Deterioration in Muscle Mass and Physical Function Differs According to Weight Loss History in Cancer Cachexia
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Changes from Baseline to Week 6 (End of Trial) in Muscle Mass and PF Outcomes
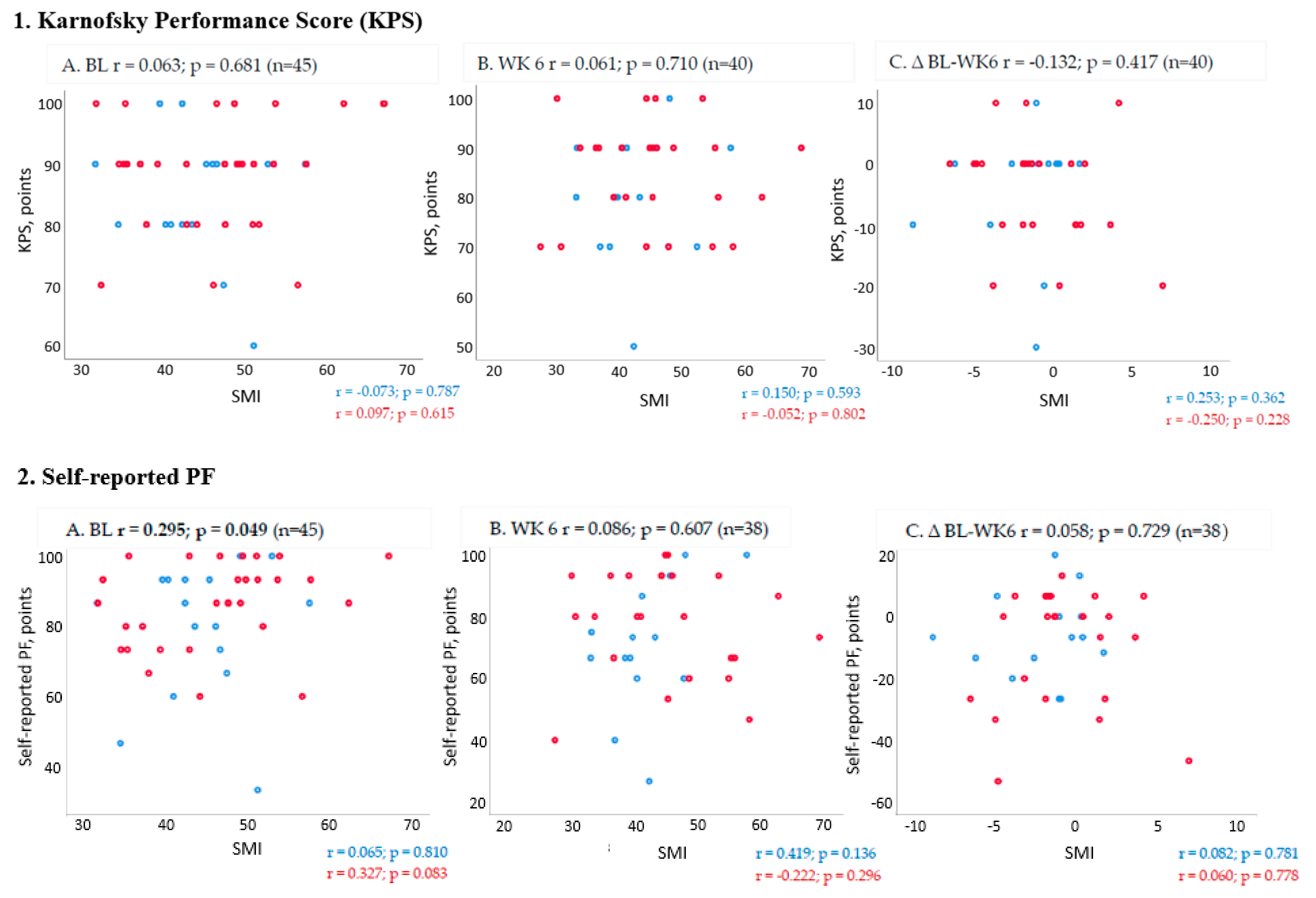
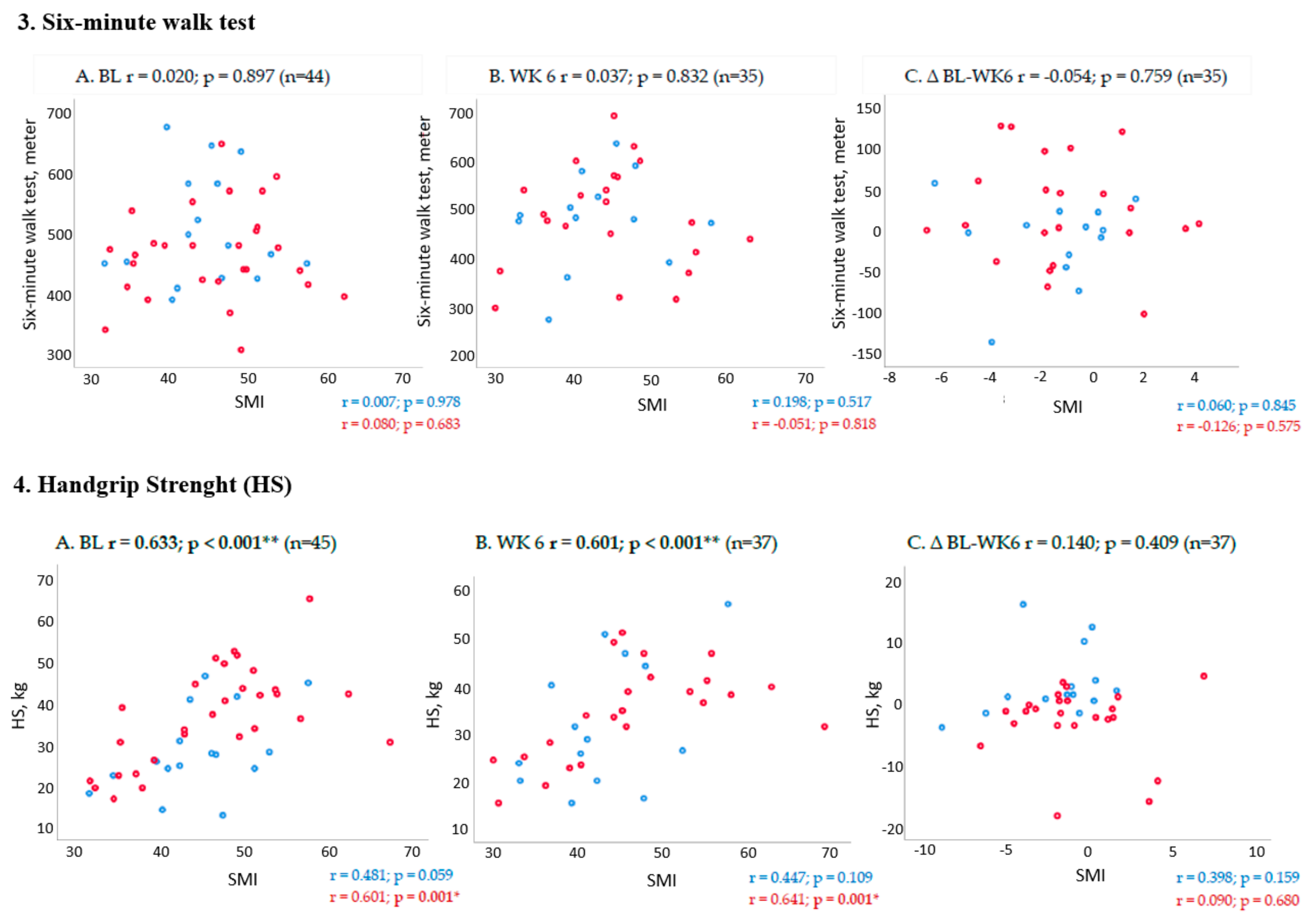
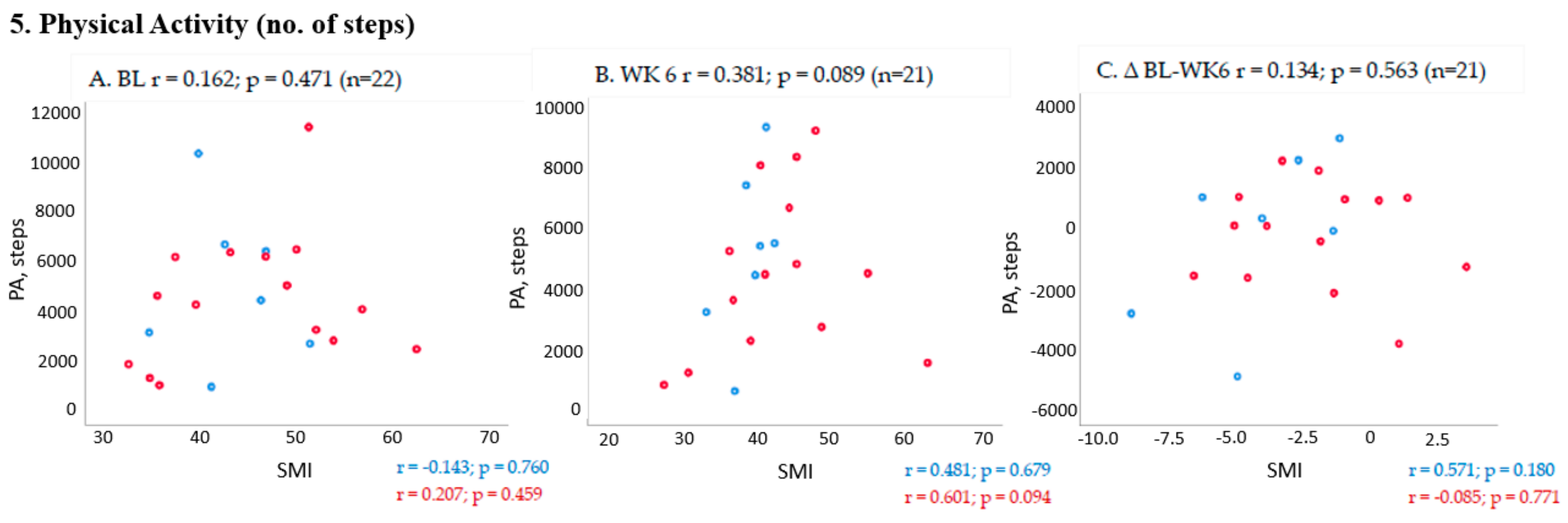
2.3. Association between Changes in Muscle Mass and PF Outcomes from Baseline to Week 6 (End of Trial)
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Data Collection Procedures
4.3. Measures of Muscle Mass
4.4. Measures of Physical Function
4.5. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Vagnildhaug, O.M.; Balstad, T.R.; Almberg, S.S.; Brunelli, C.; Knudsen, A.K.; Kaasa, S.; Thronaes, M.; Laird, B.; Solheim, T.S. A cross-sectional study examining the prevalence of cachexia and areas of unmet need in patients with cancer. Support. Care Cancer 2018, 26, 1871–1880. [Google Scholar] [CrossRef]
- Fearon, K.C. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Abernethy, A.P.; Currow, D.C.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef]
- Crawford, J.; Prado, C.M.; Johnston, M.A.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M.; et al. Cancer cachexia: Rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.A.; Steiner, M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef]
- Laird, B.J.A.; Balstad, T.R.; Solheim, T.S. Endpoints in clinical trials in cancer cachexia: Where to start? Curr. Opin. Support. Palliat. Care 2018, 12, 445–452. [Google Scholar] [CrossRef]
- Bye, A.; Sjoblom, B.; Wentzel-Larsen, T.; Gronberg, B.H.; Baracos, V.E.; Hjermstad, M.J.; Aass, N.; Bremnes, R.M.; Flotten, O.; Jordhoy, M. Muscle mass and association to quality of life in non-small cell lung cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 759–767. [Google Scholar] [CrossRef]
