Evaluation of the Accuracy of Liquid-Based Oral Brush Cytology in Screening for Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robert Koch-Institut. Krebs in Deutschland 2013/2014; Robert Koch-Institut: Berlin, Germany, 2014. [Google Scholar]
- Gleich, L.L.; Salamone, F.N. Molecular genetics of head and neck cancer. Cancer Control 2002, 9, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Sabiha, B.; Jan, H.U.; Haider, S.A.; Khan, A.A.; Ali, S.S. Genetic etiology of oral cancer. Oral Oncol. 2017, 70, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Field, J.K.; Tanzawa, H. Genetic aberrations in oral or head and neck squamous cell carcinoma 2: Chromosomal aberrations. Oral Oncol. 2000, 36, 311–327. [Google Scholar] [CrossRef]
- Scully, C.; Field, J.K.; Tanzawa, H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol. 2000, 36, 256–263. [Google Scholar] [CrossRef]
- Boffetta, P.; Mashberg, A.; Winkelmann, R.; Garfinkel, L. Carcinogenic effect of tobacco smoking and alcohol drinking on anatomic sites of the oral cavity and oropharynx. Int. J. Cancer 1992, 52, 530–533. [Google Scholar] [CrossRef]
- Kato, I.; Nomura, A.M. Alcohol in the aetiology of upper aerodigestive tract cancer. Eur. J. Cancer B Oral Oncol. 1994, 30, 75–81. [Google Scholar] [CrossRef]
- Lewin, F.; Norell, S.E.; Johansson, H.; Gustavsson, P.; Wennerberg, J.; Biorklund, A.; Rutqvist, L.E. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: A population-based case-referent study in Sweden. Cancer 1998, 82, 1367–1375. [Google Scholar] [CrossRef]
- Obe, G.; Ristow, H. Mutagenic, cancerogenic and teratogenic effects of alcohol. Mutat. Res. 1979, 65, 229–259. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Doody, D.R.; Fitzgibbons, E.D.; Ricks, S.; Porter, P.L.; Chen, C. Oral squamous cell cancer risk in relation to alcohol consumption and alcohol dehydrogenase-3 genotypes. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1137–1144. [Google Scholar]
