Glucose Depletion Enhances the Stem Cell Phenotype and Gemcitabine Resistance of Cholangiocarcinoma Organoids through AKT Phosphorylation and Reactive Oxygen Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Organoid Culture and Glucose Conditions
2.2. Drugs
2.3. Western Blotting
2.4. Quantitative RT-PCR
2.5. Flow Cytometry
2.6. Measurement of ROS and Antioxidant Capacity
2.7. Statistics
3. Results
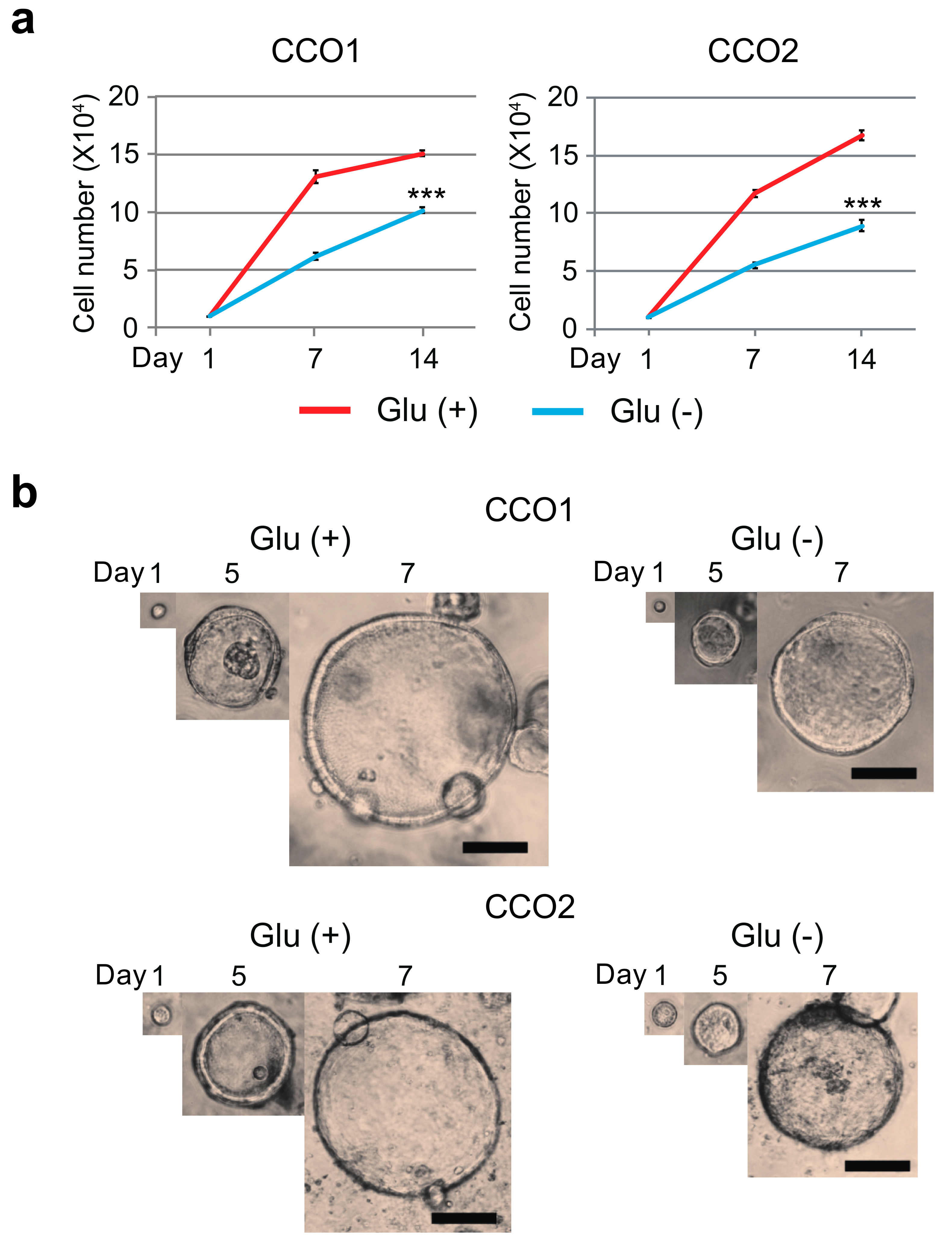
3.1. Glucose-Free Condition Reduce the Growth Activity of Cholangiocarcinoma Organoids
3.2. Higher Expression of Stem Cell Markers in Cholangiocarcinoma Organoids Cultured under Glucose-Free Condition
3.3. Reduction of Gemcitabine Sensitivity in Cholangiocarcinoma Organoids under Glucose-Free Condition
3.4. Increase of AKT Phosphorylation in Cholangiocarcinoma Organoids Cultured under Glucose-Free Condition
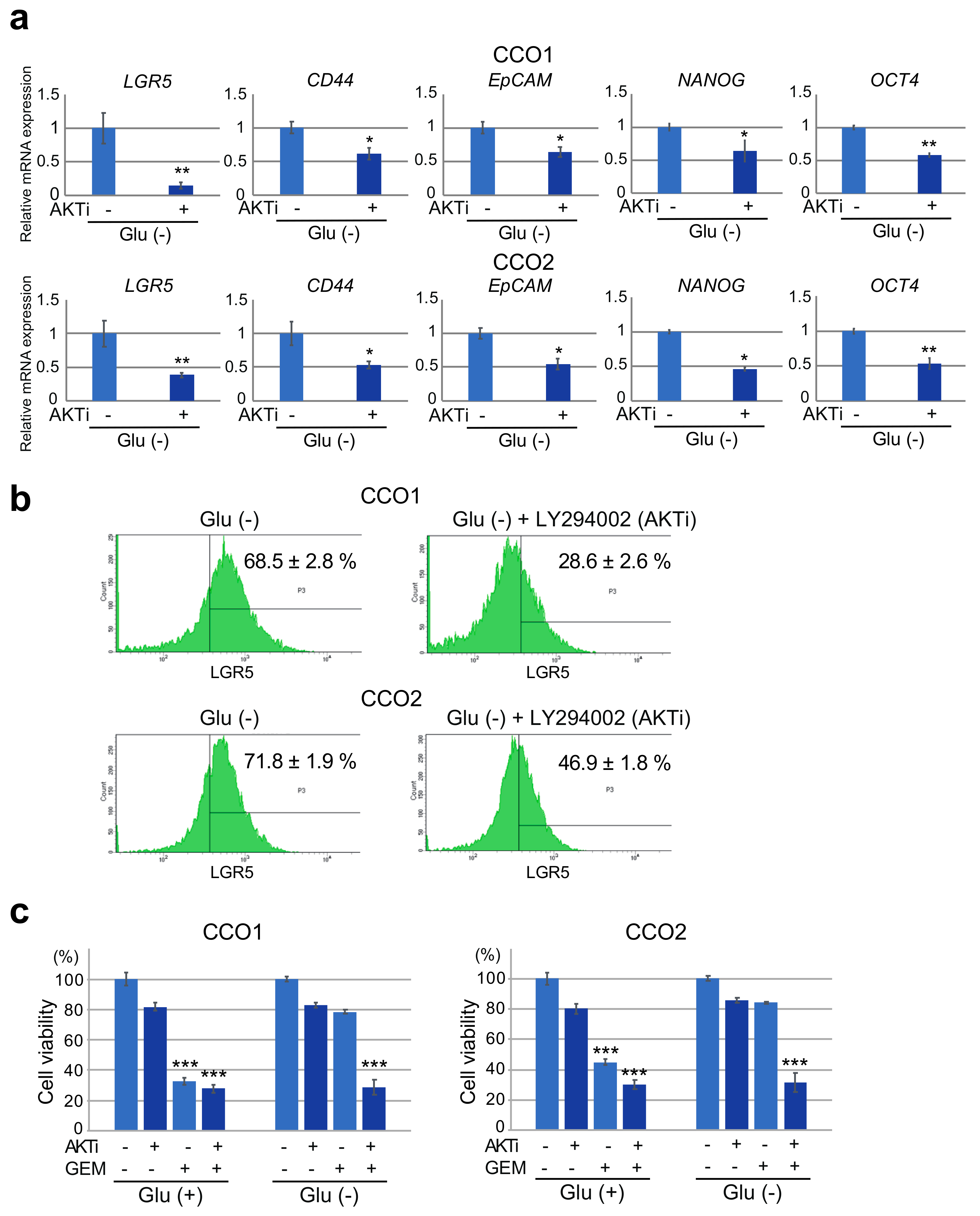
3.5. Inhibition of AKT Phosphorylation under Glucose-Free Condition Leads to A Decrease in Stemness and Resistance to Gemcitabine in Cholangiocarcinoma Organoids
3.6. Stemness and Gemcitabine Resistance of Cholangiocarcinoma Organoids Cultured under Glucose-Free Condition are Mediated by Reactive Oxygen Species (ROS)
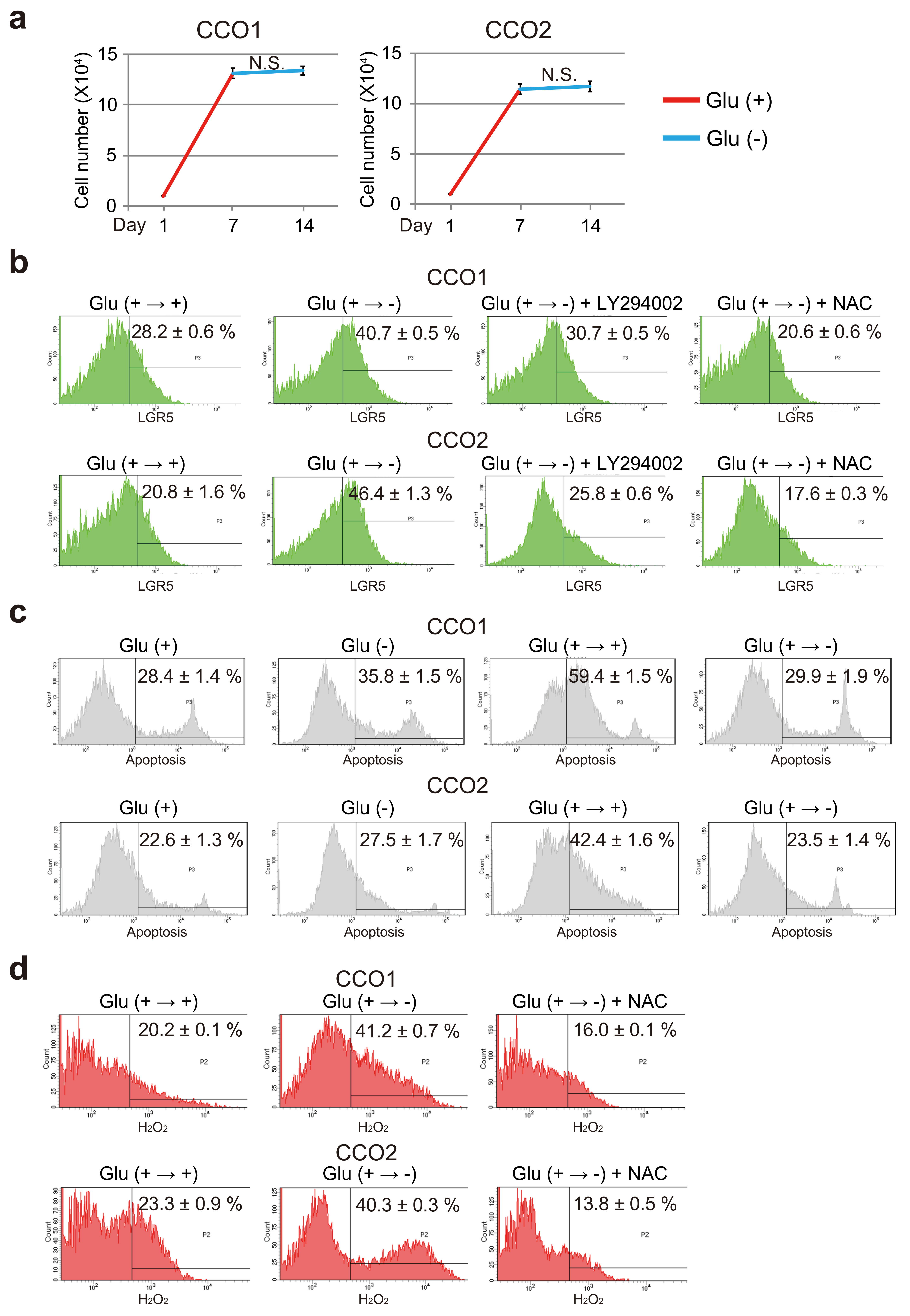
3.7. AKT Phosphorylation and ROS Increase the Stemness of Cholangiocarcinoma Organoids Transferred from Glucose-Sufficient to Glucose-Free Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CCO | cholangiocarcinoma organoid |
| EGF | epidermal growth factor |
| ICC | intrahepatic cholangiocarcinoma |
| NAC | N-acetylcysteine |
| NOS | nitric oxide synthase |
| NOX | NADPH oxidase |
| PI3K | phosphatidylinositol 3-kinase |
| Rspo1 | R-spondin 1 |
