Molecular Mechanisms Underlying Autophagy-Mediated Treatment Resistance in Cancer
Abstract
1. Introduction
2. Autophagy Contributes to Treatment Resistance in Cancer
2.1. Autophagy and Resistance Against Chemotherapy
| Name | Mechanism of Action | Targeted Stage in Autophagy | Ref. |
|---|---|---|---|
| Inhibitors that target late stages of autophagy | |||
| Lys05 | Lysosomotropic agent |
| [46,47] |
| Chloroquine (CQ) | Lysosomotropic agent |
| [48,49] |
| Hydroxychloroquine (HCQ) | |||
| Quinacrine (QNX) | |||
| IITZ-01 | Lysosomotropic agent |
| [50] |
| ROC-325 | unknown |
| [51,52,53] |
| Bafilomycin A1 (Baf A1) | Vacuolar ATPase inhibitor |
| [54] |
| ECDD-S27 | Vacuolar ATPase inhibitor |
| [55] |
| Ca-5f | Unknown |
| [56] |
| EACC | Syntaxin 17 (STX17) translocation inhibitor |
| [57] |
| MHY1485 | Mammalian target of rapamycin (mTOR) activator |
| [58] |
| Pepstatin A | Acid protease inhibitor |
| [59,60] |
| E64-d | Acid protease inhibitor |
| [59,60] |
| Alpha-hederin | Unknown |
| [61] |
| Inhibitors that target early stages of autophagy | |||
| SB02024 | Vacuolar protein sorting protein 34 (Vps34) inhibitor |
| [62] |
| SAR405 | Vacuolar protein sorting protein 18 and 34 (Vps18 and Vps34) inhibitor |
| [63] |
| PIK-III | Vacuolar protein sorting protein 34 (Vps34) inhibitor |
| [64] |
| Autophinib | Vacuolar protein sorting protein 34 (Vps34) inhibitor |
| [65] |
| SBI-0206965 | Unc-51-like kinase 1 (ULK1) inhibitor |
| [66] |
| ULK-100, ULK-101 | Unc-51-like kinase 1 (ULK1) inhibitor |
| [67] |
| LY294002 | Phosphoinositide 3-kinase (PI3) inhibitor |
| [68,69] |
| 3-Methyladenine (3-MA) | Phosphoinositide 3-kinase (PI3) inhibitor |
| [70] |
| Wortmannin | Phosphoinositide 3-kinase (PI3) inhibitor |
| [68,71,72] |
| Spautin-1 | Ubiquitin Specific Peptidase 10 and 13 (USP10 and USP13) inhibitor |
| [73,74] |
| NSC185058 | Autophagy-related (ATG) protein 4A and 4B (ATG4A and ATG4B) inhibitor |
| [75] |
| UAMC-2526 | Autophagy-related (ATG) protein 4B (ATG4B) inhibitor |
| [76] |
| DMP-1 | unknown |
| [77] |
| Inhibitors that target both early and late stages of autophagy | |||
| Tioconazole | Autophagy-related (ATG) protein 4A and 4B (ATG4A and ATG4B) inhibitor |
| [78] |
| LV-320 | Autophagy-related (ATG) protein 4A and 4B (ATG4A and ATG4B) inhibitor |
| [79] |
| S130 | Autophagy-related (ATG) protein 4A and 4B (ATG4A and ATG4B) inhibitor |
| [80] |
| Xanthium strumarium Fruit Extract | Autophagy-related (ATG) protein 4B (ATG4B) inhibitor |
| [81] |
| Verteporfin | unknown |
| [82] |
| Cancer Type | Chemotherapeutic Agent | Mode of Autophagy Inhibition | Ref. | |
|---|---|---|---|---|
| Pharmacological | Genetic | |||
| Bladder cancers | Cisplatin, Gemcitabine, Mitomycin, Pirarubicin | CQ, HCQ, Baf A1, Wortmannin, 3-MA | ATG7 shRNA, ATG12 shRNA, BECN1 shRNA, BECN1 siRNA, ATG3 siRNA | [83,84,85,86,87] |
| Bone cancers | Doxorubicin, Cisplatin, Methotrexate, Paclitaxel | Spautin-1, 3-MA, CQ | BECN1 shRNA, BECN1-targeting deoxyribozyme, miR-410 (ATG16L knockdown), ATG14 siRNA, ATG7 siRNA | [88,89,90,91,92,93,94,95,96,97,98] |
| Breast cancers | 5-Fluorouracil, Doxorubicin, Docetaxel, Adriamycin, Cyclophosphamide, Epirubicin, Paclitaxel, Cisplatin, Gemcitabine | Baf A1, CQ, HCQ, 3-MA, Verteporfin | ATG5 shRNA, ATG5 siRNA, ATG7 siRNA, FIP200 shRNA, ATG13 shRNA, BECN1 shRNA, BECN1 siRNA, BECN1 CRISPR/Cas9 KO, BNIP3L CRISPR/Cas9 KO, AMBRA1 shRNA | [99,100,101,102,103,104,105,106,107] |
| Cervical cancers | Cisplatin, Oncothermia, Paclitaxel | 3-MA, CQ, Baf A1 | BECN1 siRNA, ATG5 siRNA, ATG7 siRNA | [108,109,110,111,112] |
| Colorectal cancers | 5-FU, Oxaliplatin | CQ, 3-MA, Baf A1 | ATG7 siRNA, BECN1 siRNA, ATG5 siRNA | [113,114,115,116] |
| Endometrial cancers | Paclitaxel, Cisplatin, Resveratrol, Oncothermia | CQ, 3-MA | BECN1 shRNA, ATG5 siRNA, ATG7 siRNA | [117,118,119,120,121] |
| Gastric cancers | Vincristine, 5-fluorouracil, Cisplatin, Bufalin, Matrine, Oxaliplatin, Cinobufagin | CQ, 3-MA, Baf A1 | miR-23b (ATG12 knockdown), ATG5 siRNA | [122,123,124,125,126,127,128,129,130,131,132] |
| Gliomas | Temozolomide, Vorinostat | HCQ, CQ, 3-MA, QNX, Baf A1, ATG4B inhibitor (NSC185058) | LC3A siRNA, LC3B siRNA, TFEB siRNA, ATG4C shRNA, ATG4B shRNA | [133,134,135,136,137,138,139,140] |
| Head and neck cancers | Paclitaxel, Cisplatin, Nedaplatin | 3-MA, CQ, Baf A1 | LC3B siRNA, ATG3 siRNA, ATG5 siRNA, ATG6 siRNA, ATG7 siRNA | [141,142,143,144,145,146] |
| Liver cancers | Epirubicin, Oxaliplatin, Mitomycin, Cisplatin, Doxorubicin | 3-MA, CQ, Baf A1 | ATG4B shRNA, ATG5 siRNA, ATG5 shRNA, ATG7 shRNA, LC3 shRNA, miR-101 (RAB5A, STMN1, ATG4B knockdown) | [147,148,149,150,151,152,153,154,155] |
| Lung cancers | Paclitaxel, Camptothecin, Cisplatin, 5-fluorouracil, Gemcitabine, Pterostilbene | 3-MA, CQ, Alpha-hederin, Baf A1, ATG4B inhibitors (Compound 1, 17) | ATG7 siRNA, BECN1 siRNA, ATG5 siRNA | [61,141,156,157,158,159,160,161,162,163] |
| Neuroblastomas | Vincristine, Doxorubicin, Cisplatin, Paclitaxel | HCQ, 3-MA | ATG5 shRNA, ATG5 siRNA, BECN1 siRNA, TRP14 siRNA | [164,165,166] |
| Ovarian cancers | Cisplatin, Carboplatin, Vincristine, Gemcitabine, Ifosfamide, Docetaxel, Paclitaxel | 3-MA, CQ, Quinacrine | ATG5 siRNA, BECN1 siRNA, AMBRA1 shRNA, ATG5 CRISPR/Cas9 KO, miR-204 (LC3B and ATG7 knockdown) | [167,168,169,170,171,172,173,174,175,176] |
| Pancreatic cancers | Doxorubicin, Gemcitabine, Docetaxel | CQ, Verteporfin | ATG7 siRNA, ATG12 siRNA, USP22 siRNA, miR-23b (ATG12 knockdown), miR-29a (ATG9A and TFEB knockdown), miR-29c (USP22 knockdown), miR-137 (ATG5 knockdown) | [177,178,179,180,181,182,183,184,185] |
| Skin cancers | Temozolomide, Cisplatin | CQ, HCQ, LY294002 | ATG5 shRNA | [186,187,188] |
2.2. Autophagy and Resistance Against Targeted Agents
2.3. Clinical Trials
3. Crosstalk between Autophagy and Diverse Signaling Pathways Contributes to Treatment Resistance in Cancer
3.1. The Contributions of Autophagy and Major Signaling Pathways to Pro-Survival Responses that Resist Anti-Cancer Therapies—Guilt by Association?
3.1.1. Mitogen-Activated Protein Kinase (MAPK) Signaling
The Role of MAPK Signaling in Cancer Progression
MAPK Signaling and Autophagy: Connections to Treatment Resistance in Cancers
3.1.2. Protein Kinase B/AKT (PKB/AKT) Pathway
The Role of PKB/AKT Signaling in Cancer Progression
AKT Signaling and Autophagy: Connections to Treatment Resistance in Cancers
3.2. The Cellular Recycling and Self-Degradative Functions of Autophagy Contribute to Chemoresistance in Cancers
3.2.1. The p62-KEAP1-NRF2 Axis
p62-KEAP1-NRF2 Signaling in Cancers
Crosstalk between Autophagy Pathway and the p62-KEAP1-NRF2 Axis in Cancer Progression and Chemoresistance
3.2.2. The FOXO3A-PUMA Axis
FOXO3A-PUMA Signaling in Cancer
Crosstalk between Autophagy Pathway and the FOXO3A-PUMA Axis in Cancer Progression and Chemoresistance
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Cloonan, S.M.; Choi, A.M.K. Autophagy: A critical regulator of cellular metabolism and homeostasis. Mol. Cells 2013, 36, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.; Kimmelman, A.C.; White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016, 30, 1913–1930. [Google Scholar] [CrossRef] [PubMed]
- Santana-Codina, N.; Mancias, J.D.; Kimmelman, A.C. The Role of Autophagy in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Vessoni, A.T.; Filippi-Chiela, E.C.; Menck, C.F.; Lenz, G. Autophagy and genomic integrity. Cell Death Differ. 2013, 20, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, A.G.; Havaki, S.; Gorgoulis, V.G. DNA Damage Response and Autophagy: A Meaningful Partnership. Front. Genet. 2016, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Pedro, J.M.B.-S.; Kroemer, G. Autophagy Mediates Tumor Suppression via Cellular Senescence. Trends Cell Biol. 2016, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.S.; Gao, Z.R.; Zhang, Q.; Tang, X.F.; Lv, Y.F.; Zhang, Z.S.; Zhang, Y.; Tan, Q.L.; Peng, D.B.; Jiang, D.M.; et al. TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/AKT/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis. J. Exp. Clin. Cancer Res. 2018, 37, 188. [Google Scholar] [CrossRef] [PubMed]
- Slobodnyuk, K.; Radic, N.; Ivanova, S.; Llado, A.; Trempolec, N.; Zorzano, A.; Nebreda, A.R. Autophagy-induced senescence is regulated by p38α signaling. Cell Death Dis. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ramirez, L.; Vodnala, S.K.; Nini, R.; Hunter, K.W.; Green, J.E. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, X.; Pan, H. Active autophagy in the tumor microenvironment: A novel mechanism for cancer metastasis (Review). Oncol. Lett. 2013, 5, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Kimmelman, A.C.; White, E. Autophagy and Tumor Metabolism. Cell Metab. 2017, 25, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.M.; Dass, C.R. Metabolic stress and cancer: Is autophagy the common denominator and a feasible target? J. Pharm. Pharmacol. 2014, 66, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yu, Z.; Chen, Z.; Yang, L.; Ma, M.; Lu, S.; Wang, C.; Teng, C.; Nie, Y. Zinc chelator TPEN induces pancreatic cancer cell death through causing oxidative stress and inhibiting cell autophagy. J. Cell. Physiol 2019, 234, 20648–20661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xu, G.; Wang, Y.; Xu, Z.; Liu, X.; Xu, X.; Ren, G.; Tian, K. Oxidative stress induced autophagy in cancer associated fibroblast enhances proliferation and metabolism of colorectal cancer cells. Cell Cycle 2017, 16, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Teng, X.; Laddha, S.V.; Ma, S.; Van Nostrand, S.C.; Yang, Y.; Khor, S.; Chan, C.S.; Rabinowitz, J.D.; White, E. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 2016, 30, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, H.; Hilgendorf, S.; Vellenga, E.; Bremer, E.; Wiersma, V.R. The multifaceted role of autophagy in cancer and the microenvironment. Med. Res. Rev. 2019, 39, 517–560. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Kriel, J.; Shubha Priya, B.; Basappa; Shivananju, N.S.; Loos, B. Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization. Biochem. Pharmacol. 2018, 147, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ji, Z.; Xu, C.; Zhu, J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: A systematic review and meta-analysis. Medicine 2018, 97, e12912. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Kroemer, G. Therapeutic modulation of autophagy: Which disease comes first? Cell Death Differ. 2019, 26, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Seebacher, N.; Shi, H.; Kan, Q.; Duan, Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8, 84559–84571. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Woods, R.J.; Read, A.F. How to Use a Chemotherapeutic Agent When Resistance to It Threatens the Patient. PLoS Biol. 2017, 15, e2001110. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, N. Drug resistance: The next target for cancer treatment. BMJ 2019, 365, l2228. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.M.; Gevertz, J.L.; Sontag, E.D. A mathematical approach to differentiate spontaneous and induced evolution to drug resistance during cancer treatment. bioRxiv 2018. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Taha, R.; Omri, H.E. Current Opinions on Chemoresistance: An Overview. Bioinformation 2018, 14, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Panda, M.; Biswal, B.K. Cell signaling and cancer: A mechanistic insight into drug resistance. Mol. Biol. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, H.; Borden, K. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Parseghian, C.M.; Napolitano, S.; Loree, J.M.; Kopetz, S. Mechanisms of Innate and Acquired Resistance to anti-EGFR therapy: A Review of Current Knowledge with a Focus on Rechallenge Therapies. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Kulkarni, P. The Genetic/Non-genetic Duality of Drug ‘Resistance’ in Cancer. Trends Cancer 2018, 4, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hammerlindl, H.; Schaider, H. Tumor cell-intrinsic phenotypic plasticity facilitates adaptive cellular reprogramming driving acquired drug resistance. J. Cell Commun. Signal. 2018, 12, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Gini, B.; Wykosky, J.; Zanca, C.; Mischel, P.S.; Furnari, F.B.; Cavenee, W.K. A tale of two approaches: Complementary mechanisms of cytotoxic and targeted therapy resistance may inform next-generation cancer treatments. Carcinogenesis 2013, 34, 725–738. [Google Scholar] [CrossRef] [PubMed]
- McAfee, Q.; Zhang, Z.; Samanta, A.; Levi, S.M.; Ma, X.-H.; Piao, S.; Lynch, J.P.; Uehara, T.; Sepulveda, A.R.; Davis, L.E.; et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc. Natl. Acad. Sci. USA 2012, 109, 8253–8258. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Winkler, J.D. Lys05: A new lysosomal autophagy inhibitor. Autophagy 2012, 8, 1383–1384. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Cho, H.-Y.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H.; Chen, T.C. Quinoline-based antimalarial drugs: A novel class of autophagy inhibitors. Neurosurg Focus 2015, 38, E12. [Google Scholar] [CrossRef] [PubMed]
