H.; Kim, Y.H.; Beggs, H.E.; Reichardt, L.F.; Wang, R. Endothelial fak is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 2006, 172, 151–162. [Google Scholar] [CrossRef]
- Peng, X.; Ueda, H.; Zhou, H.; Stokol, T.; Shen, T.L.; Alcaraz, A.; Nagy, T.; Vassalli, J.D.; Guan, J.L. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc. Res. 2004, 64, 421–430. [Google Scholar] [CrossRef]
- Tavora, B.; Batista, S.; Reynolds, L.E.; Jadeja, S.; Robinson, S.; Kostourou, V.; Hart, I.; Fruttiger, M.; Parsons, M.; Hodivala-Dilke, K.M. Endothelial fak is required for tumour angiogenesis. EMBO Mol. Med. 2010, 2, 516–528. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Kaur, A.; Cance, W. Cloning and characterization of the promoter region of human focal adhesion kinase gene: Nuclear factor kappa b and p53 binding sites. Biochim. Biophys. Acta 2004, 1678, 111–125. [Google Scholar] [CrossRef]
- Anaganti, S.; Fernandez-Cuesta, L.; Langerod, A.; Hainaut, P.; Olivier, M. P53-dependent repression of focal adhesion kinase in response to estradiol in breast cancer cell-lines. Cancer Lett. 2011, 300, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.M.; Finch, R.; Kweh, F.; Massoll, N.A.; Campbell-Thompson, M.; Wallace, M.R.; Cance, W.G. P53 regulates fak expression in human tumor cells. Mol. Carcinog. 2008, 47, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Almeida, E.A.; Schlaepfer, D.D.; Dazin, P.; Aizawa, S.; Damsky, C.H. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 1998, 143, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.M.; Finch, R.; Cance, W.G. Direct interaction of the n-terminal domain of focal adhesion kinase with the n-terminal transactivation domain of p53. J. Biol. Chem. 2005, 280, 25008–25021. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Chen, X.L.; Lim, Y.; Hanson, D.A.; Vo, T.T.; Howerton, K.; Larocque, N.; Fisher, S.J.; Schlaepfer, D.D.; Ilic, D. Nuclear fak promotes cell proliferation and survival through ferm-enhanced p53 degradation. Mol. Cell 2008, 29, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R. Cordyceps: A traditional chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.L.; Ko, B.S.; Liu, T.A.; Liang, S.M.; Liu, C.C.; Lu, Y.J.; Tzean, S.S.; Shen, T.L.; Liou, J.Y. Cordycepin suppresses integrin/fak signaling and epithelial-mesenchymal transition in hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. 2014, 14, 29–34. [Google Scholar] [CrossRef]
- Wu, W.C.; Hsiao, J.R.; Lian, Y.Y.; Lin, C.Y.; Huang, B.M. The apoptotic effect of cordycepin on human oec-m1 oral cancer cell line. Cancer Chemother. Pharmacol. 2007, 60, 103–111. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, W.J.; Moon, S.K. Cordycepin suppresses tnf-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother. Res. PTR 2010, 24, 1755–1761. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.C.; Lin, Y.T.; Huang, S.H.; Wang, S.M. Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-parp pathway. J. Agric. Food Chem. 2010, 58, 11645–11652. [Google Scholar] [CrossRef]
- Lee, H.J.; Burger, P.; Vogel, M.; Friese, K.; Bruning, A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Investig. New Drugs 2012, 30, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Stellrecht, C.M.; Gandhi, V. Rna-directed agent, cordycepin, induces cell death in multiple myeloma cells. Br. J. Haematol. 2008, 140, 682–691. [Google Scholar] [CrossRef]
- Matsuda, H.; Akaki, J.; Nakamura, S.; Okazaki, Y.; Kojima, H.; Tamesada, M.; Yoshikawa, M. Apoptosis-inducing effects of sterols from the dried powder of cultured mycelium of cordyceps sinensis. Chem. Pharm. Bull. 2009, 57, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Jin, C.Y.; Park, C.; Hong, S.H.; Kim, G.Y.; Jeong, Y.K.; Lee, J.D.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol. In Vitro Int. J. Publ. Assoc. Bibra 2011, 25, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kodama, E.N.; McCaffrey, R.P.; Yusa, K.; Mitsuya, H. Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TDT+) leukemic cells. Biochem. Pharmacol. 2000, 59, 273–281. [Google Scholar] [CrossRef]