- Naito, T.; Okayama, T.; Aoyama, T.; Ohashi, T.; Masuda, Y.; Kimura, M.; Shiozaki, H.; Murakami, H.; Kenmotsu, H.; Taira, T.; et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer 2017, 17, 571. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Argiles, J.M.; Baracos, V.E.; Bernabei, R.; Coats, A.; Crawford, J.; Deutz, N.E.; Doehner, W.; Evans, W.J.; Ferrucci, L.; et al. Request for regulatory guidance for cancer cachexia intervention trials. J. Cachexia Sarcopenia Muscle 2015, 6, 272–274. [Google Scholar] [CrossRef]
- Srinath, R.; Dobs, A. Enobosarm (GTx-024, S-22): A potential treatment for cachexia. Future Oncol. 2014, 10, 187–194. [Google Scholar] [CrossRef]
- Obling, S.R.; Wilson, B.V.; Pfeiffer, P.; Kjeldsen, J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin. Nutr. 2019, 38, 182–190. [Google Scholar] [CrossRef]
- Brown, J.C.; Cespedes Feliciano, E.M.; Caan, B.J. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 9, 1200–1208. [Google Scholar] [CrossRef]
- Ramage, M.I.; Skipworth, R.J.E. The relationship between muscle mass and function in cancer cachexia: Smoke and mirrors? Curr. Opin. Support. Palliat. Care 2018, 12, 439–444. [Google Scholar] [CrossRef]
- Gale, N.; Wasley, D.; Roberts, S.; Backx, K.; Nelson, A.; van Deursen, R.; Byrne, A. A longitudinal study of muscle strength and function in patients with cancer cachexia. Support. Care Cancer 2018, 27, 131–137. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef]
- Yates, J.W.; Chalmer, B.; McKegney, F.P. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980, 45, 2220–2224. [Google Scholar] [CrossRef]
- Skipworth, R.J.; Stene, G.B.; Dahele, M.; Hendry, P.O.; Small, A.C.; Blum, D.; Kaasa, S.; Trottenberg, P.; Radbruch, L.; Strasser, F.; et al. Patient-focused endpoints in advanced cancer: Criterion-based validation of accelerometer-based activity monitoring. Clin. Nutr. 2011, 30, 812–821. [Google Scholar] [CrossRef]