| RT-PCR | quantitative reverse transcription-polymerase chain reaction |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Weinhouse, S.; Warburg, O.; Burk, D.; Schade, A.L. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Potter, V.R. The biochemical approach to the cancer problem. Fed. Proc. 1958, 17, 691–697. [Google Scholar] [PubMed]
- Hirayama, A.; Kami, K.; Sugimoto, M.; Sugawara, M.; Toki, N.; Onozuka, H.; Kinoshita, T.; Saito, N.; Ochiai, A.; Tomita, M.; et al. Quantitative Metabolome Profiling of Colon and Stomach Cancer Microenvironment by Capillary Electrophoresis Time-of-Flight Mass Spectrometry. Cancer Res. 2009, 69, 4918–4925. [Google Scholar] [CrossRef] [PubMed]
- Heddleston, J.M.; Li, Z.; Lathia, J.D.; Bao, S.; Hjelmeland, A.B.; Rich, J.N. Hypoxia inducible factors in cancer stem cells. Br. J. Cancer 2010, 102, 789–795. [Google Scholar] [CrossRef]
- Ueki, S.; Murakami, Y.; Yamada, S.; Kimura, M.; Saito, Y.; Saito, H. microRNA-mediated resistance to hypoglycemia in the HepG2 human hepatoma cell line. BMC Cancer 2016, 16, 732. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 13–21.e1. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Bonfanti, P.; Boj, S.F.; Sato, T.; Loomans, C.J.M.; Van De Wetering, M.; Sojoodi, M.; Li, V.S.W.; Schuijers, J.; Gracanin, A.; et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013, 32, 2708–2721. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nakaoka, T.; Sakai, K.; Muramatsu, T.; Toshimitsu, K.; Kimura, M.; Kanai, T.; Sato, T.; Saito, H. Inhibition of DNA Methylation Suppresses Intestinal Tumor Organoids by Inducing an Anti-Viral Response. Sci. Rep. 2016, 6, 25311. [Google Scholar] [CrossRef]
- Nakaoka, T.; Saito, Y.; Shimamoto, Y.; Muramatsu, T.; Kimura, M.; Kanai, Y.; Saito, H. Cluster microRNAs miR-194 and miR-215 suppress the tumorigenicity of intestinal tumor organoids. Cancer Sci. 2017, 108, 678–684. [Google Scholar] [CrossRef]