- Guntuku, L.; Gangasani, J.K.; Thummuri, D.; Borkar, R.M.; Manavathi, B.; Ragampeta, S.; Vaidya, J.R.; Sistla, R.; Vegi, N.G.M. IITZ-01, a novel potent lysosomotropic autophagy inhibitor, has single-agent antitumor efficacy in triple-negative breast cancer in vitro and in vivo. Oncogene 2019, 38, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, S.T.; Han, Y.; Visconte, V.; Przychodzen, B.; Espitia, C.M.; Phillips, J.; Anwer, F.; Advani, A.; Carraway, H.E.; Kelly, K.R.; et al. The novel autophagy inhibitor ROC-325 augments the antileukemic activity of azacitidine. Leukemia 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Carew, J.S.; Espitia, C.M.; Zhao, W.; Han, Y.; Visconte, V.; Phillips, J.; Nawrocki, S.T. Disruption of Autophagic Degradation with ROC-325 Antagonizes Renal Cell Carcinoma Pathogenesis. Clin. Cancer Res. 2017, 23, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Carew, J.S.; Nawrocki, S.T. Drain the lysosome: Development of the novel orally available autophagy inhibitor ROC-325. Autophagy 2017, 13, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef] [PubMed]
- Paha, J.; Kanjanasirirat, P.; Munyoo, B.; Tuchinda, P.; Suvannang, N.; Nantasenamat, C.; Boonyarattanakalin, K.; Kittakoop, P.; Srikor, S.; Kongklad, G.; et al. A novel potent autophagy inhibitor ECDD-S27 targets vacuolar ATPase and inhibits cancer cell survival. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiang, P.; Yu, J.; Miao, Y.; Chen, Z.; Qu, J.; Zhao, Q.; Chen, Z.; Liu, Y.; Yao, X.; et al. Identification of compound CA-5f as a novel late-stage autophagy inhibitor with potent anti-tumor effect against non-small cell lung cancer. Autophagy 2019, 15, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Manjithaya, R. A reversible autophagy inhibitor blocks autophagosome-lysosome fusion by preventing Stx17 loading onto autophagosomes. MBoC 2019, 30, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Park, Y.J.; Park, J.Y.; Jeong, H.O.; Kim, D.H.; Ha, Y.M.; Kim, J.M.; Song, Y.M.; Heo, H.S.; Yu, B.P.; et al. Inhibitory effect of mTOR activator MHY1485 on autophagy: Suppression of lysosomal fusion. PLoS ONE 2012, 7, e43418. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Tsuchihara, K.; Fujii, S.; Sugiyama, M.; Goya, T.; Atomi, Y.; Ueno, T.; Ochiai, A.; Esumi, H. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007, 67, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.E.; Kim, Y.S.; Jung, J.W.; Kwon, S.J.; Park, D.S.; Cha, B.K.; Oh, S.H.; Yoon, K.H.; Jeong, E.T.; Kim, H.R. Inhibition of autophagy potentiates pemetrexed and simvastatin-induced apoptotic cell death in malignant mesothelioma and non-small cell lung cancer cells. Oncotarget 2015, 6, 29482–29496. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wang, K.; Li, Q.; Zou, Y.; Chen, B.; Gong, Q.; Ho, H.I.; Yin, T.; Zhang, F.; Lu, Y.; et al. The Novel Autophagy Inhibitor Alpha-Hederin Promoted Paclitaxel Cytotoxicity by Increasing Reactive Oxygen Species Accumulation in Non-Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3221. [Google Scholar] [CrossRef] [PubMed]
- Dyczynski, M.; Yu, Y.; Otrocka, M.; Parpal, S.; Braga, T.; Henley, A.B.; Zazzi, H.; Lerner, M.; Wennerberg, K.; Viklund, J.; et al. Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 (Vps34) improves the sensitivity of breast cancer cells to Sunitinib. Cancer Lett. 2018, 435, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Ronan, B.; Flamand, O.; Vescovi, L.; Dureuil, C.; Durand, L.; Fassy, F.; Bachelot, M.-F.; Lamberton, A.; Mathieu, M.; Bertrand, T.; et al. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat. Chem. Biol. 2014, 10, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Dowdle, W.E.; Nyfeler, B.; Nagel, J.; Elling, R.A.; Liu, S.; Triantafellow, E.; Menon, S.; Wang, Z.; Honda, A.; Pardee, G.; et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014, 16, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Robke, L.; Laraia, L.; Carnero Corrales, M.A.; Konstantinidis, G.; Muroi, M.; Richters, A.; Winzker, M.; Engbring, T.; Tomassi, S.; Watanabe, N.; et al. Phenotypic Identification of a Novel Autophagy Inhibitor Chemotype Targeting Lipid Kinase VPS34. Angew. Chem. Int. Ed. Engl. 2017, 56, 8153–8157. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Chun, M.G.H.; Vamos, M.; Zou, H.; Rong, J.; Miller, C.J.; Lou, H.J.; Raveendra-Panickar, D.; Yang, C.C.; Sheffler, D.J.; et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell 2015, 59, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Celano, S.L.; Solitro, A.R.; Gunaydin, H.; Scott, M.; O’Hagan, R.C.; Shumway, S.D.; Fuller, P.; MacKeigan, J.P. A Potent and Selective ULK1 Inhibitor Suppresses Autophagy and Sensitizes Cancer Cells to Nutrient Stress. iScience 2018, 8, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Blommaart, E.F.; Krause, U.; Schellens, J.P.; Meijer, A.J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997, 243, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gao, Y.; Wang, D.; Xu, Z.; Sun, W.; Ren, P. Autophagy Inhibitor (LY294002) and 5-fluorouracil (5-FU) Combination-Based Nanoliposome for Enhanced Efficacy Against Esophageal Squamous Cell Carcinoma. Nanoscale Res. Lett. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, YT.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, A.; Wymann, M.P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: The role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993, 296, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, L.; Zheng, H.; Mao, C.; Hu, W.; Xiong, K.; Wang, F.; Liu, C. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol. Sin. 2013, 34, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Li, S.; Qin, Y.; Wang, X.; Yang, Y.; Bai, H.; Zhou, L.; Zhao, C.; Wang, C. Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int. J. Oncol. 2014, 44, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, H.; Kim, M.; Xu, L.; Li, Y.; Zhang, L.; Cai, Y.; Norberg, H.V.; Zhang, T.; Furuya, T.; et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 2011, 147, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.; Wang, S.K.; Habibzadegah-Tari, P.; Law, B.; Ostrov, D.; Li, M.; Yin, X.-M.; Kim, J.-S.; Horenstein, N.; Dunn, W.A. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy 2014, 10, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.; Cleenewerck, M.; Vangestel, C.; Lyssens, S.; Declercq, W.; Timmermans, J.-P.; Stroobants, S.; Augustyns, K.; De Meyer, G.R.Y.; Van Der Veken, P.; et al. ATG4B inhibitors with a benzotropolone core structure block autophagy and augment efficiency of chemotherapy in mice. Biochem. Pharmacol. 2017, 138, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Robke, L.; Rodrigues, T.; Schröder, P.; Foley, D.J.; Bernardes, G.J.L.; Laraia, L.; Waldmann, H. Discovery of 2,4-dimethoxypyridines as novel autophagy inhibitors. Tetrahedron 2018, 74, 4531–4537. [Google Scholar] [CrossRef]
- Liu, P.F.; Tsai, K.L.; Hsu, C.J.; Tsai, W.L.; Cheng, J.S.; Chang, H.W.; Shiau, C.W.; Goan, Y.G.; Tseng, H.H.; Wu, C.H.; et al. Drug Repurposing Screening Identifies Tioconazole as an ATG4 Inhibitor that Suppresses Autophagy and Sensitizes Cancer Cells to Chemotherapy. Theranostics 2018, 8, 830–845. [Google Scholar] [CrossRef] [PubMed]
- Bosc, D.; Vezenkov, L.; Bortnik, S.; An, J.; Xu, J.; Choutka, C.; Hannigan, A.M.; Kovacic, S.; Loo, S.; Clark, P.G.K.; et al. A new quinoline-based chemical probe inhibits the autophagy-related cysteine protease ATG4B. Sci. Rep. 2018, 8, 11653. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hong, L.; Xu, J.; Zhong, G.; Gu, Q.; Gu, Q.; Guan, Y.; Zheng, X.; Dai, Q.; Luo, X.; et al. Discovery of a small molecule targeting autophagy via ATG4B inhibition and cell death of colorectal cancer cells in vitro and in vivo. Autophagy 2019, 15, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Liu, P.F.; Tsai, W.L.; Hu, W.H.; Hu, Y.C.; Yang, H.C.; Lin, W.Y.; Weng, J.R.; Shu, C.W. Xanthium strumarium Fruit Extract Inhibits ATG4B and Diminishes the Proliferation and Metastatic Characteristics of Colorectal Cancer Cells. Toxins 2019, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Donohue, E.; Tovey, A.; Vogl, A.W.; Arns, S.; Sternberg, E.; Young, R.N.; Roberge, M. Inhibition of autophagosome formation by the benzoporphyrin derivative verteporfin. J. Biol. Chem. 2011, 286, 7290–7300. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Lin, Y.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I.S. Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des. Dev. Ther. 2017, 11, 1517–1533. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Jha, V.; Singh, S.K.; Bhattacharyya, S. Autophagy inhibition suppresses the tumorigenic potential of cancer stem cell enriched side population in bladder cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 2073–2086. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yang, X.; Gu, W.; Liu, Y.; Li, X.; Huang, X.; Zhu, X.; Tao, Y.; Gou, X.; He, W. HMGB1-mediated autophagy attenuates gemcitabine-induced apoptosis in bladder cancer cells involving JNK and ERK activation. Oncotarget 2017, 8, 71642–71656. [Google Scholar] [CrossRef] [PubMed]
- Mani, J.; Vallo, S.; Rakel, S.; Antonietti, P.; Gessler, F.; Blaheta, R.; Bartsch, G.; Michaelis, M.; Cinatl, J.; Haferkamp, A.; et al. Chemoresistance is associated with increased cytoprotective autophagy and diminished apoptosis in bladder cancer cells treated with the BH3 mimetic (-)-Gossypol (AT-101). BMC Cancer 2015, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, X.; Liu, C.; Gu, P.; Li, Z.; Wu, S.; Xu, K.; Lin, T.; Huang, J. Pirarubicin induces an autophagic cytoprotective response through suppression of the mammalian target of rapamycin signaling pathway in human bladder cancer cells. Biochem. Biophys. Res. Commun. 2015, 460, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, C.; Yu, B.; Chen, B.; Liu, Z.; Hou, C.; Wang, F.; Shen, H.; Chen, Z. Autophagic degradation of FOXO3a represses the expression of PUMA to block cell apoptosis in cisplatin-resistant osteosarcoma cells. Am. J. Cancer Res. 2017, 7, 1407–1422. [Google Scholar] [PubMed]
- Zhang, W.; Li, Q.; Song, C.; Lao, L. Knockdown of autophagy-related protein 6, Beclin-1, decreases cell growth, invasion, and metastasis and has a positive effect on chemotherapy-induced cytotoxicity in osteosarcoma cells. Tumour Biol. 2015, 36, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Schott, C.R.; Ludwig, L.; Mutsaers, A.J.; Foster, R.A.; Wood, G.A. The autophagy inhibitor spautin-1, either alone or combined with doxorubicin, decreases cell survival and colony formation in canine appendicular osteosarcoma cells. PLoS ONE 2018, 13, e0206427. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, W.; Zhou, Y.; Zhang, C. Inhibition of beclin1 affects the chemotherapeutic sensitivity of osteosarcoma. Int. J. Clin. Exp. Pathol. 2014, 7, 7114–7122. [Google Scholar] [PubMed]
- Chen, R.; Li, X.; He, B.; Hu, W. MicroRNA-410 regulates autophagy-related gene ATG16L1 expression and enhances chemosensitivity via autophagy inhibition in osteosarcoma. Mol. Med. Rep. 2017, 15, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tao, L.; Shen, C.; Liu, B.; Yang, Z.; Tao, H. Silencing of Barkor/ATG14 sensitizes osteosarcoma cells to cisplatin-induced apoptosis. Int. J. Mol. Med. 2014, 33, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, W.; Tao, L.; Liu, B.; Yang, Z.; Tao, H. Chloroquine blocks the autophagic process in cisplatin-resistant osteosarcoma cells by regulating the expression of p62/SQSTM1. Int. J. Mol. Med. 2013, 32, 448–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, M.; Jung, J.Y.; Choi, S.; Lee, H.; Morales, L.D.; Koh, J.T.; Kim, S.H.; Choi, Y.D.; Choi, C.; Slaga, T.J.; et al. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy 2017, 13, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yuan, H.; Yi, F.; Meng, C.; Zhu, Q. Autophagy prevents doxorubicin-induced apoptosis in osteosarcoma. Mol. Med. Rep. 2014, 9, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.G.; Kim, Y.J.; Park, J.E.; Lee, K.Y.; Yoo, Y.H.; Kim, J.M. Cytoprotective role of autophagy during paclitaxel-induced apoptosis in Saos-2 osteosarcoma cells. Int. J. Oncol. 2013, 42, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, C.; Li, G.; Chen, T.; Li, J.; Huang, Z. Paxilitaxel induces apoptosis accompanied by protective autophagy in osteosarcoma cells through hypoxia-inducible factor-1α pathway. Mol. Med. Rep. 2015, 12, 3681–3687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chittaranjan, S.; Bortnik, S.; Dragowska, W.H.; Xu, J.; Abeysundara, N.; Leung, A.; Go, N.E.; DeVorkin, L.; Weppler, S.A.; Gelmon, K.; et al. Autophagy inhibition augments the anticancer effects of epirubicin treatment in anthracycline-sensitive and -resistant triple-negative breast cancer. Clin. Cancer Res. 2014, 20, 3159–3173. [Google Scholar] [CrossRef] [PubMed]