- Zain, R.B.; Gupta, P.C.; Warnakulasuriya, S.; Shrestha, P.; Ikeda, N.; Axell, T. Oral lesions associated with betel quid and tobacco chewing habits. Oral Dis. 1997, 3, 204–205. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y. Human papillomavirus infection in oral potentially malignant disorders and cancer. Arch. Oral Biol. 2017, 83, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, V.; Reid, M.; Hatton, E.; Merzianu, M.; Rigual, N.; Marshall, J.; Gill, S.; Frustino, J.; Wilding, G.; Loree, T.; et al. Human papillomavirus types 16 and 18 in epithelial dysplasia of oral cavity and oropharynx: A meta-analysis, 1985–2010. Oral Oncol. 2011, 47, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.; Koch, W.; Trotti, A.; Sidransky, D. Head and neck cancer. N. Engl. J. Med. 2001, 345, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Kramer, I.R.; Lucas, R.B.; Pindborg, J.J.; Sobin, L.H. Definition of leukoplakia and related lesions: An aid to studies on oral precancer. Oral Surg. Oral Med. Oral Pathol. 1978, 46, 518–539. [Google Scholar]
- Eckert, A.W.; Lautner, M.H.; Dempf, R.; Schubert, J.; Bilkenroth, U. Prognostic factors for oral squamous cell carcinoma. Chirurg 2009, 80, 138–143. [Google Scholar] [CrossRef]
- Stell, P.M.; Wood, G.D.; Scott, M.H. Early oral cancer: Treatment by biopsy excision. Br. J. Oral Surg. 1982, 20, 234–238. [Google Scholar] [CrossRef]
- Kowalski, L.P.; Carvalho, A.L. Influence of time delay and clinical upstaging in the prognosis of head and neck cancer. Oral Oncol. 2001, 37, 94–98. [Google Scholar] [CrossRef]
- Alsarraf, A.; Kujan, O.; Farah, C.S. The utility of oral brush cytology in the early detection of oral cancer and oral potentially malignant disorders: A systematic review. J. Oral. Pathol. Med. 2018, 47, 104–116. [Google Scholar] [CrossRef]
- Alsarraf, A.; Kujan, O.; Farah, C.S. Liquid-based oral brush cytology in the diagnosis of oral leukoplakia using a modified Bethesda Cytology system. J. Oral Pathol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Casparis, S.; Borm, J.M.; Tomic, M.A.; Burkhardt, A.; Locher, M.C. Transepithelial Brush Biopsy—Oral CDx(R)—A Noninvasive Method for the Early Detection of Precancerous and Cancerous Lesions. J. Clin. Diagn. Res. JCDR 2014, 8, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, J.J. Improving detection of precancerous and cancerous oral lesions. Computer-assisted analysis of the oral brush biopsy. U.S. Collaborative OralCDx Study Group. J. Am. Dent. Assoc. 