- Helbostad, J.L.; Holen, J.C.; Jordhoy, M.S.; Ringdal, G.I.; Oldervoll, L.; Kaasa, S. European Association for Palliative Care Research, N. A first step in the development of an international self-report instrument for physical functioning in palliative cancer care: A systematic literature review and an expert opinion evaluation study. J. Pain Symptom Manag. 2009, 37, 196–205. [Google Scholar] [CrossRef]
- Stene, G.B.; Kaasa, S.; Helbostad, J.L. Assessment of Physical Functioning in Palliative Cancer Patients. In European Journal of Palliative Care; Hayward Medical Communications: Fordham, UK, 2008. [Google Scholar]
- Stephens, N.A.; Gray, C.; MacDonald, A.J.; Tan, B.H.; Gallagher, I.J.; Skipworth, R.J.; Ross, J.A.; Fearon, K.C.; Greig, C.A. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clin. Nutr. 2012, 31, 499–505. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wang, Z.; Baumgartner, R.N.; Ross, R. Human body composition: Advances in models and methods. Annu. Rev. Nutr. 1997, 17, 527–558. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Mourtzakis, M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl. Physiol. Nutr. Metab. 2012, 37, 811–821. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef]
- Schaafsma, J.; Osoba, D. The Karnofsky Performance Status Scale re-examined: A cross-validation with the EORTC-C30. Qual. Life Res. 1994, 3, 413–424. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K. European Organization for Research and Treatment of Cancer QLQ-C30 Scoring Manual, 3rd ed.; EORTC Quality of Life Group: Brussels, Belgium, 1995. [Google Scholar]
- Cella, D.; Hahn, E.A.; Dineen, K. Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual. Life Res. 2002, 11, 207–221. [Google Scholar] [CrossRef]