- Thomadaki, H.; Tsiapalis, C.M.; Scorilas, A. The effect of the polyadenylation inhibitor cordycepin on human molt-4 and daudi leukaemia and lymphoma cell lines. Cancer Chemother. Pharmacol. 2008, 61, 703–711. [Google Scholar] [CrossRef]
- Jen, C.Y.; Lin, C.Y.; Huang, B.M.; Leu, S.F. Cordycepin induced ma-10 mouse leydig tumor cell apoptosis through caspase-9 pathway. Evid. Based Complement. Altern. Med. ECAM 2011, 2011, 984537. [Google Scholar] [CrossRef]
- Liao, Y.; Ling, J.; Zhang, G.; Liu, F.; Tao, S.; Han, Z.; Chen, S.; Chen, Z.; Le, H. Cordycepin induces cell cycle arrest and apoptosis by inducing DNA damage and up-regulation of p53 in leukemia cells. Cell Cycle 2015, 14, 761–771. [Google Scholar] [CrossRef]
- Ko, B.S.; Lu, Y.J.; Yao, W.L.; Liu, T.A.; Tzean, S.S.; Shen, T.L.; Liou, J.Y. Cordycepin regulates gsk-3beta/beta-catenin signaling in human leukemia cells. PLoS ONE 2013, 8, e76320. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Liu, J.; Xu, P.; Zhang, X.M.; Tian, Y.Y.; Xue, Y.M.; Gao, X.Y.; Liu, Y.; Wang, J.H. Synergistic effect of hmgb1 knockdown and cordycepin in the K562 human chronic myeloid leukemia cell line. Mol. Med. Rep. 2015, 12, 4462–4468. [Google Scholar] [CrossRef]
- Ko, B.S.; Chang, T.C.; Chen, C.H.; Liu, C.C.; Kuo, C.C.; Hsu, C.; Shen, Y.C.; Shen, T.L.; Golubovskaya, V.M.; Chang, C.C.; et al. Bortezomib suppresses focal adhesion kinase expression via interrupting nuclear factor-kappa b. Life Sci. 2010, 86, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.M.; Finch, R.; Zheng, M.; Kurenova, E.V.; Cance, W.G. The 7-amino-acid site in the proline-rich region of the n-terminal domain of p53 is involved in the interaction with fak and is critical for p53 functioning. Biochem. J. 2008, 411, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Semela, D.; Dufour, J.F. Angiogenesis and hepatocellular carcinoma. J. Hepatol. 2004, 41, 864–880. [Google Scholar] [CrossRef]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. Fak signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Fischer, T.; Puder, S.; Kunschmann, T.; Soetje, B.; Ziegler, W.H. Focal adhesion kinase activity is required for actomyosin contractility-based invasion of cells into dense 3D matrices. Sci. Rep. 2017, 7, 42780. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.J.; Ko, B.S.; Hsu, C.; Chang, T.C.; Chen, S.C.; Wang, J.; Liou, J.Y. Overexpressed focal adhesion kinase predicts a higher incidence of extrahepatic metastasis and worse survival in hepatocellular carcinoma. Hum. Pathol. 2009, 40, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995, 9, 1149–1163. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, P.; Harper, J.W.; Elledge, S.J.; Leder, P. Mice lacking P21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef]
- Macleod, K.F.; Sherry, N.; Hannon, G.; Beach, D.; Tokino, T.; Kinzler, K.; Vogelstein, B.; Jacks, T. P53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995, 9, 935–944. [Google Scholar] [CrossRef]
- Lee, S.Y.; Debnath, T.; Kim, S.K.; Lim, B.O. Anti-cancer effect and apoptosis induction of cordycepin through dr3 pathway in the human colonic cancer cell HT-29. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 439–447. [Google Scholar] [CrossRef]