- Saito, Y.; Nakaoka, T.; Muramatsu, T.; Ojima, H.; Sukeda, A.; Sugiyama, Y.; Uchida, R.; Furukawa, R.; Kitahara, A.; Sato, T.; et al. Induction of differentiation of intrahepatic cholangiocarcinoma cells to functional hepatocytes using an organoid culture system. Sci. Rep. 2018, 8, 2821. [Google Scholar] [CrossRef]
- Yamada, S.; Takashina, Y.; Watanabe, M.; Nagamine, R.; Saito, Y.; Kamada, N.; Saito, H. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget 2018, 9, 9925–9939. [Google Scholar] [CrossRef]
- Yamada, S.; Kamada, N.; Amiya, T.; Nakamoto, N.; Nakaoka, T.; Kimura, M.; Saito, Y.; Ejima, C.; Kanai, T.; Saito, H. Gut microbiota-mediated generation of saturated fatty acids elicits inflammation in the liver in murine high-fat diet-induced steatohepatitis. BMC Gastroenterol. 2017, 17, 136. [Google Scholar] [CrossRef]
- Owada, S.; Shimoda, Y.; Tsuchihara, K.; Esumi, H. Critical Role of H2O2 Generated by NOX4 during Cellular Response under Glucose Deprivation. PLoS ONE 2013, 8, e56628. [Google Scholar] [CrossRef]
- Esumi, H.; Lu, J.; Kurashima, Y.; Hanaoka, T. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-methyl-qu inolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci. 2004, 95, 685–690. [Google Scholar] [CrossRef]
- Izuishi, K.; Kato, K.; Ogura, T.; Kinoshita, T.; Esumi, H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000, 60, 6201–6207. [Google Scholar] [PubMed]
- Koshikawa, N.; Hayashi, J.; Nakagawara, A.; Takenaga, K. Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J. Biol. Chem. 2009, 284, 33185–33194. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, R.; Hu, Z.; Tian, Y.; Zhu, Y.; Gu, L.; Zhou, L. PI3K and ERK-induced Rac1 activation mediates hypoxia-induced HIF-1alpha expression in MCF-7 breast cancer cells. PLoS ONE 2011, 6, e25213. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Potential value and limitation of dual inhibitors of PI3K and mTOR in the treatment of cancer. Curr. Cancer Drug Targets 2013, 13, 117–120. [Google Scholar] [CrossRef]