- Schmidt, K.; Vogt, L.; Thiel, C.; Jager, E.; Banzer, W. Validity of the six-minute walk test in cancer patients. Int. J. Sports Med. 2013, 34, 631–636. [Google Scholar] [CrossRef]
- Maddocks, M.; Byrne, A.; Johnson, C.D.; Wilson, R.H.; Fearon, K.C.; Wilcock, A. Physical activity level as an outcome measure for use in cancer cachexia trials: A feasibility study. Support. Care Cancer 2010, 18, 1539–1544. [Google Scholar] [CrossRef]
| Variables/Statistics | All Patients (n = 46) | No/Pre-Cachexia (n = 16) | * Cachexia (n = 30) |
|---|---|---|---|
| Age, mean (SD) | 59.8 (8.4) | 60.4 (9.3) | 59.5 (8.3) |
| Gender, male, n (%) | 26 (57) | 6 (38) | 20 (67) |
| Diagnosis, n (%) | |||
| NSCLC; Stage III | 5 (11) | 2 (12.5) | 3 (10) |
| NSCLC, Stage VI | 21 (46) | 9 (56) | 12 (40) |
| Pancreas, Stage I–III | 11(24) | 3 (19) | 8 (27) |
| Pancreas, Stage IV | 9 (19) | 2 (12.5) | 7 (23) |
| Weight loss, %, mean | 6.1 (7.1) | 0.3 (6.5) | 9.3 (5.1) |
| C-reactive protein, mg/L, mean | 25.7 (32.3) | 11.8 (16.9) | 32,5 (35.9) |
| BMI, kg/m2, mean (SD) | 23.8 (3.6) | 24.6 (3.5) | 23,3 (3.6) |
| SMI, cm2/h2, mean | |||
| Women | 39.0 (5.4) | 41.5 (5.7) | 36.2 (3.5) |
| Men | 50.3 (6.5) | 49.3 (5.2) | 50.6 (6.9) |
| Grip strength, kg, mean | |||
| Women | 24.3 (5.8) | 23.8 (4.8) | 24.8 (6.9) |
| Men | 40.7 (10.1) | 35.6 (13.0) | 42.2 (8.9) |
| KPS, mean (SD) | 87.2 (9.6) | 85 (10.3) | 88.3 (9.1) |
| self-PF score, mean | 84.2 (15,2) | 79.6 (19.2) | 86.7 (12.3) |
| 6-min walk test, meter, mean | 481.6 (83.0) | 505.5 (90.9) | 468.4 (76.7) |
| PA, No. of steps, mean | 4549.9 (2759.8) | 4865.9 (3141.2) | 4402.5 (2668.9) |
| Co-morbidities, n (%) | |||
| Cerebrovascular disease | 1 (2) | 0 | 1 (3) |
| Chronic pulmonary disease | 5 (11) | 3 (19) | 2 (7) |
| Liver disease | 1 (2) | 1 (6) | 0 |
| Diabetes mellitus | 5 (11) | 0 | 5 (17) |
| Other | 18 (39) | 7 (44) | 11 (37) |
| None | 14 (30) | 5 (31) | 9 (30) |