- Das, C.K.; Linder, B.; Bonn, F.; Rothweiler, F.; Dikic, I.; Michaelis, M.; Cinatl, J.; Mandal, M.; Kögel, D. BAG3 Overexpression and Cytoprotective Autophagy Mediate Apoptosis Resistance in Chemoresistant Breast Cancer Cells. Neoplasia 2018, 20, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Lefort, S.; Joffre, C.; Kieffer, Y.; Givel, A.-M.; Bourachot, B.; Zago, G.; Bieche, I.; Dubois, T.; Meseure, D.; Vincent-Salomon, A.; et al. Inhibition of autophagy as a new means of improving chemotherapy efficiency in high-LC3B triple-negative breast cancers. Autophagy 2014, 10, 2122–2142. [Google Scholar] [CrossRef] [PubMed]
- Aydinlik, S.; Erkisa, M.; Cevatemre, B.; Sarimahmut, M.; Dere, E.; Ari, F.; Ulukaya, E. Enhanced cytotoxic activity of doxorubicin through the inhibition of autophagy in triple negative breast cancer cell line. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yeo, S.; Wang, C.; Chen, S.; Sun, S.; Haas, M.A.; Tu, W.; Jin, F.; Guan, J.-L. Autophagy inhibition re-sensitizes pulse stimulation-selected paclitaxel-resistant triple negative breast cancer cells to chemotherapy-induced apoptosis. Breast Cancer Res. Treat. 2015, 149, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, A.; Wang, M.; Liu, W.; Wang, C.; Xie, X.; Chen, X.; Dong, J.; Li, M. Enhanced Autophagy reveals vulnerability of P-gp mediated Epirubicin Resistance in Triple Negative Breast Cancer Cells. Apoptosis 2016, 21, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, G.; El Bouchtaoui, M.; Sophie, T.; Leboeuf, C.; de Bazelaire, C.; Ratajczak, P.; Giacchetti, S.; de Roquancourt, A.; Bertheau, P.; Verneuil, L.; et al. Targeting autophagic cancer stem-cells to reverse chemoresistance in human triple negative breast cancer. Oncotarget 2017, 8, 35205–35221. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Wang, L.; Luo, J.; Zhu, H.W.; Cai, Z.W. Ambra1 modulates the sensitivity of breast cancer cells to epirubicin by regulating autophagy via ATG12. Cancer Sci. 2018, 109, 3129–3138. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, M.; Song, Y.; Chen, L.; Xiao, P.; Wan, X.; Dai, F.; Shen, P. The cytoprotective role of gemcitabine-induced autophagy associated with apoptosis inhibition in triple-negative MDA-MB-231 breast cancer cells. Int. J. Mol. Med. 2014, 34, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gong, F.; Chen, Y.; Jiang, Y.; Liu, J.; Yu, M.; Zhang, S.; Wang, M.; Xiao, G.; Liao, H. Autophagy promotes paclitaxel resistance of cervical cancer cells: Involvement of Warburg effect activated hypoxia-induced factor 1-α-mediated signaling. Cell Death Dis. 2014, 5, e1367. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, J.H.; Zhang, J.; Zhang, N.; Wang, Z.H. Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells. Cancer Res. Treat. 2014, 47, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Han, G.H.; Shin, H.-Y.; Lee, E.-J.; Cho, H.; Chay, D.B.; Kim, J.-H. Combined treatment with modulated electro-hyperthermia and an autophagy inhibitor effectively inhibit ovarian and cervical cancer growth. Int. J. Hyperthermia 2019, 36, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Leisching, G.; Loos, B.; Botha, M.; Engelbrecht, A.M. A Nontoxic Concentration of Cisplatin Induces Autophagy in Cervical Cancer: Selective Cancer Cell Death with Autophagy Inhibition as an Adjuvant Treatment. Int. J. Gynecol. Cancer 2015, 25, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.M.; Li, Z.G. Blockage of cisplatin-induced autophagy sensitizes cervical cancer cells to cisplatin. Genet. Mol. Res. 2015, 14, 16905–16912. [Google Scholar] [CrossRef] [PubMed]
- Selvakumaran, M.; Amaravadi, R.K.; Vasilevskaya, I.A.; O’Dwyer, P.J. Autophagy Inhibition Sensitizes Colon Cancer Cells to Antiangiogenic and Cytotoxic Therapy. Clin. Cancer Res. 2013, 19, 2995–3007. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tsuno, N.H.; Sunami, E.; Tsurita, G.; Kawai, K.; Okaji, Y.; Nishikawa, T.; Shuno, Y.; Hongo, K.; Hiyoshi, M.; et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer 2010, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, N.; Faried, A.; Tsutsumi, S.; Kuwano, H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur. J. Cancer 2010, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, G.; Xiao, C.; Wang, X.; Wang, Y.; Song, G.; Liu, X.; Chen, J.; Lu, H.; Guo, W.; et al. Loss of SHMT2 mediates 5-FU chemoresistance by inducing autophagy in colorectal cancer. bioRxiv 2019, 680892. [Google Scholar]
- Liu, S.; Li, X. Autophagy inhibition enhances sensitivity of endometrial carcinoma cells to paclitaxel. Int. J. Oncol. 2015, 46, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Oda, K.; Wada-Hiraike, O.; Sone, K.; Inaba, K.; Ikeda, Y.; Miyasaka, A.; Kashiyama, T.; Tanikawa, M.; Arimoto, T.; et al. The anti-malarial chloroquine suppresses proliferation and overcomes cisplatin resistance of endometrial cancer cells via autophagy inhibition. Gynecol. Oncol. 2015, 137, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Oda, K.; Wada-Hiraike, O.; Sone, K.; Inaba, K.; Ikeda, Y.; Makii, C.; Miyasaka, A.; Kashiyama, T.; Tanikawa, M.; et al. Autophagy inhibition augments resveratrol-induced apoptosis in Ishikawa endometrial cancer cells. Oncol. Lett. 2016, 12, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Hu, X.; Wu, B.; Jiang, H.; Young, C.Y.F.; Pang, Y.; Yuan, H. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011, 307, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, H.; Li, D.; Li, M. Role of Endometrial Autophagy in Physiological and Pathophysiological Processes. J. Cancer 2019, 10, 3459–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Q.; Pang, J.; Jin, H.; Li, H.; Yang, X. Blocking autophagy enhances the pro-apoptotic effect of bufalin on human gastric cancer cells through endoplasmic reticulum stress. Biol. Open 2017, 6, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, Z.; Shang, Y.; Jiang, X.; Dong, J.; Yu, P.; Nie, Y.; Zhao, Q. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015, 6, e1766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Fang, N.; Liu, X.M.; Xiong, S.P.; Liao, Y.Q.; Jin, W.J.; Song, R.F.; Wan, Y.Y. Antitumor activity of chloroquine in combination with Cisplatin in human gastric cancer xenografts. Asian Pac. J. Cancer Prev. 2015, 16, 3907–3912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.Q.; He, B.; Fang, N.; Lu, S.; Liao, Y.Q.; Wan, Y.-Y. Autophagy inhibition sensitizes cisplatin cytotoxicity in human gastric cancer cell line SGC7901. Asian Pac. J. Cancer Prev. 2013, 14, 4685–4688. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Ma, H.; Chen, X.; Liu, T.; Jiao, Z.; He, W.; Wang, F.; Liu, X.; Zeng, X. Protective role of autophagy in matrine-induced gastric cancer cell death. Int. J. Oncol. 2013, 42, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Pan, D.; Zhang, S.W.; Xie, D.Y.; Zheng, X.L.; Chen, H. Autophagy regulates chemoresistance of gastric cancer stem cells via the Notch signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3402–3407. [Google Scholar] [PubMed]
- Dong, X.; Wang, Y.; Zhou, Y.; Wen, J.; Wang, S.; Shen, L. Aquaporin 3 facilitates chemoresistance in gastric cancer cells to cisplatin via autophagy. Cell Death Discov. 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qu, X.J.; Liu, Y.P.; Xu, Y.Y.; Liu, J.; Hou, K.Z.; Zhang, Y. Protective autophagy antagonizes oxaliplatin-induced apoptosis in gastric cancer cells. Chin. J. Cancer 2011, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Xie, W.J.; Pan, D.; Chen, H.; Zhang, L. Inhibition of autophagy by bafilomycin A1 promotes chemosensitivity of gastric cancer cells. Tumor Biol. 2015, 37, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Luo, M.; Ni, X.; Wu, J.; Wang, S.; Ma, Y.; Yu, J. Autophagy Facilitates Metadherin-Induced Chemotherapy Resistance Through the AMPK/ATG5 Pathway in Gastric Cancer. Cell. Physiol. Biochem. 2018, 46, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Lu, B.; Tian, Q.; Zhang, H.; Wu, M.; Guo, H.; Zhang, Q.; Li, X.; Zhou, T.; Wang, Y. Inhibition of autophagy enhances cinobufagin-induced apoptosis in gastric cancer. Oncol. Rep. 2019, 41, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Buccarelli, M.; Marconi, M.; Pacioni, S.; Pascalis, I.D.; D’Alessandris, Q.G.; Martini, M.; Ascione, B.; Malorni, W.; Larocca, L.M.; Pallini, R.; et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lohitesh, K.; Saini, H.; Srivastava, A.; Mukherjee, S.; Roy, A.; Chowdhury, R. Autophagy inhibition potentiates SAHA-mediated apoptosis in glioblastoma cells by accumulation of damaged mitochondria. Oncol. Rep. 2018, 39, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Cho, H.-Y.; Jahanian, A.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H.; Chen, T.C. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg Focus 2014, 37, E12. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.S.; Hosoda, R.; Akiyama, Y.; Sebori, R.; Wanibuchi, M.; Mikami, T.; Sugino, T.; Suzuki, K.; Maruyama, M.; Tsukamoto, M.; et al. Chloroquine potentiates temozolomide cytotoxicity by inhibiting mitochondrial autophagy in glioma cells. J. Neurooncol. 2015, 122, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, H.K.; Lee, N.H.; Yi, H.Y.; Kim, H.S.; Hong, S.H.; Hong, Y.K.; Joe, Y.A. The synergistic effect of combination temozolomide and chloroquine treatment is dependent on autophagy formation and p53 status in glioma cells. Cancer Lett. 2015, 360, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T.; Germano, I.M.; Komata, T.; Ito, H.; Kondo, Y.; Kondo, S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004, 11, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Kim, C.K.; Alvarez, A.A.; Pangeni, R.P.; Wan, X.; Song, X.; Shi, T.; Yang, Y.; Sastry, N.; Horbinski, C.M.; et al. MST4 Phosphorylation of ATG4B Regulates Autophagic Activity, Tumorigenicity, and Radioresistance in Glioblastoma. Cancer Cell 2017, 32, 840–855.e8. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zeng, W.; Chen, Y.; Li, H.; Wang, J.; Cheng, Q.; Yu, J.; Zhou, H.; Liu, Z.; Xiao, J.; et al. Knockdown ATG4C inhibits gliomas progression and promotes temozolomide chemosensitivity by suppressing autophagic flux. J. Exp. Clin. Cancer Res. 2019, 38, 298. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lin, C.W.; Yang, S.F.; Sheu, G.T.; Yu, Y.Y.; Chen, M.K.; Chiou, H.L. A combination of pterostilbene with autophagy inhibitors exerts efficient apoptotic characteristics in both chemosensitive and chemoresistant lung cancer cells. Toxicol. Sci. 2014, 137, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, W.; Peng, X.; Xie, J.; Li, H.; Tan, G. Inhibition of autophagy results in a reversal of taxol resistance in nasopharyngeal carcinoma by enhancing taxol-induced caspase-dependent apoptosis. Am. J. Transl. Res. 2017, 9, 1934–1942. [Google Scholar] [PubMed]
- Aga, T.; Endo, K.; Tsuji, A.; Aga, M.; Moriyama-Kita, M.; Ueno, T.; Nakanishi, Y.; Hatano, M.; Kondo, S.; Sugimoto, H.; et al. Inhibition of autophagy by chloroquine makes chemotherapy in nasopharyngeal carcinoma more efficient. Auris Nasus Larynx 2019, 46, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Li, G.; Liu, C.; Ren, S.; Deng, T.; Zhang, S.; Tian, Y.; Liu, Y.; Qiu, Y. Autophagy inhibition impairs the epithelial-mesenchymal transition and enhances cisplatin sensitivity in nasopharyngeal carcinoma. Oncol. Lett. 2017, 13, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Li, L.; Nie, D.; Tao, Q.; Wu, J.; Fan, J.; Lin, C.; Zhao, S.; Ju, D. Inhibition of Autophagy Potentiated the Antitumor Effect of Nedaplatin in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. PLoS ONE 2015, 10, e0135236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W. Effect of autophagy inhibitor 3-methyladenine combined with TP chemotherapy on nasopharyngeal carcinoma and EGFR and VEGF levels in tissues. Int. J. Clin. Exp. Med. 2019, 12, 6403–6410. [Google Scholar]