- Bu, Z.; Ji, J. Therapeutic implications of mTOR inhibitors in the treatment of gastric cancer. Curr. Cancer Drug Targets 2013, 13, 121–125. [Google Scholar] [CrossRef]
- Cho, D.C.; Mier, J.W. Dual inhibition of PI3-kinase and mTOR in renal cell carcinoma. Curr. Cancer Drug Targets 2013, 13, 126–142. [Google Scholar] [CrossRef]
- ElFiky, A.A.; Jiang, Z. The PI3 kinase signaling pathway in prostate cancer. Curr. Cancer Drug Targets 2013, 13, 157–164. [Google Scholar] [CrossRef]
- Chen, J.; Shao, R.; Li, F.; Monteiro, M.; Liu, J.; Xu, Z.P.; Gu, W. PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1317–1326. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Liu, L.; Wise, D.R.; Diehl, J.A.; Simon, M.C. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J. Biol. Chem. 2008, 283, 31153–31162. [Google Scholar] [CrossRef]
- Bensaad, K.; Cheung, E.C.; Vousden, K.H. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009, 28, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, Y.; Dusting, G.J. NADPH Oxidase-Mediated Redox Signaling: Roles in Cellular Stress Response, Stress Tolerance, and Tissue Repair. Pharmacol. Rev. 2011, 63, 218–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martindale, J.L.; Liu, Y.; Holbrook, N.J. The cellular response to oxidative stress: Influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998, 333 Pt 2, 291–300. [Google Scholar] [CrossRef]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Harrison, D.G. Dual role of reactive oxygen species in vascular growth. Circ. Res. 1999, 85, 562–563. [Google Scholar] [CrossRef] [PubMed]