1999, 130, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Lingen, M.W.; Kalmar, J.R.; Karrison, T.; Speight, P.M. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008, 44, 10–22. [Google Scholar] [CrossRef]
- Macey, R.; Walsh, T.; Brocklehurst, P.; Kerr, A.R.; Liu, J.L.; Lingen, M.W.; Ogden, G.R.; Warnakulasuriya, S.; Scully, C. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst. Rev. 2015, Cd010276. [Google Scholar] [CrossRef]
- Goodson, M.L.; Smith, D.R.; Thomson, P.J. Efficacy of oral brush biopsy in potentially malignant disorder management. J. Oral Pathol. Med. 2017, 46, 896–901. [Google Scholar] [CrossRef]
- Kujan, O.; Desai, M.; Sargent, A.; Bailey, A.; Turner, A.; Sloan, P. Potential applications of oral brush cytology with liquid-based technology: Results from a cohort of normal oral mucosa. Oral Oncol. 2006, 42, 810–818. [Google Scholar] [CrossRef]
- Onofre, M.A.; Sposto, M.R.; Navarro, C.M.; Motta, M.E.; Turatti, E.; Almeida, R.T. Potentially malignant epithelial oral lesions: Discrepancies between clinical and histological diagnosis. Oral Dis. 1997, 3, 148–152. [Google Scholar] [CrossRef]
- Remmerbach. Evaluation der Zytologischen Diagnostik und Adjuvanter Methoden an Präparaten Oraler Bürstenbiopsien zur Sekundärprävention von Lippen- und Oropharynxkarzinomen; Univ. Habil.-Schr.: Leipzig, Germany, 2006; p. 249. [Google Scholar]
- Remmerbach, T.W.; Hemprich, A.; Böcking, A. Minimally invasive brush-biopsy: Innovative method for early diagnosis of oral squamous cell carcinoma. Schweiz Monatsschr Zahnmed 2007, 117, 926–940. [Google Scholar]
- Remmerbach, T.W.; Pomjanski, N.; Bauer, U.; Neumann, H. Liquid-based versus conventional cytology of oral brush biopsies: A split-sample pilot study. Clin. Oral Investig. 2017, 21, 2493–2498. [Google Scholar] [CrossRef]