- Zhang, N.; Wu, Y.; Lyu, X.; Li, B.; Yan, X.; Xiong, H.; Li, X.; Huang, G.; Zeng, Y.; Zhang, Y.; et al. HSF1 upregulates ATG4B expression and enhances epirubicin-induced protective autophagy in hepatocellular carcinoma cells. Cancer Lett. 2017, 409, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.B.; Hui, B.; Shi, Y.H.; Zhou, J.; Peng, Y.F.; Gu, C.-Y.; Yang, H.; Shi, G.-M.; Ke, A.-W.; Wang, X.-Y.; et al. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin. Cancer Res. 2011, 17, 6229–6238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, K.; Ma, Y.; Yang, H.; Zhang, Y.; Kong, X.; Wei, L. Autophagy inhibits chemotherapy-induced apoptosis through downregulating Bad and Bim in hepatocellular carcinoma cells. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Zhou, L.; Wang, W.; Liu, Z.; Cheng, Y.; Li, J.; Wei, H. Autophagy Plays a Critical Role in Insulin Resistance- Mediated Chemoresistance in Hepatocellular Carcinoma Cells by Regulating the ER Stress. J. Cancer 2018, 9, 4314–4324. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Xiong, E.M.; Ge, L.; Wan, Y.Y.; Zhang, C.L.; Du, F.Y.; Xu, M.; Bhat, R.A.; Jin, J.; Gong, A.H. Egr-1 promotes hypoxia-induced autophagy to enhance chemo-resistance of hepatocellular carcinoma cells. Exp. Cell Res. 2016, 340, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fan, X.; Tang, M.; Chen, R.; Wang, H.; Jia, R.; Zhou, X.; Jing, W.; Wang, H.; Yang, Y.; et al. Osteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells. Cancer Lett. 2016, 383, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.T.; Song, K.; Zhou, J.; Shi, Y.H.; Liu, W.R.; Shi, G.M.; Gao, Q.; Wang, X.Y.; Ding, Z.B.; Fan, J. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, Y.; Wang, Y.; Zhang, C.; Zhang, H.; Huang, C.; Jiang, H.; Wang, X.; Li, X. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2013, 29, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Du, T.; Zhang, Z.; Du, F.; Jin, J.; Gong, A. Knockdown of autophagy-related gene LC3 enhances the sensitivity of HepG2 cells to epirubicin. Exp. Ther. Med. 2015, 9, 1271–1276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Datta, S.; Choudhury, D.; Das, A.; Mukherjee, D.D.; Dasgupta, M.; Bandopadhyay, S.; Chakrabarti, G. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of β-catenin pathway. Apoptosis 2019, 24, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Uchibori, M.; Suyama, M.; Fujita, M.; Arai, Y.; Hu, D.; Xia, S.; Ma, B.; Kabir, A.; Kamatari, Y.O.; et al. Novel Atg4B inhibitors potentiate cisplatin therapy in lung cancer cells through blockade of autophagy. Computational Toxicology 2019, 12, 100095. [Google Scholar] [CrossRef]
- Chen, K.; Shi, W. Autophagy regulates resistance of non-small cell lung cancer cells to paclitaxel. Tumour Biol. 2016, 37, 10539–10544. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Hsu, S.H.; Hsu, H.W.; Huang, K.C.; Liu, W.; Wu, C.Y.; Huang, W.P.; Chen, J.Y.F.; Chen, B.H.; Chiu, C.C. Human non-small cell lung cancer cells can be sensitized to camptothecin by modulating autophagy. Int. J. Oncol. 2018, 53, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Liu, G.; Tian, G. Autophagy inhibition of cancer stem cells promotes the efficacy of cisplatin against non-small cell lung carcinoma. Ther. Adv. Respir. Dis. 2019, 13, 1753466619866097. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, X.; Sun, H.; Zhang, J.; Yan, M.; Zhang, H. Autophagy inhibition promotes 5-fluorouraci-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A549 cells. PLoS ONE 2013, 8, e56679. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lo, Y.L.; Hsu, C.; Wu, Y.T.; Liao, Z.X.; Wu, W.J.; Chen, Y.J.; Kao, C.; Chiu, C.C.; Wang, L.F. CS-PEI/Beclin-siRNA Downregulate Multidrug Resistance Proteins and Increase Paclitaxel Therapeutic Efficacy against NSCLC. Mol. Ther. Nucleic Acids 2019, 17, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Zhou, H.; Wang, W.; Luo, Y.; Yang, H.; Yi, H. Inhibition of autophagy promotes cisplatin-induced apoptotic cell death through Atg5 and Beclin 1 in A549 human lung cancer cells. Mol. Med. Rep. 2018, 17, 6859–6865. [Google Scholar] [CrossRef] [PubMed]
- Belounis, A.; Nyalendo, C.; Le Gall, R.; Imbriglio, T.V.; Mahma, M.; Teira, P.; Beaunoyer, M.; Cournoyer, S.; Haddad, E.; Vassal, G.; et al. Autophagy is associated with chemoresistance in neuroblastoma. BMC Cancer 2016, 16, 891. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Sun, M.; Yin, Z.; Qian, J. High mobility group box 1-mediated autophagy promotes neuroblastoma cell chemoresistance. Oncol. Rep. 2015, 34, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Yang, K.; Ye, L.; You, Z.; Chen, R.; Liu, Y.; He, Y. Suberoylanilide hydroxamic acid sensitizes neuroblastoma to paclitaxel by inhibiting thioredoxin-related protein 14-mediated autophagy. Cancer Sci. 2017, 108, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, G.S. Role of autophagy in cisplatin resistance in ovarian cancer cells. J. Biol. Chem. 2014, 289, 17163–17173. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Jaramillo, M.C.; Zhang, Z.; Zheng, Y.; Yao, M.; Zhang, D.D.; Yi, X. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Mol. Med. Rep. 2015, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yu, L.; Wei, W.; Lin, X.; Hou, X.; Tian, Y. shRNA-mediated AMBRA1 knockdown reduces the cisplatin-induced autophagy and sensitizes ovarian cancer cells to cisplatin. J. Toxicol. Sci. 2016, 41, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, A.; Pilotto, G.; Mazzoldi, E.L.; Nicoletto, M.O.; Frezzini, S.; Pastò, A.; Amadori, A. Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis. 2017, 8, e2943. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Liu, X.; Liu, Y.; Kong, D.; Liu, X.; Zhong, R.; Ma, S. Inhibition of autophagy sensitizes MDR-phenotype ovarian cancer SKVCR cells to chemotherapy. Biomed. Pharmacother. 2016, 82, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Roy, D.; Kalogera, E.; Mondal, S.; Wen, X.; He, X.; Dowdy, S.; Shridhar, V. Quinacrine promotes autophagic cell death and chemosensitivity in ovarian cancer and attenuates tumor growth. Oncotarget 2015, 6, 36354–36369. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.J.; Jaramillo, M.C.; Zhang, Z.B.; Zheng, Y.X.; Yao, M.; Zhang, D.D.; Yi, X.F. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 1502–1513. [Google Scholar] [PubMed]
- Tang, J.; Zhu, J.; Ye, Y.; Liu, Y.; He, Y.; Zhang, L.; Tang, D.; Qiao, C.; Feng, X.; Li, J.; et al. Inhibition LC3B can increase chemosensitivity of ovarian cancer cells. Cancer Cell Int. 2019, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J.H.; Jin, L.; Sui, Y.X.; Han, L.L.; Huang, Y. Effect of autophagy-related beclin1 on sensitivity of cisplatin-resistant ovarian cancer cells to chemotherapeutic agents. Asian Pac. J. Cancer Prev. 2015, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-F.; Wang, X.-Y.; Fu, Z.-Q.; Peng, Q.-H.; Zhang, J.-Y.; Ye, F.; Fu, Y.-F.; Zhou, C.-Y.; Lu, W.-G.; Cheng, X.-D.; et al. TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy 2015, 11, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, C.; He, R.; Zhou, M.; Liu, Y.; Guo, X.; Wang, M.; Zhu, F.; Qin, R.; Li, X. Danthron suppresses autophagy and sensitizes pancreatic cancer cells to doxorubicin. Toxicol In Vitro 2019, 54, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Zhao, Y.; Zhang, M.; He, R.Z.; Shi, X.H.; Guo, X.J.; Shi, C.J.; Peng, F.; Wang, M.; Shen, M.; et al. Inhibition of Autophagy by Deguelin Sensitizes Pancreatic Cancer Cells to Doxorubicin. Int. J. Mol. Sci. 2017, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Cheng, X.; Kuang, J.; Feng, H.; Chen, L.; Liang, J.; Shen, X.; Yuen, S.; Peng, C.; Shen, B.; et al. CQ sensitizes human pancreatic cancer cells to gemcitabine through the lysosomal apoptotic pathway via reactive oxygen species. Mol. Oncol. 2018, 12, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hu, C.; Cao, H.; Wu, X.; Wang, R.; Lu, H.; Li, H.; Chen, H. MicroRNA-29c Increases the Chemosensitivity of Pancreatic Cancer Cells by Inhibiting USP22 Mediated Autophagy. Cell. Physiol. Biochem. 2018, 47, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Huang, F.Z.; Xu, H.B.; Sun, J.C.; Wang, C.F. MicroRNA-137 inhibits autophagy and chemosensitizes pancreatic cancer cells by targeting ATG5. Int. J. Biochem. Cell Biol. 2019, 111, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, W.; Zhi, X.; Liu, H.; Zhou, Y.; Chen, B.W.; Hu, L.; Shen, J.; Zheng, X.; Zhang, S.; et al. USP9X inhibition improves gemcitabine sensitivity in pancreatic cancer by inhibiting autophagy. Cancer Lett. 2018, 436, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, W.; Tang, G.; Wang, H.; Wu, M.; Yu, W.; Zhou, Z.; Mou, Y.; Liu, X. Targeted Codelivery of Docetaxel and Atg7 siRNA for Autophagy Inhibition and Pancreatic Cancer Treatment. ACS Appl. Bio Mater. 2019, 2, 1168–1176. [Google Scholar] [CrossRef]
- Donohue, E.; Thomas, A.; Maurer, N.; Manisali, I.; Zeisser-Labouebe, M.; Zisman, N.; Anderson, H.J.; Ng, S.S.W.; Webb, M.; Bally, M.; et al. The autophagy inhibitor verteporfin moderately enhances the antitumor activity of gemcitabine in a pancreatic ductal adenocarcinoma model. J. Cancer 2013, 4, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.J.; Willy, J.A.; Quirin, K.A.; Wek, R.C.; Korc, M.; Yin, X.-M.; Kota, J. Novel role of miR-29a in pancreatic cancer autophagy and its therapeutic potential. Oncotarget 2016, 7, 71635–71650. [Google Scholar] [CrossRef] [PubMed]
- Ryabaya, O.O.; Inshakov, A.N.; Egorova, A.V.; Emelyanova, M.A.; Nasedkina, T.V.; Zasedatelev, A.S.; Khochenkov, D.A.; Stepanova, E.V. Autophagy inhibitors chloroquine and LY294002 enhance temozolomide cytotoxicity on cutaneous melanoma cell lines in vitro. Anticancer Drugs 2017, 28, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-H.; Piao, S.; Wang, D.; McAfee, Q.W.; Nathanson, K.L.; Lum, J.J.; Li, L.Z.; Amaravadi, R.K. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin. Cancer Res. 2011, 17, 3478–3489. [Google Scholar] [CrossRef] [PubMed]
- Cervia, D.; Assi, E.; De Palma, C.; Giovarelli, M.; Bizzozero, L.; Pambianco, S.; Di Renzo, I.; Zecchini, S.; Moscheni, C.; Vantaggiato, C.; et al. Essential role for acid sphingomyelinase-inhibited autophagy in melanoma response to cisplatin. Oncotarget 2016, 7, 24995–25009. [Google Scholar] [CrossRef] [PubMed]
- Fitzwalter, B.E.; Towers, C.G.; Sullivan, K.D.; Andrysik, Z.; Hoh, M.; Ludwig, M.; O’Prey, J.; Ryan, K.M.; Espinosa, J.M.; Morgan, M.J.; et al. Autophagy Inhibition Mediates Apoptosis Sensitization in Cancer Therapy by Relieving FOXO3a Turnover. Dev. Cell 2018, 44, 555–565.e3. [Google Scholar] [CrossRef] [PubMed]
- Joo, W.D.; Visintin, I.; Mor, G. Targeted cancer therapy - Are the days of systemic chemotherapy numbered? Maturitas 2013, 76, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Shen, L. Molecular targeted therapy of cancer: The progress and future prospect. Front. Lab. Med. 2017, 1, 69–75. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, G.; Li, N.; Su, R.; Chen, X.; Zhong, L. Autophagy Inhibition Promotes Bevacizumab-induced Apoptosis and Proliferation Inhibition in Colorectal Cancer Cells. J. Cancer 2018, 9, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, J.; Liu, Z.; Pan, L.; Xu, G. Autophagy activation promotes bevacizumab resistance in glioblastoma by suppressing AKT/mTOR signaling pathway. Oncol. Lett. 2018, 15, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.; Shao, Y.; Shi, J.; Wang, W.; Chen, W.; Ye, Z.; Jiang, J.; Fang, Q.; Zhang, G.; et al. Hydroxychloroquine potentiates the anti-cancer effect of bevacizumab on glioblastoma via the inhibition of autophagy. Biomed. Pharmacother 2019, 118, 109339. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, D.; Sun, K.; Wang, J.; Liu, Y.; Song, J.; Zhao, Q.; Zhang, S.; Deng, W.; Zhao, X.; et al. Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J. Mol. Med. (Berl) 2013, 91, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; DeLay, M.; Jahangiri, A.; Molinaro, A.M.; Rose, S.D.; Carbonell, W.S.; Aghi, M.K. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012, 72, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martin, A.; Oliveras-Ferraros, C.; Menendez, J.A. Autophagy Facilitates the Development of Breast Cancer Resistance to the Anti-HER2 Monoclonal Antibody Trastuzumab. PLoS ONE 2009, 4, e6251. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.E.; Reidel, S.I.; Bal de Kier, J.E.D.; Jasnis, M.A.; Fiszman, G.L. Autophagy Protects from Trastuzumab-Induced Cytotoxicity in HER2 Overexpressing Breast Tumor Spheroids. PLoS ONE 2015, 10, e0137920. [Google Scholar] [CrossRef] [PubMed]
- Bortnik, S.; Choutka, C.; Horlings, H.M.; Leung, S.; Baker, J.H.; Lebovitz, C.; Dragowska, W.H.; Go, N.E.; Bally, M.B.; Minchinton, A.I.; et al. Identification of breast cancer cell subtypes sensitive to ATG4B inhibition. Oncotarget 2016, 7, 66970–66988. [Google Scholar] [CrossRef] [PubMed]
- Cufí, S.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Corominas-Faja, B.; Urruticoechea, A.; Martin-Castillo, B.; Menendez, J.A. Autophagy-related gene 12 (ATG12) is a novel determinant of primary resistance to HER2-targeted therapies: Utility of transcriptome analysis of the autophagy interactome to guide breast cancer treatment. Oncotarget 2012, 3, 1600–1614. [Google Scholar] [CrossRef] [PubMed]
- Eckers, J.; Skiba, J.; Senthilkumar, G.; Nickel, K.P.; Swick, A.D.; Kimple, R.J. Abstract 1344: Autophagy contributes to therapeutic resistance in head and neck cancer. Cancer Res. 2018, 78, 1344. [Google Scholar]