- Gào, X.; Schöttker, B. Reduction–oxidation pathways involved in cancer development: A systematic review of literature reviews. Oncotarget 2017, 8, 51888–51906. [Google Scholar] [CrossRef]
- Nakanishi, A.; Wada, Y.; Kitagishi, Y.; Matsuda, S. Link between PI3K/AKT/PTEN Pathway and NOX Proteinin Diseases. Aging Dis. 2014, 5, 203–211. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, N.; Saito, Y.; Manabe, H.; Nakaoka, T.; Uchida, R.; Furukawa, R.; Muramatsu, T.; Sugiyama, Y.; Kimura, M.; Saito, H. Glucose Depletion Enhances the Stem Cell Phenotype and Gemcitabine Resistance of Cholangiocarcinoma Organoids through AKT Phosphorylation and Reactive Oxygen Species. Cancers 2019, 11, 1993. https://doi.org/10.3390/cancers11121993
Yoshikawa N, Saito Y, Manabe H, Nakaoka T, Uchida R, Furukawa R, Muramatsu T, Sugiyama Y, Kimura M, Saito H. Glucose Depletion Enhances the Stem Cell Phenotype and Gemcitabine Resistance of Cholangiocarcinoma Organoids through AKT Phosphorylation and Reactive Oxygen Species. Cancers. 2019; 11(12):1993. https://doi.org/10.3390/cancers11121993
Chicago/Turabian StyleYoshikawa, Nao, Yoshimasa Saito, Hiroki Manabe, Toshiaki Nakaoka, Ryoei Uchida, Ryo Furukawa, Toshihide Muramatsu, Yuko Sugiyama, Masaki Kimura, and Hidetsugu Saito. 2019. "Glucose Depletion Enhances the Stem Cell Phenotype and Gemcitabine Resistance of Cholangiocarcinoma Organoids through AKT Phosphorylation and Reactive Oxygen Species" Cancers 11, no. 12: 1993. https://doi.org/10.3390/cancers11121993
APA StyleYoshikawa, N., Saito, Y., Manabe, H., Nakaoka, T., Uchida, R., Furukawa, R., Muramatsu, T., Sugiyama, Y., Kimura, M., & Saito, H. (2019). Glucose Depletion Enhances the Stem Cell Phenotype and Gemcitabine Resistance of Cholangiocarcinoma Organoids through AKT Phosphorylation and Reactive Oxygen Species. Cancers, 11(12), 1993. https://doi.org/10.3390/cancers11121993