- Kujan, O.; Pemberton, M.N.; Schwarz, M.; Sloan, P. Evaluation of an innovative oral brush for potential applications using liquid based cytology. J. Oral Sci. 2018, 60, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Olms, C.; Hix, N.; Neumann, H.; Yahiaoui-Doktor, M.; Remmerbach, T.W. Clinical comparison of liquid-based and conventional cytology of oral brush biopsies: A randomized controlled trial. Head Face Med. 2018, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, E.; Schwarzmann, P.; Kirkpatrick, M.; Fox, W.; Heinzerling, R.H.; Geyer, J.W.; Knesel, E.A. The false negative rate in cervical cytology. Comparison of monolayers to conventional smears 1. Acta Cytol. 1996, 40, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Navone, R.; Burlo, P.; Pich, A.; Pentenero, M.; Broccoletti, R.; Marsico, A.; Gandolfo, S. The impact of liquid-based oral cytology on the diagnosis of oral squamous dysplasia and carcinoma. Cytopathology 2007, 18, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Dolens Eda, S.; Nakai, F.V.; Santos Parizi, J.L.; Alborghetti Nai, G. Cytopathology: A useful technique for diagnosing oral lesions?: A systematic literature review. Diagn. Cytopathol. 2013, 41, 505–514. [Google Scholar] [CrossRef]
- Burkhardt, A.; Schwarz-Furlan, S. Abrasive cytohistology of squamous epithelial lesions. Transl. Res. Oral Oncol. 2018, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Böcking, A.; Freudenberg, N. Standardisierte Befunderstellung in der extragenitalen Zytologie. Pathologe 1998, 19, 235–236. [Google Scholar]
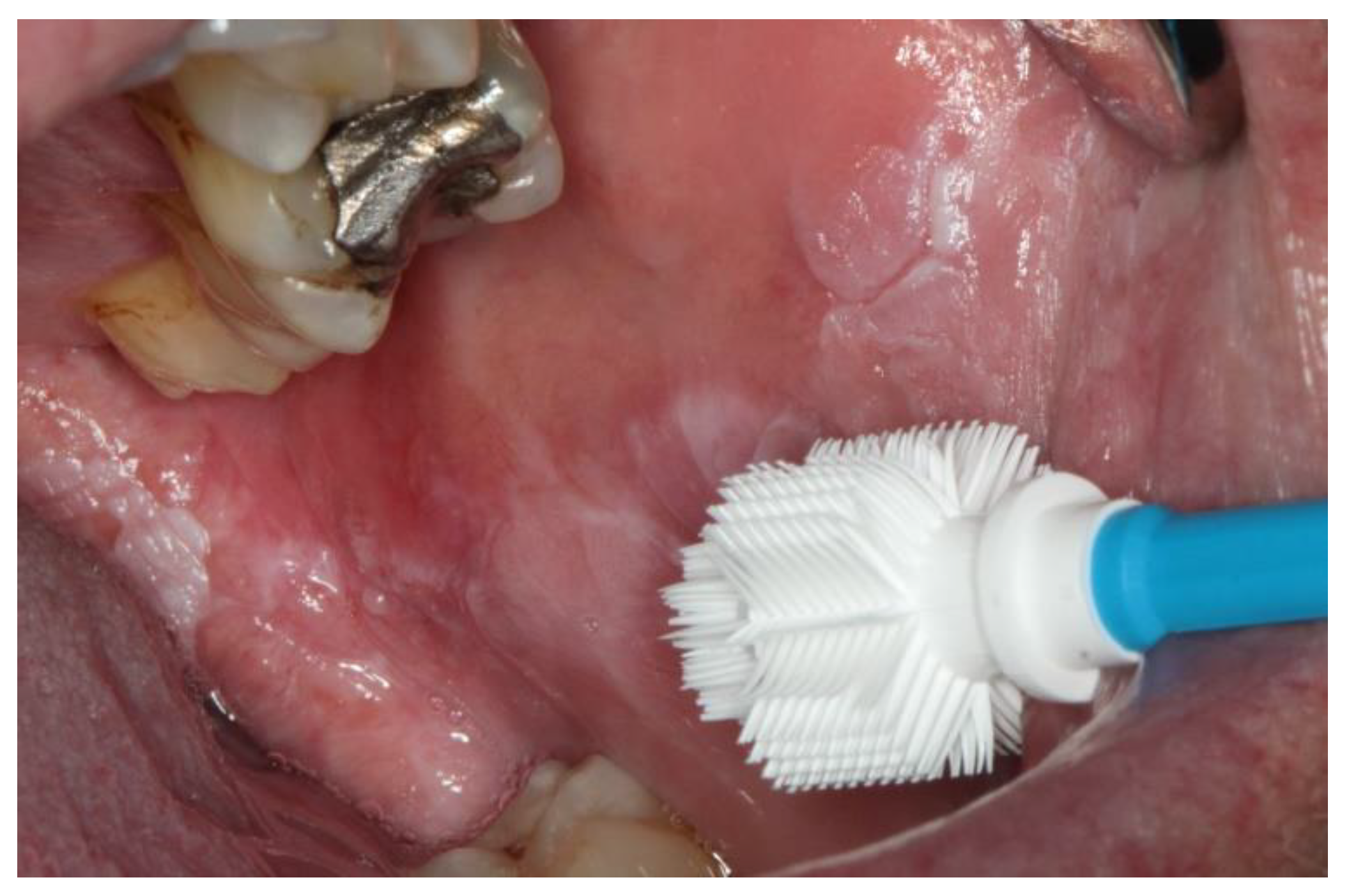

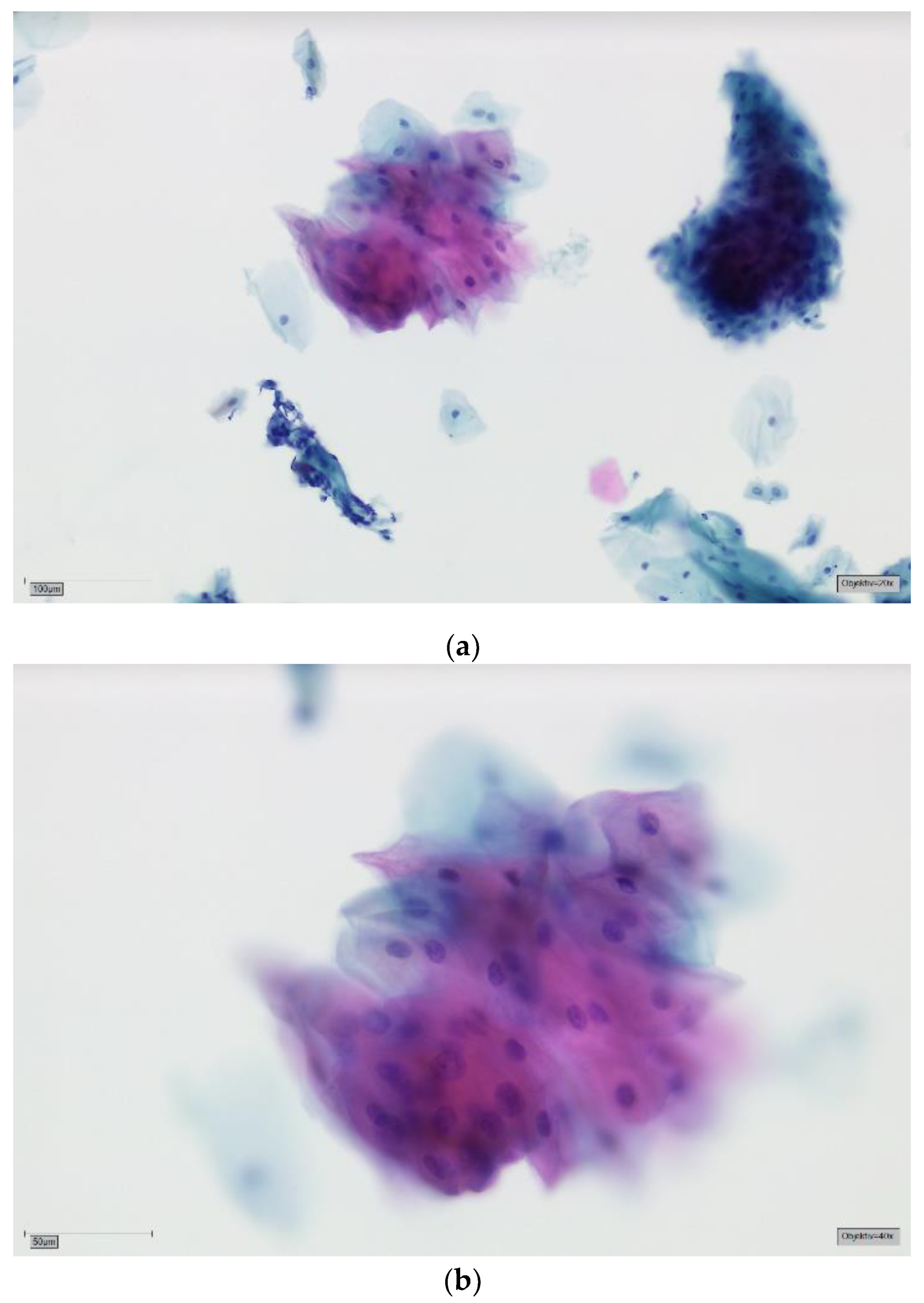


| Result Brush | Sample Size and Average Age (Years) | ||
|---|---|---|---|
| Male | Female | Total | |
| Positive | 63 | 26 | 89 |
| 59.3 | 67.7 | 61.7 | |
| Suspicious | 23 | 33 | 56 |
| 67.9 | 66.9 | 67.3 | |
| Doubtful | 65 | 85 | 150 |
| 60.3 | 69.0 | 65.3 | |
| Negative | 457 | 600 | 1057 |
| 58.5 | 62.4 | 60.7 | |
| Total | 608 | 744 | 1352 |
| 59.1 | 63.5 | 61.6 | |
| Diagnosis | Frequency | |
|---|---|---|
| Absolute | Relative | |
| SCC | 105 | 7.8% |
| Lichen | 260 | 19.2% |
| Lichen erosivus | 138 | 10.2% |
| Leukoplakia | 297 | 22.0% |
| Proliferative verrucous leukoplakia | 20 | 1.5% |
| Erythroplakia | 29 | 2.1% |