- Li, X.; Fan, Z. The EGFR antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1α and Bcl-2 and activating the beclin-1/hVps34 complex. Cancer Res. 2010, 70, 5942–5952. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Pan, T.; Fan, Z. Roles of autophagy in cetuximab-mediated cancer therapy against EGFR. Autophagy 2010, 6, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yao, Y.; Xu, G.; Zhou, C.; Zhang, Y.; Sun, J.; Jiang, R.; Shao, Q.; Chen, Y. CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Braggio, D.; Koller, D.; Jin, F.; Siva, N.; Zewdu, A.; Lopez, G.; Batte, K.; Casadei, L.; Welliver, M.; Strohecker, A.; et al. Autophagy inhibition overcomes sorafenib resistance in S45F-mutated desmoid tumors. Cancer 2019, 125. [Google Scholar] [CrossRef] [PubMed]
- Eritja, N.; Chen, B.-J.; Rodríguez-Barrueco, R.; Santacana, M.; Gatius, S.; Vidal, A.; Martí, M.D.; Ponce, J.; Bergadà, L.; Yeramian, A.; et al. Autophagy orchestrates adaptive responses to targeted therapy in endometrial cancer. Autophagy 2017, 13, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Ding, Z.-B.; Zhou, J.; Hui, B.; Shi, G.-M.; Ke, A.-W.; Wang, X.-Y.; Dai, Z.; Peng, Y.-F.; Gu, C.-Y.; et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 2011, 7, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, M.; Wang, Y.; Chen, Y.; He, C.; Zhang, X.; Yang, T.; Lu, Y.; You, J.; Lee, R.J.; et al. Enhancing anti-tumor efficiency in hepatocellular carcinoma through the autophagy inhibition by miR-375/sorafenib in lipid-coated calcium carbonate nanoparticles. Acta Biomater 2018, 72, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhu, H.; Gu, D.; Pan, X.; Qian, L.; Xue, B.; Yang, D.; Zhou, J.; Shan, Y. MiRNA-30a-mediated autophagy inhibition sensitizes renal cell carcinoma cells to sorafenib. Biochem. Biophys. Res. Commun. 2015, 459, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, K.; Wang, H.; Dai, Y. Inhibition of Autophagy by Chloroquine Enhances the Antitumor Efficacy of Sorafenib in Glioblastoma. Cell Mol. Neurobiol. 2016, 36, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, Z.; Jiang, L.; Sui, X.; You, L.; Shou, J.; Jing, Z.; Xie, J.; Ge, W.; Cai, X.; et al. Autophagy inhibition sensitizes hepatocellular carcinoma to the multikinase inhibitor linifanib. Sci. Rep. 2014, 4, 6683. [Google Scholar] [CrossRef] [PubMed]
- DeVorkin, L.; Hattersley, M.; Kim, P.; Ries, J.; Spowart, J.; Anglesio, M.S.; Levi, S.M.; Huntsman, D.G.; Amaravadi, R.K.; Winkler, J.D.; et al. Autophagy Inhibition Enhances Sunitinib Efficacy in Clear Cell Ovarian Carcinoma. Mol. Cancer Res. 2017, 15, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Xu, Y.-Z.; Lu, W.-J.; Li, Y.-H.; Tan, S.-S.; Lin, H.-J.; Wu, T.-M.; Li, Y.; Wang, S.-Y.; Zhao, Y.-L. Chloroquine potentiates the anticancer effect of sunitinib on renal cell carcinoma by inhibiting autophagy and inducing apoptosis. Oncol. Lett. 2018, 15, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lu, D.; Xuan, M.J.; Hu, W. Antitumor effect of sunitinib in human prostate cancer cells functions via autophagy. Exp. Ther. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, T.; Blank, A.; Pantasis, S.; Normand, L.; Bill, R.; Krebs, P.; Tschan, M.P.; Marinoni, I.; Perren, A. Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-dependent Mechanism. Mol. Cancer Ther. 2017, 16, 2502–2515. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.K.; Shouman, S.; El-Demerdash, E.; Elgendy, M.; Abdel-Naim, A.B. Chloroquine synergizes sunitinib cytotoxicity via modulating autophagic, apoptotic and angiogenic machineries. Chem. Biol. Interact. 2014, 217, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, K.; Ma, Q.; Yu, Q.; Liu, C.; Ndege, I.; Wang, X.; Yu, Z. Autophagy inhibitor facilitates gefitinib sensitivity in vitro and in vivo by activating mitochondrial apoptosis in triple negative breast cancer. PLoS ONE 2017, 12, e0177694. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Lee, K.-H.; Lee, H.S.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Concurrent Autophagy Inhibition Overcomes the Resistance of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Human Bladder Cancer Cells. Int. J. Mol. Sci. 2017, 18, 321. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Hu, X.; Yu, C.; Zhou, L.; Ding, Z.; Zhou, M. Gefitinib-mediated apoptosis is enhanced via inhibition of autophagy by chloroquine diphosphate in cutaneous squamous cell carcinoma cells. Oncol. Lett. 2019, 18, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.C.; Wu, M.-Y.; Hwang, M.-H.; Chang, Y.-T.; Huang, H.-J.; Lin, A.M.-Y.; Yang, J.C.-H. Chloroquine Enhances Gefitinib Cytotoxicity in Gefitinib-Resistant Nonsmall Cell Lung Cancer Cells. PLoS ONE 2015, 10, e0119135. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-T.; Li, W.-C.; Gao, S.; Wang, F.; Li, X.-Q.; Yu, H.-Q.; Fan, L.-L.; Wei, W.; Wang, H.; Sun, G.-P. Autophagy Inhibition Overcomes the Antagonistic Effect Between Gefitinib and Cisplatin in Epidermal Growth Factor Receptor Mutant Non--Small-Cell Lung Cancer Cells. Clin. Lung Cancer 2015, 16, e55–e66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, Y.Z.; Du, X.; Peng, X.N.; Shen, Q.M. MiRNA-153-3p promotes gefitinib-sensitivity in non-small cell lung cancer by inhibiting ATG5 expression and autophagy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2444–2452. [Google Scholar] [PubMed]
- Meng, J.; Chang, C.; Chen, Y.; Bi, F.; Ji, C.; Liu, W. EGCG overcomes gefitinib resistance by inhibiting autophagy and augmenting cell death through targeting ERK phosphorylation in NSCLC. Onco Targets Ther. 2019, 12, 6033–6043. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Tian, Y.; Li, W.; Li, D.; Zhao, T.; Yang, Y.; Li, Q.; Chen, S.; Yang, Y.; Zhang, Z.; et al. ERK inhibition represses gefitinib resistance in non-small cell lung cancer cells. Oncotarget 2018, 9, 12020–12034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, G.; Shi, Y.; Su, C.; Chang, J. Co-delivery of Gefitinib and chloroquine by chitosan nanoparticles for overcoming the drug acquired resistance. J. Nanobiotechnol. 2015, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.C.; Yen, Y. Inhibition of autophagy by MPT0L145, a novel PIK3C3 inhibitor, to overcome drug resistance in cancer cells. JCO 2018, 36, e14583. [Google Scholar] [CrossRef]
- Dragowska, W.H.; Weppler, S.A.; Wang, J.C.; Wong, L.Y.; Kapanen, A.I.; Rawji, J.S.; Warburton, C.; Qadir, M.A.; Donohue, E.; Roberge, M.; et al. Induction of autophagy is an early response to gefitinib and a potential therapeutic target in breast cancer. PLoS ONE 2013, 8, e76503. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Pan, H.; Chen, Y.; Sun, J.; Wang, Y.; Li, J.; Ge, W.; Feng, L.; Lin, X.; Wang, X.; et al. EGFR Tyrosine Kinase Inhibitors Activate Autophagy as a Cytoprotective Response in Human Lung Cancer Cells. PLoS ONE 2011, 6, e18691. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Jiao, L.; Lin, C.; Lu, C.; Zhang, K.; Hu, C.; Ye, J.; Zhang, D.; Wu, H.; et al. Protective autophagy decreases osimertinib cytotoxicity through regulation of stem cell-like properties in lung cancer. Cancer Lett. 2019, 452, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Jiao, L.; Lin, C.; Lu, C.; Zhang, K.; Hu, C.; Ye, J.; Zhang, D.; Feng, M.; et al. Autophagy determines osimertinib resistance through regulation of stem cell-like properties in EGFR-mutant lung cancer. bioRxiv 2018, 330092. [Google Scholar]
- Fleisher, B.; Mody, H.; Werkman, C.; Bihorel, S. Chloroquine and Osimertinib Induce Synergic Reduction in Cell Viability through Autophagy/Apoptosis Crosstalk. FASEB J. 2019, 33, 675. [Google Scholar]
- Zhang, Z.; Zhang, M.; Liu, H.; Yin, W. AZD9291 promotes autophagy and inhibits PI3K/AKT pathway in NSCLC cancer cells. J. Cell. Biochem. 2019, 120, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Jiang, J.; Xie, N.; Zhou, L.; Huang, Z.; Zhang, L.; Qin, S.; Fu, S.; Peng, L.; Gao, W.; et al. MCT1 relieves osimertinib-induced CRC suppression by promoting autophagy through the LKB1/AMPK signaling. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Lam, S.K.; Mak, J.C.W.; Zheng, C.Y.; Ho, J.C.-M. Erlotinib-induced autophagy in epidermal growth factor receptor mutated non-small cell lung cancer. Lung Cancer 2013, 81, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, T.; Dong, X.; Li, Z.; Wu, G.; Zhang, R. Autophagy inhibition facilitates erlotinib cytotoxicity in lung cancer cells through modulation of endoplasmic reticulum stress. Int. J. Oncol. 2016, 48, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ling, Y.-H.; Sironi, J.; Schwartz, E.L.; Perez-Soler, R.; Piperdi, B. The autophagy inhibitor chloroquine overcomes the innate resistance of wild-type EGFR non-small-cell lung cancer cells to erlotinib. J. Thorac. Oncol. 2013, 8, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Eimer, S.; Belaud-Rotureau, M.-A.; Airiau, K.; Jeanneteau, M.; Laharanne, E.; Véron, N.; Vital, A.; Loiseau, H.; Merlio, J.-P.; Belloc, F. Autophagy inhibition cooperates with erlotinib to induce glioblastoma cell death. Cancer Biol. Ther. 2011, 11, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Sobhakumari, A.; Schickling, B.M.; Love-Homan, L.; Raeburn, A.; Fletcher, E.V.M.; Case, A.J.; Domann, F.E.; Miller, F.J.; Simons, A.L. NOX4 mediates cytoprotective autophagy induced by the EGFR inhibitor erlotinib in head and neck cancer cells. Toxicol. Appl. Pharmacol. 2013, 272, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Dermawan, J.K.T.; Gurova, K.; Pink, J.; Dowlati, A.; De, S.; Narla, G.; Sharma, N.; Stark, G.R. Quinacrine overcomes resistance to erlotinib by inhibiting FACT, NF-κB, and cell-cycle progression in non-small cell lung cancer. Mol. Cancer Ther. 2014, 13, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Haas, N.B.; Appleman, L.J.; Stein, M.; Redlinger, M.; Wilks, M.; Xu, X.; Onorati, A.; Kalavacharla, A.; Kim, T.; Zhen, C.J.; et al. Autophagy Inhibition to Augment mTOR Inhibition: A Phase I/II Trial of Everolimus and Hydroxychloroquine in Patients with Previously Treated Renal Cell Carcinoma. Clin. Cancer Res. 2019, 25, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Lui, A.; New, J.; Ogony, J.; Thomas, S.; Lewis-Wambi, J. Everolimus downregulates estrogen receptor and induces autophagy in aromatase inhibitor-resistant breast cancer cells. BMC Cancer 2016, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Rosich, L.; Xargay-Torrent, S.; López-Guerra, M.; Campo, E.; Colomer, D.; Roué, G. Counteracting autophagy overcomes resistance to everolimus in mantle cell lymphoma. Clin. Cancer Res. 2012, 18, 5278–5289. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Tian, X.; Wang, Q.; He, W.; Fan, J.; Gou, X. Attenuation of everolimus-induced cytotoxicity by a protective autophagic pathway involving ERK activation in renal cell carcinoma cells. Drug Des. Dev. Ther. 2018, 12, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-F.; Lin, Y.-C.; Yang, S.-C.; Tsai, T.-F.; Chen, H.-E.; Chou, K.-Y.; Hwang, T.I.-S. Autophagy inhibition enhances RAD001-induced cytotoxicity in human bladder cancer cells. Drug Des. Dev. Ther. 2016, 10, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Santini, D.; Zappavigna, S.; Lombardi, A.; Misso, G.; Boccellino, M.; Desiderio, V.; Vitiello, P.P.; Di Lorenzo, G.; Zoccoli, A.; et al. Antagonistic effects of chloroquine on autophagy occurrence potentiate the anticancer effects of everolimus on renal cancer cells. Cancer Biol. Ther. 2015, 16, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Rosich, L.; Colomer, D.; Roué, G. Autophagy controls everolimus (RAD001) activity in mantle cell lymphoma. Autophagy 2013, 9, 115–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singla, M.; Bhattacharyya, S. Autophagy as a potential therapeutic target during epithelial to mesenchymal transition in renal cell carcinoma: An in vitro study. Biomed. Pharmacother. 2017, 94, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Nozawa, H.; Hiyoshi, M.; Tada, N.; Murono, K.; Nirei, T.; Emoto, S.; Kishikawa, J.; Iida, Y.; Sunami, E.; et al. Temsirolimus and chloroquine cooperatively exhibit a potent antitumor effect against colorectal cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; White, E.P.; Mehnert, J.M. Coordinate Autophagy and mTOR Pathway Inhibition Enhances Cell Death in Melanoma. PLoS ONE 2013, 8, e55096. [Google Scholar] [CrossRef] [PubMed]
- Bray, K.; Mathew, R.; Lau, A.; Kamphorst, J.J.; Fan, J.; Chen, J.; Chen, H.-Y.; Ghavami, A.; Stein, M.; DiPaola, R.S.; et al. Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PLoS ONE 2012, 7, e41831. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, X.; Zhang, Z.; Xing, Y.; Kong, X. Inhibition of autophagy enhances apoptosis induced by the PI3K/AKT/mTor inhibitor NVP-BEZ235 in renal cell carcinoma cells. Cell Biochem. Funct. 2013, 31, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Shi, G.; Jin, J.; Guo, H.; Guo, X.; Luo, F.; Song, Y.; Jia, X. Dual PI3K/mTOR inhibitor NVP-BEZ235-induced apoptosis of hepatocellular carcinoma cell lines is enhanced by inhibitors of autophagy. Int. J. Mol. Med. 2013, 31, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jin, Z.; Yu, K.; Liu, Q. NVP-BEZ235-induced autophagy as a potential therapeutic approach for multiple myeloma. Am. J. Transl. Res. 2019, 11, 87–105. [Google Scholar] [PubMed]