| Randomized to multimodal treatment, n (%) | 25 (54) | 9 (56) | 16 (54) |
| All Patients | No/Pre-Cachexia | Cachexia | ||
|---|---|---|---|---|
| SMI, cm2/m2 | n | 40 | 15 | 25 |
| Baseline | 45.7 (8.6) | 44.6 (6.8) | 46.4 (9.6) | |
| 6 weeks | 44.4 (9.1) | 42.6 (6.8) | 45.4 (10.3) | |
| Within group | ∆ | −1.3 (3.1) CI: 0.3 to 2.3 | −2.0 (2.9) CI: 0.4 to 3.6 | −0.9 (3.2) CI: −0.4 to 2.2 |
| p-value | 0.010 * | 0.019 * | 0.155 | |
| Between group | ∆ | −1.0 (1.0) CI: −3.1 to 1.0 | ||
| p-value | 0.308 | |||
| KPS, score | n | 41 | 13 | 26 |
| Baseline | 87.1 (9.8) | 84.0 (9.9) | 88.9 (9.5) | |
| 6 weeks | 83.2 (11.3) | 80.0 (12.5) | 85.0 (10.3) | |
| Within group | ∆ | −3.9 (8.9) CI: 1.1 to 6.7 | −4.0 (9.9) CI: −1.5 to 9.5 | −3.8 (8.5) CI: 0.4 to 7.3 |
| p-value | 0.008 ** | 0.138 | 0.030 * | |
| Between group | ∆ | −0.2 (2.9) CI: −6.1 to 5.8 | ||
| p-value | 0.958 | |||
| Self-PF, points | n | 39 | 14 | 25 |
| Baseline | 83.8 (15.1) | 77.1 (19.3) | 87.5 (10.9) | |
| 6 weeks | 75.8 (18.7) | 70.6 (20.9) | 78.7 (17.1) | |
| Within group | ∆ | −8.0 (17.0) CI: 2.5 to 13.5 | −6.5 (13.8) CI: −1.4 to 14.5 | −8.8 (18.7) CI: 1.1 to 16.5 |
| p-value | 0.006 ** | 0.099 | 0.027 * | |
| Between group | ∆ | 2.3 (5.7) CI: −9.4 to 13.9 | ||
| p-value | 0.696 | |||
| 6MWT, meters | n | 36 | 13 | 23 |
| Baseline | 472.3 (81.9) | 492.7 (81.9) | 460.8 (81.5) | |
| 6 weeks | 482.0 (101.1) | 481.4 (97.6) | 482.4 (105.2) | |
| Within group | ∆ | 9.7 (60.2) CI: −30.0 to 10.7 | −11.3 (50.9) CI: −19.4 to 42.1 | 21.5 (62.8) CI: −48.7 to 5.6 |
| p-value | 0.341 | 0.439 | 0.114 | |
| Between group | ∆ | −32.9 (20.4) CI: −74.4 to 6.7 | ||
| p-value | 0.117 | |||
| HGS, kg | n | 38 | 14 | 24 |
| Baseline | 34.2 (11.9) | 28.7 (10.9) | 37.4 (11.5) | |
| 6 weeks | 33.6 (11.1) | 31.8 (13.5) | 34.7 (9.7) | |
| Within group | ∆ | −0.6 (6.3) CI: −1.5 to 2.6 | 3.1 (5.7) CI: −6.4 to 0.1 | −2.7 (5.6) CI: 0.4 to 5.1 |