| Other Lesions | 503 | 37.2% |
| Total | 1352 | |
| Location | Frequency All Lesions | Frequency SCC | ||
|---|---|---|---|---|
| Absolute | Relative | Absolute | Relative | |
| Border of tongue | 303 | 22.4% | 34 | 32.4% |
| Back of tongue | 26 | 1.9% | 0 | 0% |
| Floor of the mouth | 79 | 5.8% | 16 | 15.2% |
| Buccal mucosa | 384 | 28.4% | 8 | 7.6% |
| Tonsil | 8 | 0.6% | 2 | 1.9% |
| Lip/Labial mucosa | 37 | 2.7% | 4 | 3.8% |
| Palate | 106 | 7.8% | 11 | 10.5% |
| Alveolar ridge | 409 | 30.3% | 30 | 28.6% |
| Total | 1352 | 105 | ||
| Test | Accuracy | Confidence Interval (CI) | Lower Endpoint | Upper Endpoint |
|---|---|---|---|---|
| Sensitivity | 95.6% | 95% | 0.945 | 0.967 |
| Specificity | 84.9% | 95% | 0.830 | 0.868 |
| Positive predictive value | 36.6% | 95% | 0.340 | 0.392 |
| Negative predictive value | 99.5% | 95% | 0.992 | 0.999 |
| Brush Result | Cancer Diagnosis | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 77 | 12 | 89 |
| Suspicious | 15 | 41 | 56 |
| Doubtful | 16 | 134 | 150 |
| Negative | 5 | 1052 | 1057 |
| Total | 113 | 1239 | 1352 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deuerling, L.; Gaida, K.; Neumann, H.; Remmerbach, T.W. Evaluation of the Accuracy of Liquid-Based Oral Brush Cytology in Screening for Oral Squamous Cell Carcinoma. Cancers 2019, 11, 1813. https://doi.org/10.3390/cancers11111813
Deuerling L, Gaida K, Neumann H, Remmerbach TW. Evaluation of the Accuracy of Liquid-Based Oral Brush Cytology in Screening for Oral Squamous Cell Carcinoma. Cancers. 2019; 11(11):1813. https://doi.org/10.3390/cancers11111813
Chicago/Turabian StyleDeuerling, Lena, Kristin Gaida, Heinrich Neumann, and Torsten W. Remmerbach. 2019. "Evaluation of the Accuracy of Liquid-Based Oral Brush Cytology in Screening for Oral Squamous Cell Carcinoma" Cancers 11, no. 11: 1813. https://doi.org/10.3390/cancers11111813
APA StyleDeuerling, L., Gaida, K., Neumann, H., & Remmerbach, T. W. (2019). Evaluation of the Accuracy of Liquid-Based Oral Brush Cytology in Screening for Oral Squamous Cell Carcinoma. Cancers, 11(11), 1813. https://doi.org/10.3390/cancers11111813