- Yang, X.; Niu, B.; Wang, L.; Chen, M.; Kang, X.; Wang, L.; Ji, Y.; Zhong, J. Autophagy inhibition enhances colorectal cancer apoptosis induced by dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Oncol. Lett. 2016, 12, 102–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Echeverry, N.; Ziltener, G.; Barbone, D.; Weder, W.; Stahel, R.A.; Broaddus, V.C.; Felley-Bosco, E. Inhibition of autophagy sensitizes malignant pleural mesothelioma cells to dual PI3K/mTOR inhibitors. Cell Death Dis. 2015, 6, e1757. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Kruger, A.J.; Jeong, J.-Y.; Kim, J.; Shin, P.K.; Kim, S.Y.; Cho, J.Y.; Hahm, K.B.; Hong, S.P. Combination Therapy with a PI3K/mTOR Dual Inhibitor and Chloroquine Enhances Synergistic Apoptotic Cell Death in Epstein-Barr Virus-Infected Gastric Cancer Cells. Mol. Cells 2019, 42, 448–459. [Google Scholar] [PubMed]
- Xu, C.-X.; Zhao, L.; Yue, P.; Fang, G.; Tao, H.; Owonikoko, T.K.; Ramalingam, S.S.; Khuri, F.R.; Sun, S.-Y. Augmentation of NVP-BEZ235’s anticancer activity against human lung cancer cells by blockage of autophagy. Cancer Biol. Ther. 2011, 12, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Hugle, M.; Fulda, S. Dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 synergizes with chloroquine to induce apoptosis in embryonal rhabdomyosarcoma. Cancer Lett. 2015, 360, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.; Hugle, M.; Cristofanon, S.; Tchoghandjian, A.; Fulda, S. The dual PI3K/mTOR inhibitor NVP-BEZ235 and chloroquine synergize to trigger apoptosis via mitochondrial-lysosomal cross-talk. Int. J. Cancer 2013, 132, 2682–2693. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Wu, H.-C.; Wu, J.-E.; Huang, K.-Y.; Yang, S.-C.; Chen, S.-X.; Tsao, C.-J.; Hsu, K.-F.; Chen, Y.-L.; Hong, T.-M. The dual PI3K/mTOR inhibitor BEZ235 restricts the growth of lung cancer tumors regardless of EGFR status, as a potent accompanist in combined therapeutic regimens. J. Exp. Clin. Cancer Res. 2019, 38, 282. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Guo, H.; Thakur, A.; Zhang, S.; Wang, T.; Liang, Y.; Shi, P.; Gao, L.; Liu, F.; Feng, J.; et al. Blockade efficacy of MEK/ERK-dependent autophagy enhances PI3K/AKT inhibitor NVP-BKM120’s therapeutic effectiveness in lung cancer cells. Oncotarget 2016, 7, 67277–67287. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hosford, S.R.; Traphagen, N.A.; Shee, K.; Demidenko, E.; Liu, S.; Miller, T.W. Autophagy promotes escape from phosphatidylinositol 3-kinase inhibition in estrogen receptor-positive breast cancer. FASEB J. 2018, 32, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, X.; Qiao, H.; Ye, M.; Lai, X.; Yu, S.; Ding, L.; Wen, A.; Zhang, J. Protective autophagy promotes the resistance of HER2-positive breast cancer cells to lapatinib. Tumour Biol. 2016, 37, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Janser, F.A.; Adams, O.; Bütler, V.; Schläfli, A.M.; Dislich, B.; Seiler, C.A.; Kröll, D.; Langer, R.; Tschan, M.P. Her2-Targeted Therapy Induces Autophagy in Esophageal Adenocarcinoma Cells. Int. J. Mol. Sci. 2018, 19, 3069. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.P.; Mitchell, C.; Rahmani, M.; Nephew, K.P.; Grant, S.; Dent, P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol. Ther. 2009, 8, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, S.; Wang, H.; Yu, X.; Wang, Q.; Jiang, S.; Ju, D.; Ye, L.; Feng, M. Blocking autophagy improves the anti-tumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations in vitro and in vivo. Sci. Rep. 2017, 7, 4559. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Rzymski, T.; Mellor, H.R.; Pike, L.; Bottini, A.; Generali, D.; Harris, A.L. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009, 69, 4415–4423. [Google Scholar] [CrossRef] [PubMed]
- Heine, S.; Kleih, M.; Giménez, N.; Böpple, K.; Ott, G.; Colomer, D.; Aulitzky, W.E.; van der Kuip, H.; Silkenstedt, E. Cyclin D1-CDK4 activity drives sensitivity to bortezomib in mantle cell lymphoma by blocking autophagy-mediated proteolysis of NOXA. J. Hematol. Oncol. 2018, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Gopinathan, G.; Sukumar, J.T.; Gribben, J.G. Blocking autophagy prevents bortezomib-induced NF-κB activation by reducing I-κBα degradation in lymphoma cells. PLoS ONE 2012, 7, e32584. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Rhyasen, G.; Bolanos, L.; Rasch, C.; Varney, M.; Wunderlich, M.; Goyama, S.; Jansen, G.; Cloos, J.; Rigolino, C.; et al. Cytotoxic effects of bortezomib in myelodysplastic syndrome/acute myeloid leukemia depend on autophagy-mediated lysosomal degradation of TRAF6 and repression of PSMA1. Blood 2012, 120, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, M.; Jiang, J.; He, R.; Wang, M.; Guo, X.; Shen, M.; Qin, R. Combined inhibition of autophagy and Nrf2 signaling augments bortezomib-induced apoptosis by increasing ROS production and ER stress in pancreatic cancer cells. Int. J. Biol. Sci. 2018, 14, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, F.; Jiang, J.; Sun, C.; Zhong, Q.; Shen, M.; Wang, X.; Tian, R.; Shi, C.; Xu, M.; et al. Simultaneous inhibition of the ubiquitin-proteasome system and autophagy enhances apoptosis induced by ER stress aggravators in human pancreatic cancer cells. Autophagy 2016, 12, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, C.; Lu, D.; Wu, X.; Gao, L.; Zhang, W. Endoplasmic reticulum stress and autophagy participate in apoptosis induced by bortezomib in cervical cancer cells. Biotechnol. Lett. 2016, 38, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Modernelli, A.; Naponelli, V.; Giovanna Troglio, M.; Bonacini, M.; Ramazzina, I.; Bettuzzi, S.; Rizzi, F. EGCG antagonizes Bortezomib cytotoxicity in prostate cancer cells by an autophagic mechanism. Sci. Rep. 2015, 5, 15270. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Schnell, H.M.; Ruoff, K.; Goldberg, A. Rapid induction of p62 and GABARAPL1 upon proteasome inhibition promotes survival before autophagy activation. J. Cell Biol. 2018, 217, 1757–1776. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Xu, H.; Shi, C.; Jin, F.; Li, W. Profilin 1 induces drug resistance through Beclin1 complex-mediated autophagy in multiple myeloma. Cancer Sci. 2018, 109, 2706–2716. [Google Scholar] [CrossRef] [PubMed]
- Riz, I.; SHawley, T.; GHawley, R. KLF4-SQSTM1/p62-associated prosurvival autophagy contributes to carfilzomib resistance in multiple myeloma models. Oncotarget 2015, 6, 14814–14831. [Google Scholar] [CrossRef] [PubMed]
- Jarauta, V.; Jaime, P.; Gonzalo, O.; de Miguel, D.; Ramírez-Labrada, A.; Martínez-Lostao, L.; Anel, A.; Pardo, J.; Marzo, I.; Naval, J. Inhibition of autophagy with chloroquine potentiates carfilzomib-induced apoptosis in myeloma cells in vitro and in vivo. Cancer Lett. 2016, 382, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, K.; Misund, K.; Starheim, K.K.; Holien, T.; Johansson, I.; Darvekar, S.; Buene, G.; Waage, A.; Bjørkøy, G.; Sundan, A. Hydroxychloroquine potentiates carfilzomib toxicity towards myeloma cells. Oncotarget 2016, 7, 70845–70856. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy Levy, J.M.; Zahedi, S.; Griesinger, A.M.; Morin, A.; Davies, K.D.; Aisner, D.L.; Kleinschmidt-DeMasters, B.; Fitzwalter, B.E.; Goodall, M.L.; Thorburn, J.; et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. eLife 2017, 6, e19671. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Dudek-Perić, A.M.; Maes, H.; Garg, A.D.; Gabrysiak, M.; Demirsoy, S.; Swinnen, J.V.; Agostinis, P. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in Vemurafenib-resistant melanoma cells. Biochem. Pharmacol. 2015, 93, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Thompson, J.C.; Griesinger, A.M.; Amani, V.; Donson, A.M.; Birks, D.K.; Morgan, M.J.; Mirsky, D.M.; Handler, M.H.; Foreman, N.K.; et al. Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors. Cancer Discov. 2014, 4, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-H.; Piao, S.-F.; Dey, S.; McAfee, Q.; Karakousis, G.; Villanueva, J.; Hart, L.S.; Levi, S.; Hu, J.; Zhang, G.; et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Investig. 2014, 124, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kang, H.; Zhao, Y.; Min, I.; Wyrwas, B.; Moore, M.; Teng, L.; Zarnegar, R.; Jiang, X.; Fahey, T.J. Targeting Autophagy Sensitizes BRAF-Mutant Thyroid Cancer to Vemurafenib. J. Clin. Endocrinol. Metab. 2016, 102, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Sueda, T.; Sakai, D.; Kawamoto, K.; Konno, M.; Nishida, N.; Koseki, J.; Colvin, H.; Takahashi, H.; Haraguchi, N.; Nishimura, J.; et al. BRAF V600E inhibition stimulates AMP-activated protein kinase-mediated autophagy in colorectal cancer cells. Sci. Rep. 2016, 6, 18949. [Google Scholar] [CrossRef] [PubMed]
- Goulielmaki, M.; Koustas, E.; Moysidou, E.; Vlassi, M.; Sasazuki, T.; Shirasawa, S.; Zografos, G.; Oikonomou, E.; Pintzas, A. BRAF associated autophagy exploitation: BRAF and autophagy inhibitors synergise to efficiently overcome resistance of BRAF mutant colorectal cancer cells. Oncotarget 2016, 7, 9188–9221. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, C.G.; Camolotto, S.A.; Boespflug, A.M.; Guillen, K.P.; Foth, M.; Truong, A.; Schuman, S.S.; Shea, J.E.; Seipp, M.T.; Yap, J.T.; et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 2019, 25, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Verykiou, S.; Alexander, M.; Edwards, N.; Plummer, R.; Chaudhry, B.; Lovat, P.E.; Hill, D.S. Harnessing autophagy to overcome mitogen-activated protein kinase kinase inhibitor-induced resistance in metastatic melanoma. Br. J. Dermatol. 2019, 180, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.J.M.; Valdes, Y.R.; Peart, T.M.; Fazio, E.N.; Bertrand, M.; McGee, J.; Préfontaine, M.; Sugimoto, A.; DiMattia, G.E.; Shepherd, T.G. Combination of AKT inhibition with autophagy blockade effectively reduces ascites-derived ovarian cancer cell viability. Carcinogenesis 2014, 35, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Jiang, Q. Autophagy prevention sensitizes AKTi-1/2-induced anti-hepatocellular carcinoma cell activity in vitro and in vivo. Biochem. Biophys. Res. Commun. 2016, 480, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Degtyarev, M.; De Mazière, A.; Orr, C.; Lin, J.; Lee, B.B.; Tien, J.Y.; Prior, W.W.; van Dijk, S.; Wu, H.; Gray, D.C.; et al. AKT inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J. Cell Biol. 2008, 183, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, F.; Thomas, C.; Crafter, C.; Kumano, M.; Zhang, F.; Davies, B.R.; Gleave, M.E.; Zoubeidi, A. Blocked autophagy using lysosomotropic agents sensitizes resistant prostate tumor cells to the novel AKT inhibitor AZD5363. Clin. Cancer Res. 2013, 19, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Koh, D.; Na, H.; Ka, N.L.; Kim, S.; Kim, H.J.; Hong, S.; Shin, Y.K.; Seong, J.K.; Lee, M.-O. MTA1 is a novel regulator of autophagy that induces tamoxifen resistance in breast cancer cells. Autophagy 2018, 14, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Das, C.K.; Parekh, A.; Parida, P.K.; Bhutia, S.K.; Mandal, M. Lactate dehydrogenase A regulates autophagy and tamoxifen resistance in breast cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1866, 1004–1018. [Google Scholar]
- Nagelkerke, A.; Sieuwerts, A.M.; Bussink, J.; Sweep, F.C.G.J.; Look, M.P.; Foekens, J.A.; Martens, J.W.M.; Span, P.N. LAMP3 is involved in tamoxifen resistance in breast cancer cells through the modulation of autophagy. Endocr. Relat. Cancer 2014, 21, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.A.; Kwok, B.; Dragowska, W.H.; To, K.H.; Le, D.; Bally, M.B.; Gorski, S.M. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res. Treat. 2008, 112, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, T.; Li, G.; Xu, C.; Xu, Z.; Zhang, J.; He, K.; Zheng, L.; Guan, Z.; Su, X.; et al. Lower Beclin 1 downregulates HER2 expression to enhance tamoxifen sensitivity and predicts a favorable outcome for ER positive breast cancer. Oncotarget 2017, 8, 52156–52177. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, J.S.; Gaddy, V.T.; Duplantier, J.; Thandavan, S.P.; Shah, M.; Smith, M.J.; Browning, D.; Rawson, J.; Smith, S.B.; Barrett, J.T.; et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol. Cancer Ther. 2008, 7, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Tian, L.; Jung, M.; Choi, S.K.; Sun, Y.; Kim, H.; Moon, W.K. Downregulation of Choline Kinase-Alpha Enhances Autophagy in Tamoxifen-Resistant Breast Cancer Cells. PLoS ONE 2015, 10, e0141110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, A.; Roy, S.; Lazar, A.J.F.; Wang, W.-L.; McAuliffe, J.C.; Reynoso, D.; McMahon, J.; Taguchi, T.; Floris, G.; Debiec-Rychter, M.; et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc. Natl. Acad. Sci. USA 2010, 107, 14333–14338. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Mishima, Y.; Terui, Y.; Kimura, S.; Hatake, K. An Autophagy Inhibitor, Chloroquine, Overcomes an Imatinib-Resistant T315I Mutant of Bcr/Abl, Whose Latent May Exist Before Imatinib Treatment. Blood 2009, 114, 2183. [Google Scholar] [CrossRef]