| p-value | 0.578 | 0.059 | 0.026 * | |
| Between group | ∆ | 5.9 (1.9) CI:2.0 to 9.7 | ||
| p-value | 0.004 ** | |||
| PA, No. steps | n | 22 | 7 | 15 |
| Baseline | 4549.9 (2759.8) | 4865.9 (3141.2) | 4402.5 (2668.8) | |
| 6 weeks | 4897.9 (2796.2) | 5091.7 (2808.8) | 4807.4 (2884.4) | |
| Within group | ∆ | 348 (2071.6) CI: 1266.5 to 570.6) | 225.8 (2783.1) CI: 2799.7 to 2348.1 | 405.0 (1762.7) CI: −1381.1 to 571.2 |
| p-value | 0.440 | 0.837 | 0.389 | |
| Between group | ∆ | 179.2 (970.9) CI: −1846 to 2204.4 | ||
| p-value | 0.855 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stene, G.B.; Balstad, T.R.; Leer, A.S.M.; Bye, A.; Kaasa, S.; Fallon, M.; Laird, B.; Maddocks, M.; Solheim, T.S. Deterioration in Muscle Mass and Physical Function Differs According to Weight Loss History in Cancer Cachexia. Cancers 2019, 11, 1925. https://doi.org/10.3390/cancers11121925
Stene GB, Balstad TR, Leer ASM, Bye A, Kaasa S, Fallon M, Laird B, Maddocks M, Solheim TS. Deterioration in Muscle Mass and Physical Function Differs According to Weight Loss History in Cancer Cachexia. Cancers. 2019; 11(12):1925. https://doi.org/10.3390/cancers11121925
Chicago/Turabian StyleStene, Guro Birgitte, Trude Rakel Balstad, Anne Silja M. Leer, Asta Bye, Stein Kaasa, Marie Fallon, Barry Laird, Matthew Maddocks, and Tora S. Solheim. 2019. "Deterioration in Muscle Mass and Physical Function Differs According to Weight Loss History in Cancer Cachexia" Cancers 11, no. 12: 1925. https://doi.org/10.3390/cancers11121925
APA StyleStene, G. B., Balstad, T. R., Leer, A. S. M., Bye, A., Kaasa, S., Fallon, M., Laird, B., Maddocks, M., & Solheim, T. S. (2019). Deterioration in Muscle Mass and Physical Function Differs According to Weight Loss History in Cancer Cachexia. Cancers, 11(12), 1925. https://doi.org/10.3390/cancers11121925