- Pan, B.S.; Wang, Y.K.; Lai, M.S.; Mu, Y.F.; Huang, B.M. Cordycepin induced MA-10 mouse leydig tumor cell apoptosis by regulating p38 mapks and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 13372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.H.; Hueng, D.Y.; Syu, J.P.; Liao, C.C.; Wu, Y.C. Cordycepin induces apoptosis of c6 glioma cells through the adenosine 2a receptor-p53-caspase-7-parp pathway. Chem. Biol. Interact. 2014, 216, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.; Moran-Jones, K.; Sansom, O.J.; Brunton, V.G.; Frame, M.C. Fak deletion promotes p53-mediated induction of p21, DNA-damage responses and radio-resistance in advanced squamous cancer cells. PLoS ONE 2011, 6, e27806. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.P.; Sagar, S.M.; Parks, R.E., Jr. Adenosine deaminase from human erythrocytes: Purification and effects of adenosine analogs. Biochem. Pharmacol. 1975, 24, 693–701. [Google Scholar] [CrossRef]
- Lu, H.; Li, X.; Zhang, J.; Shi, H.; Zhu, X.; He, X. Effects of cordycepin on HEPG2 and ea.HY926 cells: Potential antiproliferative, antimetastatic and anti-angiogenic effects on hepatocellular carcinoma. Oncol. Lett. 2014, 7, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.R.; Yamada, T.; Taylor, B.N.; Christov, K.; King, M.L.; Majumdar, D.; Lekmine, F.; Tiruppathi, C.; Shilkaitis, A.; Bratescu, L.; et al. A cell penetrating peptide derived from azurin inhibits angiogenesis and tumor growth by inhibiting phosphorylation of VEGFR-2, FAK and AKT. Angiogenesis 2011, 14, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.M.; Ho, B.; Zheng, M.; Magis, A.; Ostrov, D.; Morrison, C.; Cance, W.G. Disruption of focal adhesion kinase and p53 interaction with small molecule compound R2 reactivated p53 and blocked tumor growth. BMC Cancer 2013, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Kisker, O.; Becker, C.M.; Prox, D.; Fannon, M.; D’Amato, R.; Flynn, E.; Fogler, W.E.; Sim, B.K.; Allred, E.N.; Pirie-Shepherd, S.R.; et al. Continuous administration of endostatin by intraperitoneally implanted osmotic pump improves the efficacy and potency of therapy in a mouse xenograft tumor model. Cancer Res. 2001, 61, 7669–7674. [Google Scholar] [PubMed]
- Gourdeau, H.; Leblond, L.; Hamelin, B.; Dong, K.; Ouellet, F.; Boudreau, C.; Custeau, D.; Richard, A.; Gilbert, M.J.; Jolivet, J. Species differences in troxacitabine pharmacokinetics and pharmacodynamics: Implications for clinical development. Clin. Cancer Res. 2004, 10, 7692–7702. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Liang, S.-M.; Wu, Y.-J.; Wu, Y.-J.; Lu, Y.-J.; Jan, Y.-J.; Ko, B.-S.; Chuang, Y.-J.; Shyue, S.-K.; Kuo, C.-C.; et al. Cordycepin Suppresses Endothelial Cell Proliferation, Migration, Angiogenesis, and Tumor Growth by Regulating Focal Adhesion Kinase and p53. Cancers 2019, 11, 168. https://doi.org/10.3390/cancers11020168
Lin Y-T, Liang S-M, Wu Y-J, Wu Y-J, Lu Y-J, Jan Y-J, Ko B-S, Chuang Y-J, Shyue S-K, Kuo C-C, et al. Cordycepin Suppresses Endothelial Cell Proliferation, Migration, Angiogenesis, and Tumor Growth by Regulating Focal Adhesion Kinase and p53. Cancers. 2019; 11(2):168. https://doi.org/10.3390/cancers11020168
Chicago/Turabian StyleLin, Yi-Ting, Shu-Man Liang, Ya-Ju Wu, Yi-Ju Wu, Yi-Jhu Lu, Yee-Jee Jan, Bor-Sheng Ko, Yung-Jen Chuang, Song-Kun Shyue, Cheng-Chin Kuo, and et al. 2019. "Cordycepin Suppresses Endothelial Cell Proliferation, Migration, Angiogenesis, and Tumor Growth by Regulating Focal Adhesion Kinase and p53" Cancers 11, no. 2: 168. https://doi.org/10.3390/cancers11020168
APA StyleLin, Y.-T., Liang, S.-M., Wu, Y.-J., Wu, Y.-J., Lu, Y.-J., Jan, Y.-J., Ko, B.-S., Chuang, Y.-J., Shyue, S.-K., Kuo, C.-C., & Liou, J.-Y. (2019). Cordycepin Suppresses Endothelial Cell Proliferation, Migration, Angiogenesis, and Tumor Growth by Regulating Focal Adhesion Kinase and p53. Cancers, 11(2), 168. https://doi.org/10.3390/cancers11020168