- Crowley, L.C.; O’Donovan, T.R.; Nyhan, M.J.; McKenna, S.L. Pharmacological agents with inherent anti-autophagic activity improve the cytotoxicity of imatinib. Oncol. Rep. 2013, 29, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Rothe, K.; Lin, H.; Lin, K.B.L.; Leung, A.; Wang, H.M.; Malekesmaeili, M.; Brinkman, R.R.; Forrest, D.L.; Gorski, S.M.; Jiang, X. The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood 2014, 123, 3622–3634. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, D.; Lee, Y. Metformin Synergistically Potentiates the Antitumor Effects of Imatinib in Colorectal Cancer Cells. Dev. Reprod. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cao, L.; Yang, L.; Kang, R.; Lotze, M.; Tang, D. microRNA 30A promotes autophagy in response to cancer therapy. Autophagy 2012, 8, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, L.; Zhao, M.; Zhu, S.; Kang, R.; Vernon, P.; Tang, D.; Cao, L. Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia 2012, 26, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; De Vera, M.E.; Buchser, W.J.; Romo de Vivar Chavez, A.; Loughran, P.; Beer Stolz, D.; Basse, P.; Wang, T.; Van Houten, B.; Zeh, H.J.; et al. Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res. 2012, 72, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Buchser, W.J.; Liang, X. Blocking the interleukin 2 (IL2)-induced systemic autophagic syndrome promotes profound antitumor effects and limits toxicity. Autophagy 2012, 8, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Chude, C.I.; Amaravadi, R.K. Targeting Autophagy in Cancer: Update on Clinical Trials and Novel Inhibitors. Int. J. Mol. Sci. 2017, 18, 1279. [Google Scholar] [CrossRef] [PubMed]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 2017, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Poklepovic, A.; Gewirtz, D.A. Outcome of early clinical trials of the combination of hydroxychloroquine with chemotherapy in cancer. Autophagy 2014, 10, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Solitro, A.R.; MacKeigan, J.P. Leaving the lysosome behind: Novel developments in autophagy inhibition. Future Med. Chem. 2015, 8, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Das, C.K.; Mandal, M. Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastasis Rev. 2018, 37, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Chiou, V.L.; Lee, J.-M.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Amit, I.; Yarden, Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim. Biophys. Acta 2007, 1773, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Wislez, M.; Zhang, J.; Iwanaga, K.; Dackor, J.; Hanna, A.E.; Kalyankrishna, S.; Cody, D.D.; Price, R.E.; Sato, M.; et al. High expression of ErbB family members and their ligands in lung adenocarcinomas that are sensitive to inhibition of epidermal growth factor receptor. Cancer Res. 2005, 65, 11478–11485. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Nordgren, H.; Sjöström, J.; Wester, K.; Villman, K.; Bengtsson, N.O.; Ostenstad, B.; Lundqvist, H.; Blomqvist, C. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br. J. Cancer 2004, 90, 2344–2348. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Phuchareon, J.; McCormick, F.; Eisele, D.W.; Tetsu, O. EGFR inhibition evokes innate drug resistance in lung cancer cells by preventing AKT activity and thus inactivating Ets-1 function. Proc. Natl. Acad. Sci. USA 2015, 112, E3855––E3863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, S.; Zhao, W.; Qin, S.; Chu, Q.; Wu, K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol. Cancer 2018, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Shih, J.Y. Management of acquired resistance to EGFR TKI–targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 2018, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Westover, D.; Zugazagoitia, J.; Cho, B.C.; Lovly, C.M.; Paz-Ares, L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 2018, 29, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ricordel, C.; Friboulet, L.; Facchinetti, F.; Soria, J.-C. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann. Oncol. 2018, 29, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Nagaria, T.S.; Shi, C.; Leduc, C.; Hoskin, V.; Sikdar, S.; Sangrar, W.; Greer, P.A. Combined targeting of Raf and Mek synergistically inhibits tumorigenesis in triple negative breast cancer model systems. Oncotarget 2017, 8, 80804–80819. [Google Scholar] [CrossRef] [PubMed]
- Khunger, A.; Khunger, M.; Velcheti, V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: Clinical evidence and experience. Ther. Adv. Respir. Dis. 2018, 12, 1753466618767611. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Luque-Cabal, M.; García-Teijido, P.; Fernández-Pérez, Y.; Sánchez-Lorenzo, L.; Palacio-Vázquez, I. Mechanisms Behind the Resistance to Trastuzumab in HER2-Amplified Breast Cancer and Strategies to Overcome It. Clin. Med. Insights Oncol. 2016, 10, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kabraji, S.; Ni, J.; Lin, N.U.; Xie, S.; Winer, E.P.; Zhao, J.J. Drug Resistance in HER2-Positive Breast Cancer Brain Metastases: Blame the Barrier or the Brain? Clin. Cancer Res. 2018, 24, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.M.; Paplomata, E.; Peake, B.M.; Sanabria, E.; Chen, Z.; Nahta, R. P38 MAPK contributes to resistance and invasiveness of HER2- overexpressing breast cancer. Curr. Med. Chem. 2014, 21, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.H.; Nam, A.-R.; Park, J.E.; Bang, J.-H.; Bang, Y.-J.; Oh, D.-Y. Resistance Mechanism against Trastuzumab in HER2-Positive Cancer Cells and Its Negation by Src Inhibition. Mol. Cancer Ther. 2017, 16, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, M.; Kojiro, E.; Baba, H. Molecular mechanism of secondary-resistant for trastuzumab in gastric cancer. JCO 2016, 34, 58. [Google Scholar] [CrossRef]
- De Gagliato, D.M.; Jardim, D.L.F.; Marchesi, M.S.P.; Hortobagyi, G.N. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget 2016, 7, 64431–64446. [Google Scholar] [CrossRef] [PubMed]
- Flem-Karlsen, K.; Tekle, C.; Øyjord, T.; Flørenes, V.A.; Mælandsmo, G.M.; Fodstad, Ø.; Nunes-Xavier, C.E. p38 MAPK activation through B7-H3-mediated DUSP10 repression promotes chemoresistance. Sci. Rep. 2019, 9, 5839. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Takazawa, Y.; Inoue, Y.; Yokouchi, Y.; Kobayashi, M.; Saiura, A.; Shibutani, T.; Ishikawa, Y. Tumor B7-H3 (CD276) Expression and Survival in Pancreatic Cancer. J. Clin. Med. 2018, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Amori, G.; Yuasa, T.; Yamamoto, S.; Yonese, J.; Ishikawa, Y. Relationship of B7-H3 expression in tumor cells and tumor vasculature with FOXP3+ regulatory T cells in renal cell carcinoma. Cancer Manag. Res. 2019, 11, 7021–7030. [Google Scholar] [CrossRef] [PubMed]
- Koul, H.K.; Pal, M.; Koul, S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef] [PubMed]
- García-Cano, J.; Roche, O.; Cimas, F.J.; Pascual-Serra, R.; Ortega-Muelas, M.; Fernández-Aroca, D.M.; Sánchez-Prieto, R. p38MAPK and Chemotherapy: We Always Need to Hear Both Sides of the Story. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Hammaker, D.; Firestein, G.S. ‘Go upstream, young man’: Lessons learned from the p38 saga. Ann. Rheum. Dis. 2010, 69 (Suppl. 1), i77–i82. [Google Scholar] [CrossRef]
- Dambach, D.M. Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr. Top. Med. Chem. 2005, 5, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Haluska, P.; Tolcher, A.W.; Erlichman, C.; Papadopoulos, K.P.; Lensing, J.L.; Beeram, M.; Molina, J.R.; Rasco, D.W.; Arcos, R.R.; et al. A First-in-Human Phase I Study of the Oral p38 MAPK Inhibitor, Ralimetinib (LY2228820 Dimesylate), in Patients with Advanced Cancer. Clin. Cancer Res. 2016, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Rosen, L.S.; Tolcher, A.W.; Papadopoulos, K.; Beeram, M.; Shi, P.; Pitou, C.; Bell, R.; Kulanthaivel, P.; Zhang, X.; et al. Phase 1 and pharmacokinetic study of LY3007113, a p38 MAPK inhibitor, in patients with advanced cancer. Investig. New Drugs 2018, 36, 629–637. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Morcillo, M.A.; Valero, M.L.L.; Callejas-Valera, J.L.; Arias-González, L.; Melgar-Rojas, P.; Galán-Moya, E.M.; García-Gil, E.; García-Cano, J.; Sánchez-Prieto, R. P38MAPK is a major determinant of the balance between apoptosis and autophagy triggered by 5-fluorouracil: Implication in resistance. Oncogene 2012, 31, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Leli, N.M.; Onorati, A.; Piao, S.; Verginadis, I.I.; Tameire, F.; Rebecca, V.W.; Chude, C.I.; Murugan, S.; Fennelly, C.; et al. ER Translocation of the MAPK Pathway Drives Therapy Resistance in BRAF-Mutant Melanoma. Cancer Discov. 2019, 9, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.L.; Stalnecker, C.A.; Zeitouni, D.; Klomp, J.E.; Peng, S.; Tikunov, A.P.; Gunda, V.; Pierobon, M.; Waters, A.M.; George, S.D.; et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 2019, 25, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Anticancer autophagy inhibitors attract ’resurgent’ interest. Nat. Rev. Drug Discov. 2019, 18, 408. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Santis, M.C.D.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.M.; Rosenbaum, B.E.; Berger, M.; Izsak, A.; Li, Y.; Illa Bochaca, I.; Vega-Saenz de Miera, E.; Wang, J.; Darvishian, F.; Zhong, H.; et al. Revisiting the Clinical and Biologic Relevance of Partial PTEN Loss in Melanoma. J. Invest. Dermatol. 2019, 139, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Catalanotti, F.; Cheng, D.T.; Shoushtari, A.N.; Johnson, D.B.; Panageas, K.S.; Momtaz, P.; Higham, C.; Won, H.H.; Harding, J.J.; Merghoub, T.; et al. PTEN Loss-of-Function Alterations Are Associated With Intrinsic Resistance to BRAF Inhibitors in Metastatic Melanoma. JCO Precision Oncology 2017, 1–15. [Google Scholar] [CrossRef]
- Paraiso, K.H.T.; Xiang, Y.; Rebecca, V.W.; Abel, E.V.; Chen, Y.A.; Munko, A.C.; Wood, E.; Fedorenko, I.V.; Sondak, V.K.; Anderson, A.R.A.; et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011, 71, 2750–2760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, Y.; Zhou, K.; Wang, L.; Yan, Z.; Liu, Y.; Xu, L.; Zhao, S.; Chu, H.; Shi, T.; et al. PIK3CA mutations confer resistance to first-line chemotherapy in colorectal cancer. Cell Death Dis. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dillon, L.M.; Miller, T.W. Therapeutic targeting of cancers with loss of PTEN function. Curr. Drug Targets 2014, 15, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. Efficacy of PI3K/AKT/mTOR pathway inhibitors for the treatment of advanced solid cancers: A literature-based meta-analysis of 46 randomised control trials. PLoS ONE 2018, 13, e0192464. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.M.; Mayer, I.A.; Arteaga, C.L. Is There a Future for AKT Inhibitors in the Treatment of Cancer? Clin. Cancer Res. 2016, 22, 2599–2601. [Google Scholar] [CrossRef] [PubMed]
- Yoshida-Ichikawa, Y.; Tanabe, M.; Tokuda, E.; Shimizu, H.; Horimoto, Y.; Miura, K.; Saito, M. Overcoming the Adverse Effects of Everolimus to Achieve Maximum Efficacy in the Treatment of Inoperable Breast Cancer: A Review of 11 Cases at Our Hospital. Case Rep. Oncol. 2018, 11, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grünwald, V.; Thompson, J.A.; Figlin, R.A.; Hollaender, N.; et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet 2008, 372, 449–456. [Google Scholar] [CrossRef]
- Chan, D.L.; Segelov, E.; Singh, S. Everolimus in the management of metastatic neuroendocrine tumours. Therap. Adv. Gastroenterol. 2017, 10, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Crazzolara, R.; Bradstock, K.F.; Bendall, L.J. RAD001 (Everolimus) induces autophagy in acute lymphoblastic leukemia. Autophagy 2009, 5, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Santanam, U.; Banach-Petrosky, W.; Abate-Shen, C.; Shen, M.M.; White, E.; DiPaola, R.S. Atg7 cooperates with Pten loss to drive prostate cancer tumor growth. Genes Dev. 2016, 30, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Kong, Y.; Shao, C.; Yue, X.; Wang, Z.; Zhang, N. Heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to pharmorubicin by promoting autophagy via PI3K/AKT pathway. J. Cell. Mol. Med. 2018, 22, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.; Gueron, G.; Elguero, B.; Giudice, J.; Salles, A.; Leskow, F.C.; Jares-Erijman, E.A.; Colombo, L.; Meiss, R.; Navone, N.; et al. Heme oxygenase 1 (HO-1) challenges the angiogenic switch in prostate cancer. Angiogenesis 2011, 14, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Halin Bergström, S.; Nilsson, M.; Adamo, H.; Thysell, E.; Jernberg, E.; Stattin, P.; Widmark, A.; Wikström, P.; Bergh, A. Extratumoral Heme Oxygenase-1 (HO-1) Expressing Macrophages Likely Promote Primary and Metastatic Prostate Tumor Growth. PLoS ONE 2016, 11, e0157280. [Google Scholar] [CrossRef] [PubMed]
- Degese, M.S.; Mendizabal, J.E.; Gandini, N.A.; Gutkind, J.S.; Molinolo, A.; Hewitt, S.M.; Curino, A.C.; Coso, O.A.; Facchinetti, M.M. Expression of heme oxygenase-1 in non-small cell lung cancer (NSCLC) and its correlation with clinical data. Lung Cancer 2012, 77, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.R.; Wang, H.M.; Liu, P.L.; Chen, Y.H.; Yang, M.C.; Chou, S.H.; Cheng, Y.J.; Yin, W.H.; Hwang, J.J.; Chong, I.W. High expression of heme oxygenase-1 is associated with tumor invasiveness and poor clinical outcome in non-small cell lung cancer patients. Cell Oncol. (Dordr) 2012, 35, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Kaveney, A.D.; Mehnert, J.M.; Tan, A.R.; Aisner, J.; Moss, R.A.; Gibbon, D.; Adams, S.; Fischer, J.; Scott, P.; Gable, C.; et al. A phase I trial of MK-2206 and hydroxychloroquine(HCQ) in solid tumors, melanoma, renal, and prostate cancer to examine the role of autophagy in tumorigenesis. JCO 2014, 32, TPS2640. [Google Scholar] [CrossRef]
- Mancias, J.D.; Kimmelman, A.C. Mechanisms of selective autophagy in normal physiology and cancer. J. Mol. Biol. 2016, 428, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kaplan, V.; Livneh, I.; Avni, N.; Fabre, B.; Ziv, T.; Kwon, Y.T.; Ciechanover, A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA 2016, 113, E7490–E7499. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, P.; Komatsu, M. p62/SQSTM1—Steering the cell through health and disease. J. Cell. Sci. 2018, 131, jcs222836. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Komatsu, M. Activation of p62/SQSTM1-KEAP1-Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Cancer. Front. Oncol. 2018, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ichimura, Y.; Taguchi, K.; Suzuki, T.; Mizushima, T.; Takagi, K.; Hirose, Y.; Nagahashi, M.; Iso, T.; Fukutomi, T.; et al. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming. Nat. Commun. 2016, 7, 12030. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Di Caro, G.; Li, N.; Wu, X.; Lytle, N.K.; Dhar, D.; Banerjee, S.; Fagman, J.B.; Browne, C.D.; et al. Stress-Activated NRF2-MDM2 Cascade Controls Neoplastic Progression in Pancreas. Cancer Cell 2017, 32, 824–839.e8. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Yu, H.; Gu, S.; Xu, Y.; Su, J.; Li, H.; Kang, J.; Cui, M. p62/SQSTM1 is involved in cisplatin resistance in human ovarian cancer cells via the Keap1-Nrf2-ARE system. Int. J. Oncol. 2014, 45, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.G.; Choi, B.H.; Ku, S.K.; Kwak, M.K. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: Implications for cancer stem cell resistance. Redox Biol. 2018, 17, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Battista, R.A.; Resnati, M.; Facchi, C.; Ruggieri, E.; Cremasco, F.; Paradiso, F.; Orfanelli, U.; Giordano, L.; Bussi, M.; Cenci, S.; et al. Autophagy mediates epithelial cancer chemoresistance by reducing p62/SQSTM1 accumulation. PLoS ONE 2018, 13, e0201621. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Fitzwalter, B.E.; Regan, D.; Goodspeed, A.; Morgan, M.J.; Liu, C.-W.; Gustafson, D.L.; Thorburn, A. Cancer Cells Upregulate NRF2 Signaling to Adapt to Autophagy Inhibition. Dev. Cell 2019, 50, 690–703.e6. [Google Scholar] [CrossRef] [PubMed]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zeng, Y.; Zhou, S.F. Role of Apoptosis in Cancer Resistance to Chemotherapy. In Current Understanding of Apoptosis—Programmed Cell Death; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Pellegrini, M.; Tsuchihara, K.; Yamamoto, K.; Hacker, G.; Erlacher, M.; Villunger, A.; Mak, T.W. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J. Exp. Med. 2006, 203, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Akhter, R.; Sanphui, P.; Biswas, S.C. The essential role of p53 Upregulated Modulator of Apoptosis and its regulation by FOXO3A transcription factor in β-amyloid induced neuron death. J. Biol. Chem. 2014. [Google Scholar] [CrossRef]
- DeVorkin, L.; Choutka, C.; Gorski, S.M. The interplay between autophagy and apoptosis. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, and Infection; Hayat, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter XXIV; Volume 3. [Google Scholar]
- Su, M.; Mei, Y.; Sinha, S. Role of the Crosstalk between Autophagy and Apoptosis in Cancer. J. Oncol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, J.; Andrysik, Z.; Staskiewicz, L.; Gump, J.; Maycotte, P.; Oberst, A.; Green, D.R.; Espinosa, J.M.; Thorburn, A. Autophagy controls the kinetics and extent of mitochondrial apoptosis by regulating PUMA levels. Cell Rep. 2014, 7, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Eijkelenboom, A.; Mokry, M.; de Wit, E.; Smits, L.M.; Polderman, P.E.; van Triest, M.H.; van Boxtel, R.; Schulze, A.; de Laat, W.; Cuppen, E.; et al. Genome-wide analysis of FOXO3 mediated transcription regulation through RNA polymerase II profiling. Mol. Syst. Biol. 2013, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Warr, M.R.; Binnewies, M.; Flach, J.; Reynaud, D.; Garg, T.; Malhotra, R.; Debnath, J.; Passegué, E. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature 2013, 494, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, W.; Chen, X.; Wang, S.; Qian, W. Oxidative stress response induced by chemotherapy in leukemia treatment. Mol. Clin. Oncol. 2018, 8, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, L.R.C.; Dutra, F.F.; Siqueira, M.S.; Paula-Neto, H.A.; Dahan, J.; Kiarely, E.; Carneiro, L.A.M.; Bozza, M.T.; Travassos, L.H. Protein aggregation as a cellular response to oxidative stress induced by heme and iron. Proc. Natl. Acad. Sci. USA 2016, 113, E7474–E7482. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Thorburn, A. Therapeutic Targeting of Autophagy. EBioMedicine 2016, 14, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.A.; Regan, D.P.; Hansen, R.J.; Maycotte, P.; Thorburn, A.; Gustafson, D.L. Autophagy Inhibition Delays Early but Not Late-Stage Metastatic Disease. J. Pharmacol. Exp. Ther. 2016, 358, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, H.; Liu, D.; Hill, C.; Ertay, A.; Li, J.; Zou, Y.; Miller, P.; White, E.; Downward, J.; et al. Autophagy inhibition specifically promotes epithelial-mesenchymal transition and invasion in RAS-mutated cancer cells. Autophagy 2019, 15, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A. Autophagy and Its Effects: Making Sense of Double-Edged Swords. PLoS Biol. 2014, 12, e1001967. [Google Scholar] [CrossRef] [PubMed]
- Lauzier, A.; Normandeau-Guimond, J.; Vaillancourt-Lavigueur, V.; Boivin, V.; Charbonneau, M.; Rivard, N.; Scott, M.S.; Dubois, C.M.; Jean, S. Colorectal cancer cells respond differentially to autophagy inhibition in vivo. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, J.; Staskiewicz, L.; Goodall, M.L.; Dimberg, L.; Frankel, A.E.; Ford, H.L.; Thorburn, A. Non-cell-autonomous Effects of Autophagy Inhibition in Tumor Cells Promote Growth of Drug-resistant Cells. Mol. Pharmacol. 2017, 91, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, L.M.; Simon, A.K.; Baehrecke, E.H. Current questions and possible controversies in autophagy. Cell Death Discov. 2015, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. The challenge of developing autophagy inhibition as a therapeutic strategy. Cancer Res. 2016, 76, 5610–5614. [Google Scholar] [CrossRef] [PubMed]
- Görgülü, K.; Diakopoulos, K.N.; Ai, J.; Schoeps, B.; Kabacaoglu, D.; Karpathaki, A.F.; Ciecielski, K.J.; Kaya-Aksoy, E.; Ruess, D.A.; Berninger, A.; et al. Levels of the Autophagy-Related 5 Protein Affect Progression and Metastasis of Pancreatic Tumors in Mice. Gastroenterology 2019, 156, 203–217.e20. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Bi, F.; You, Y.; Liu, X.; Yang, Q. Development of a Novel Autophagy-related Prognostic Signature for Serous Ovarian Cancer. J. Cancer 2018, 9, 4058–4071. [Google Scholar] [CrossRef] [PubMed]
- Bortnik, S.; Gorski, S.M. Clinical Applications of Autophagy Proteins in Cancer: From Potential Targets to Biomarkers. Int. J. Mol. Sci. 2017, 18, 1496. [Google Scholar] [CrossRef] [PubMed]
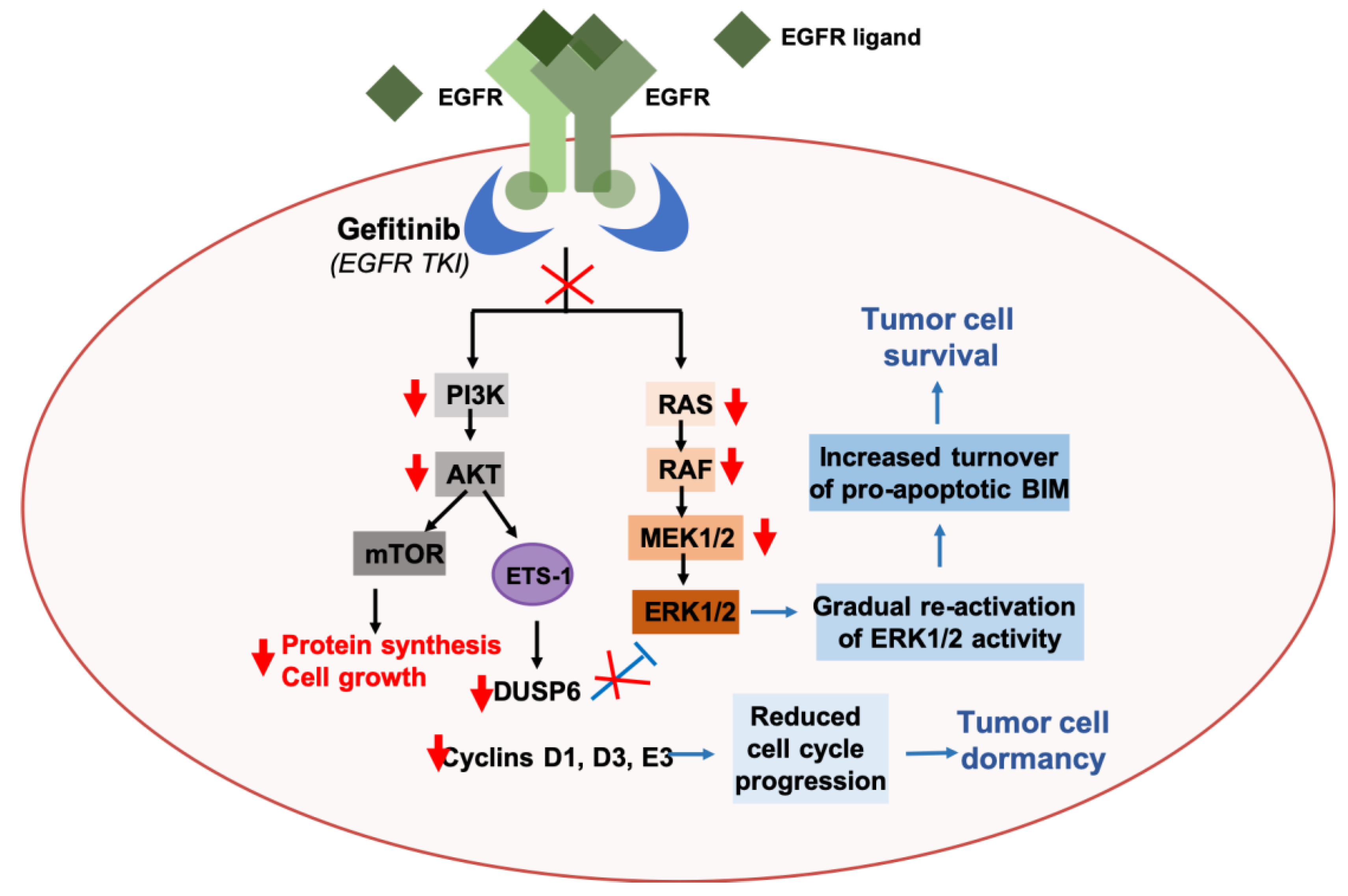
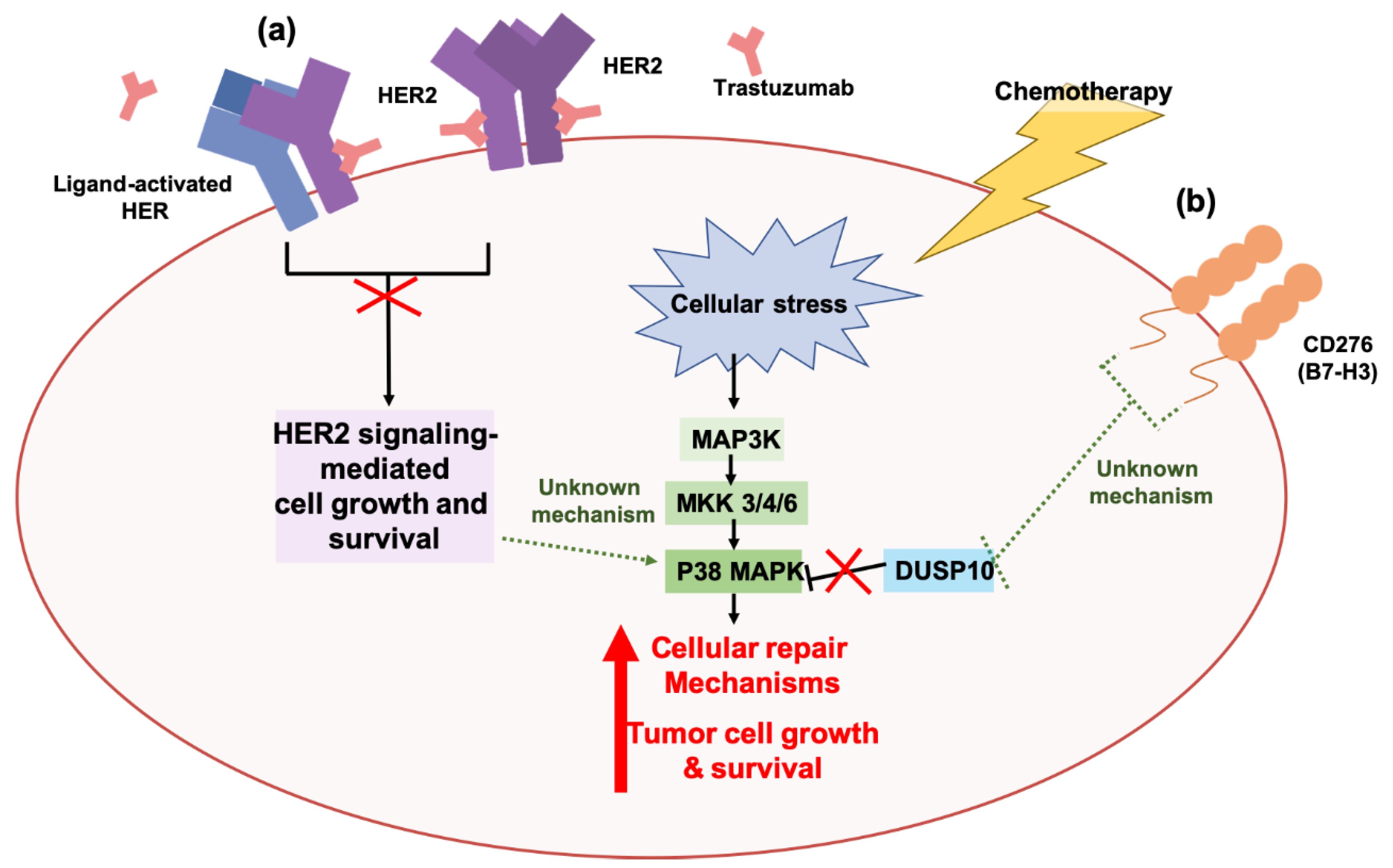
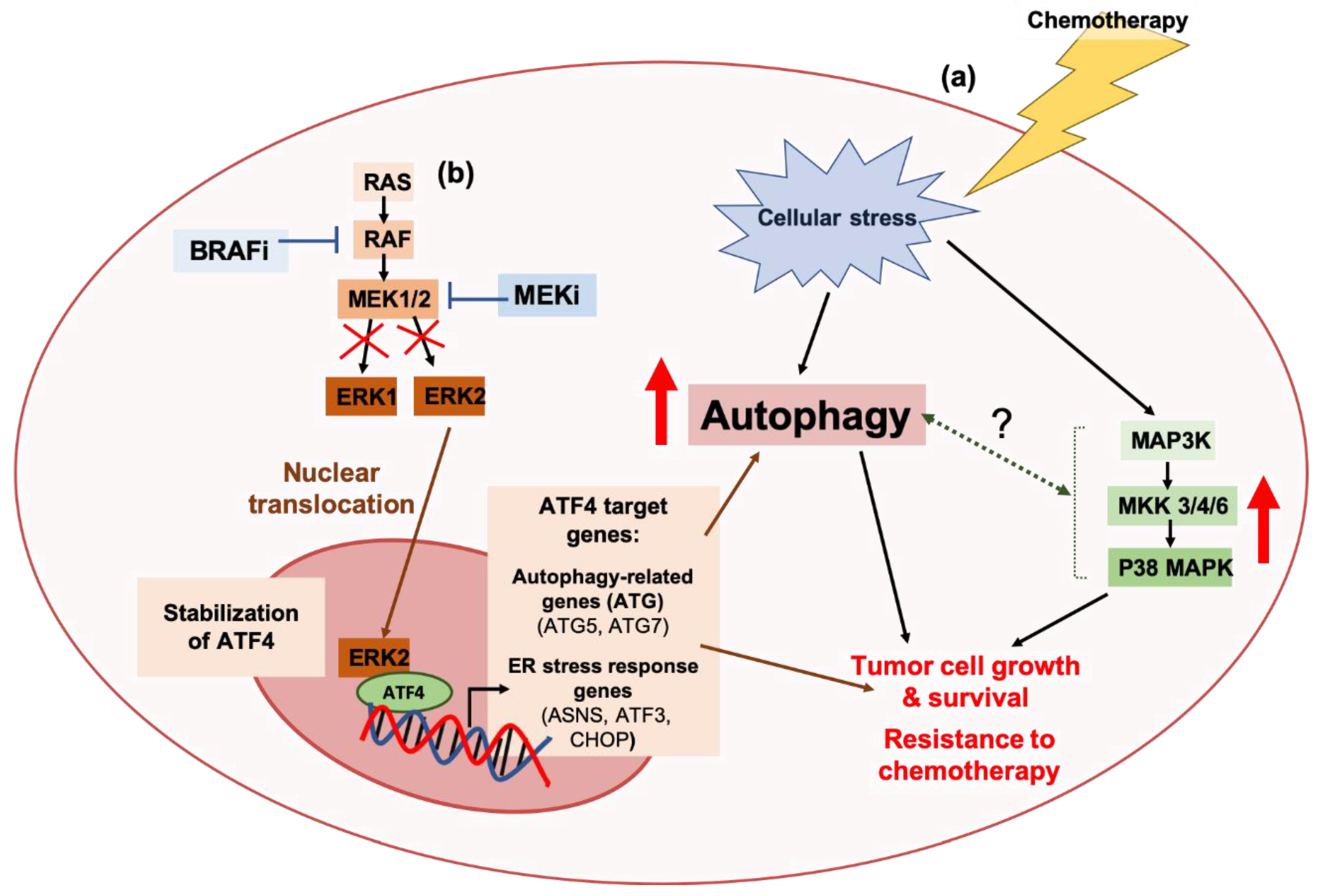
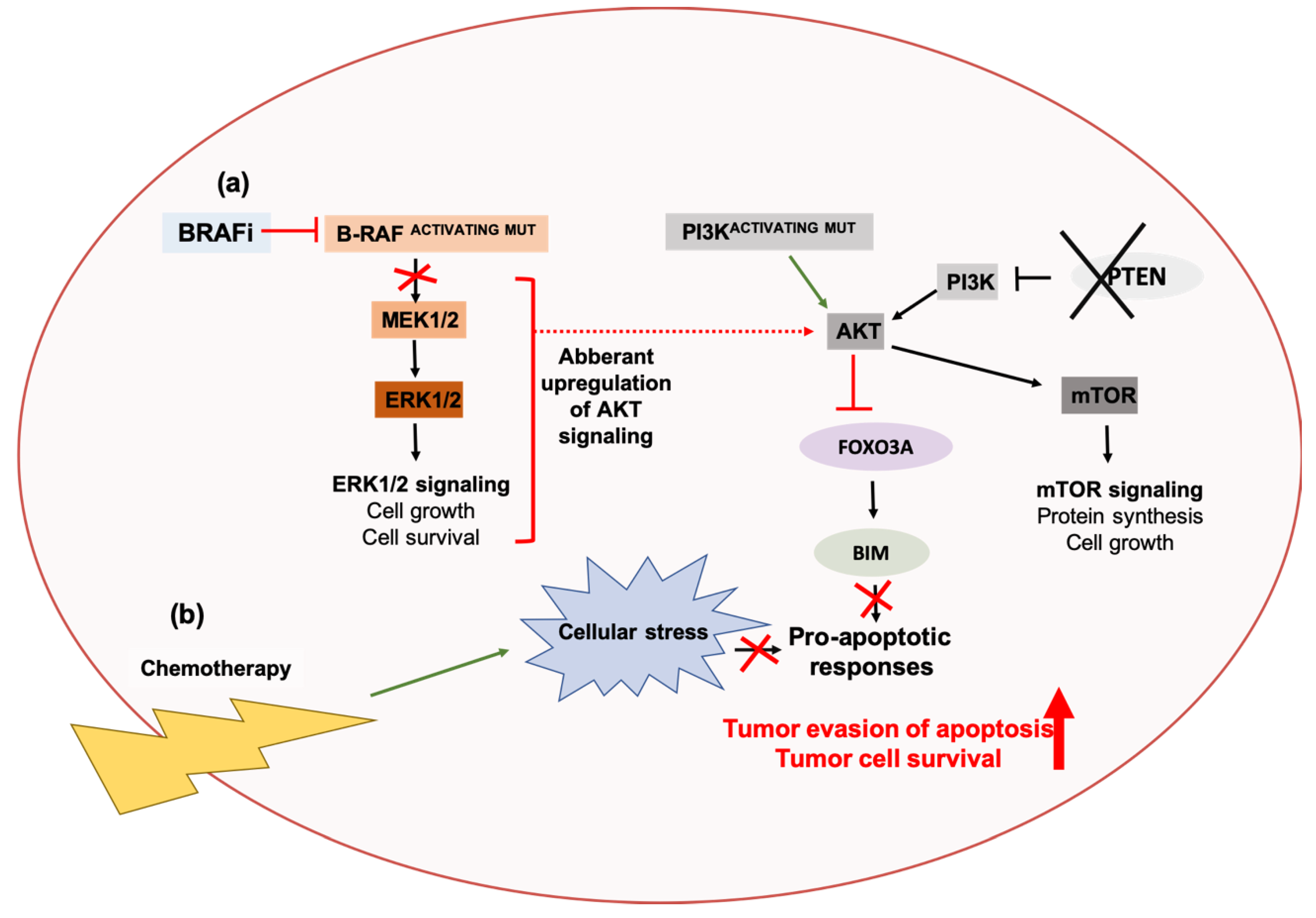
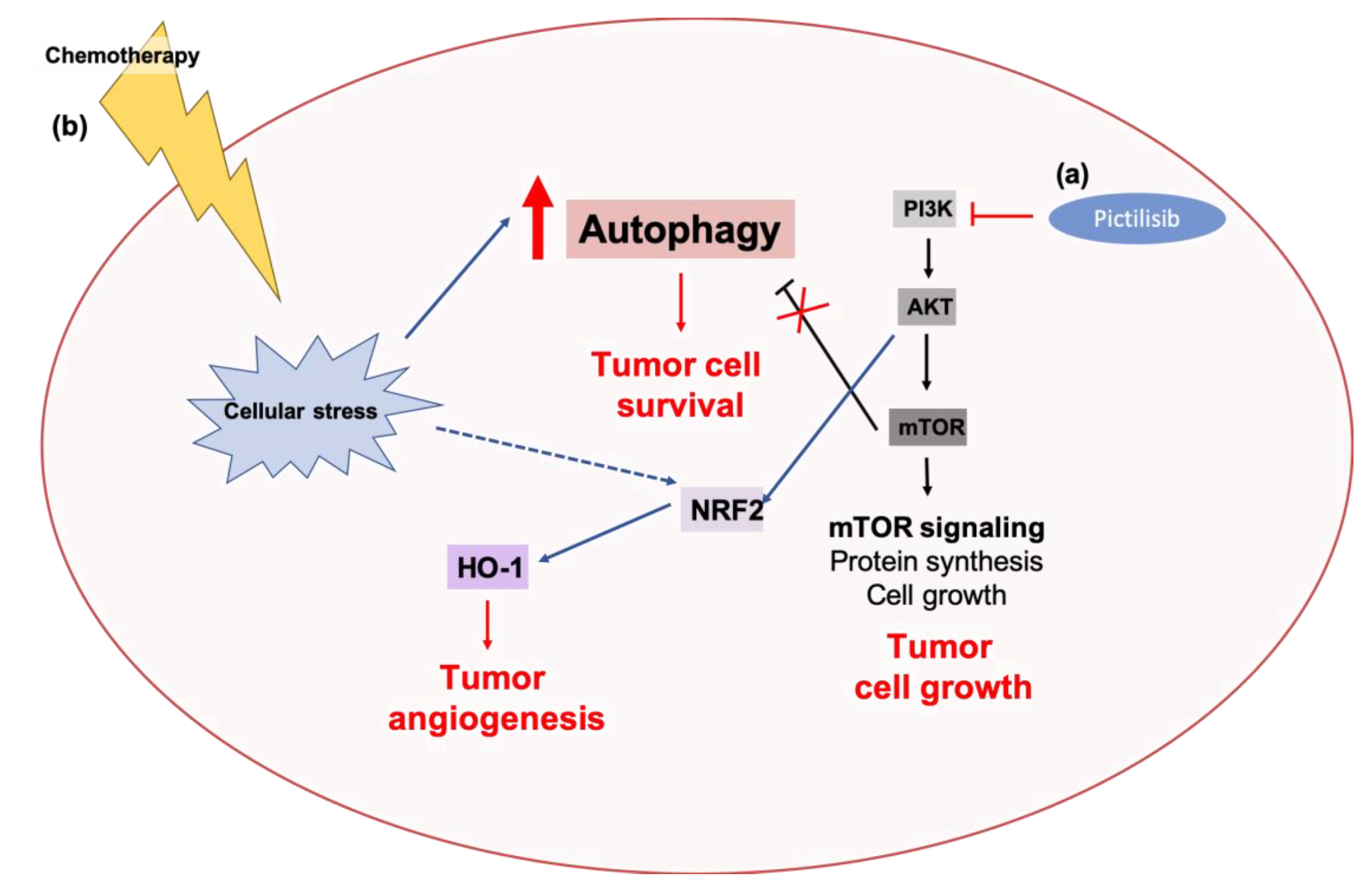

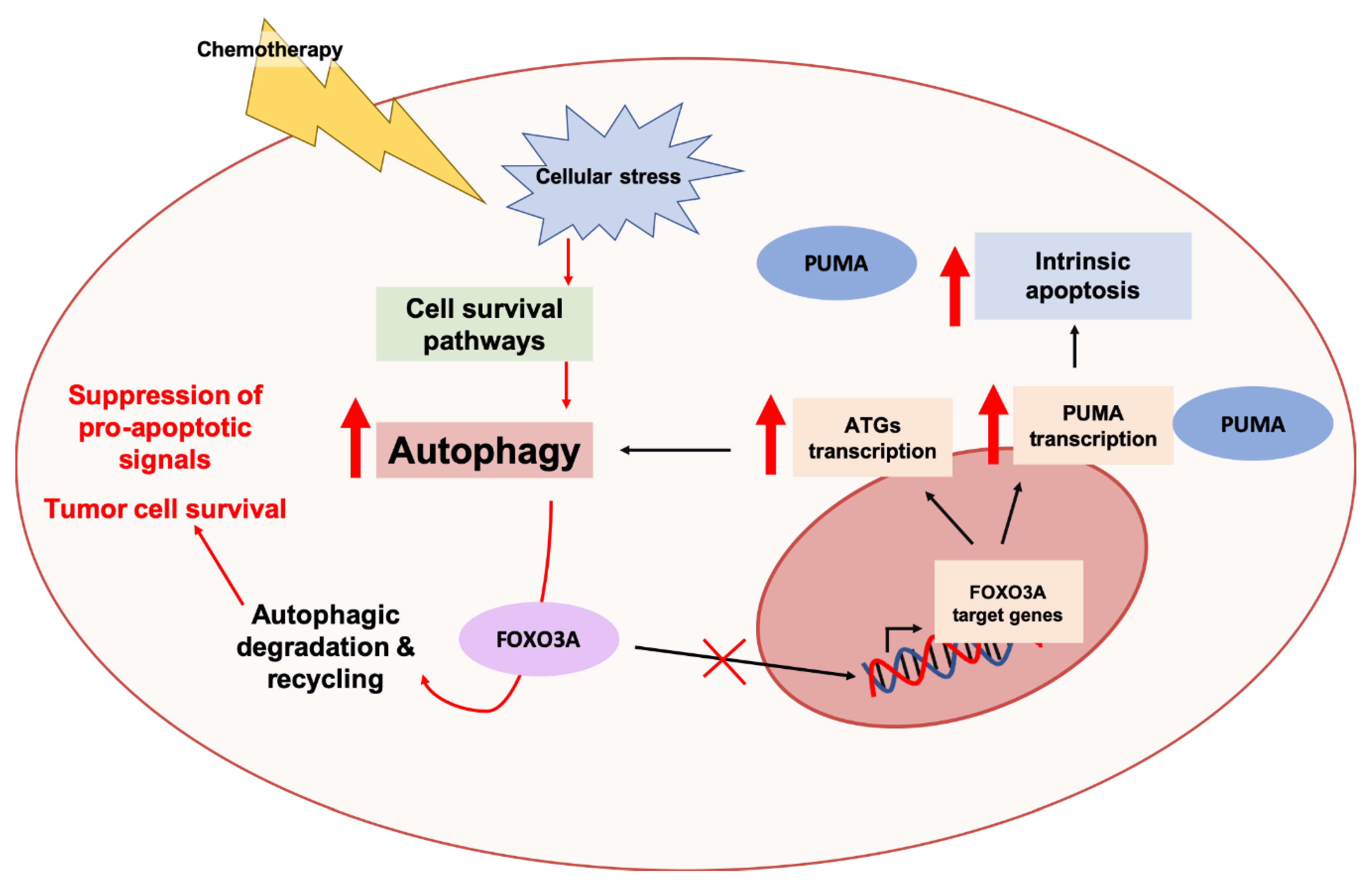
| Targeted Agent | Cancer Types | Mode of Autophagy Inhibition | Ref. | |
|---|---|---|---|---|
| Pharmacological | Genetic | |||
| Monoclonal antibodies | ||||
| Bevacizumab (VEGF (vascular endothelial growth factor) inhibitor) | Gliomas, Colorectal cancers, Liver cancers | CQ, HCQ | BECN1 siRNA (small interfering Ribonucleic Acid), ATG7 shRNA (short hairpin Ribonucleic Acid) | [113,192,193,194,195,196] |
| Trastuzumab (HER2 (human epidermal growth factor receptor 2) inhibitor) | Breast cancers | 3-MA, Baf A1 | LC3B siRNA, ATG4B siRNA, ATG12 shRNA, ATG4B siRNA | [197,198,199,200] |
| Cetuximab (EGFR (epidermal growth factor receptor) inhibitor) | Vulvar cancers, Lung cancers, Head and neck cancers | CQ, 3-MA | BECN1 siRNA, ATG7 siRNA | [201,202,203] |
| Small molecule inhibitors | ||||
| Sorafenib (multi-kinase inhibitor—VEGFRs, PDGFRs (platelet-derived growth factor receptor), RAF (Rapidly Accelerated Fibrosarcoma) kinases) | Endometrial cancers, Liver cancers, Kidney cancers, Gliomas, Desmoid tumors | CQ, 3-MA, Baf A1 | BECN1 siRNA, BECN1 shRNA, ATG5 siRNA, ATG5 shRNA, miR (microRNA)-375 (ATG7 knockdown) | [204,205,206,207,208,209,210] |
| Linifanib (multi-kinase inhibitor—VEGFRs and PDFRs) | Liver cancers | CQ, 3-MA | ATG7 siRNA, ATG5 siRNA | [211] |
| Sunitinib (Multi-kinase inhibitor—PDGFRs, VEGFRs, KIRs (Killer cell immunoglobulin like receptors), FLT-3 (fms like tyrosine kinase-3), CSF-1R (Colony stimulating factor 1 receptor), RET (rearranged during transfection) kinases) | Ovarian cancers, Kidney cancers, Prostate cancers, Pancreatic cancers | Lys05, CQ, 3-MA, Baf A1 | ATG7 siRNA, ATG5 siRNA, ATG5 shRNA, LAMP2 shRNA | [212,213,214,215,216] |
| Gefitinib (EGFR inhibitor) | Breast cancers, Bladder cancers, Skin cancers, Lung cancers, Liver cancers, Pancreatic cancers | 3-MA, Baf A1, CQ, HCQ | ATG12 siRNA, BECN1 siRNA, ATG7 siRNA, ATG5 siRNA, VPS34 siRNA, miR-153-3p (ATG5 knockdown) | [217,218,219,220,221,222,223,224,225,226,227,228] |
| Osimertinib (EGFR inhibitor) | Lung cancers, Breast cancers, Colorectal cancers | Spautin-1, CQ, 3-MA | BECN1 siRNA, ATG5 siRNA | [229,230,231,232,233] |
| Erlotinib (EGFR inhibitor) | Lung cancers, Glioblastomas, Head and neck cancers | l CQ, 3-MA, Quinacrine | ATG5 siRNA, ATG7 siRNA, BECN1 siRNA, LC3 siRNA | [228,234,235,236,237,238,239] |
| Everolimus (mTORC1 (mammalian target of rapamycin complex 1) inhibitor) | Blood cancers, Kidney cancers, Bladder cancers, Breast cancer, Neuroendocrine tumors | HCQ, CQ, Baf A1, 3-MA | - | [240,241,242,243,244,245,246,247] |
| Temsirolimus (mTORC1 inhibitor) | Colorectal cancers, Skin cancers, Kidney cancers | CQ, HCQ | ATG7 shRNA | [248,249,250] |
| Dactolisib/NVP-BEZ235 (Dual mTOR/Class I PI3K (phosphoinositide 3-kinase) inhibitor) | Kidney cancers, Liver cancers, Blood cancers, Colorectal cancers, Head and neck cancers, Gliomas, Mesotheliomas, Gastric cancers, Rhabdomyosarcomas, Neuroblastomas, Lung cancers | 3-MA, CQ, Baf A1, VPS34 inhibitor (VPS34-IN1) | ATG5 siRNA, BECN1 siRNA | [251,252,253,254,255,256,257,258,259,260] |
| Buparlisib/BKM120 (Pan-Class I PI3K inhibitor) | Lung cancers | CQ | - | [261] |
| Pictilisib/GDC-0941 (Pan-Class I PI3K inhibitor) | Breast cancers | CQ | ATG5 siRNA, ATG7 siRNA | [262] |
| Lapatinib (Dual EGFR/HER2 inhibitor) | Breast cancers, Esophageal cancers | CQ, 3-MA | ATG12 shRNA, ATG5 siRNA, BECN1 siRNA | [200,263,264,265] |
| Afatinib (EGFR/HER2/HER4 inhibitor) | Lung cancers | HCQ, 3-MA | - | [266] |
| Bortezomib (Proteasome inhibitor) | Breast cancers, Blood cancers, Pancreatic cancers, Cervical cancers, Prostate cancers, Neuroblastomas | 3-MA, HCQ, CQ, Baf A1 | LC3B siRNA, ATG5 siRNA, P62 shRNA, GABARAPL1 shRNA, BECN1 siRNA | [267,268,269,270,271,272,273,274,275,276] |
| Carfilzomib (Proteasome inhibitor) | Blood cancers, Neuroblastomas | CQ, HCQ | - | [277,278,279] |
| Vemurafenib (B-RAF inhibitor) | Brain cancers, Skin cancers, Thyroid cancers, Colorectal cancers | CQ, HCQ, Baf A1, Lys05 | ATG5 siRNA, ATG5 shRNA, ATG7 siRNA, ATG13 siRNA | [280,281,282,283,284,285,286] |
| Trametinib (MEK1/2 (mitogen-activated protein kinase kinase 1/ 2) inhibitor) | Skin cancers, Pancreatic cancers | CQ, HCQ, PIK-III | Inactive dominant-negative ATG4BC74A | [287,288] |
| AKTi-1/2 (Dual AKT 1/2 (Protein kinase B 1/ 2) inhibitor) | Ovarian cancers, Liver cancers, Prostate cancers | CQ, Spautin-1, 3-MA, Baf A1 | ATG7 siRNA, ATG5 siRNA, BECN1 siRNA, BECN1 shRNA | [289,290,291] |
| AZD5363 (AKT 1/2/3 inhibitor) | Prostate cancers | CQ, 3-MA, Baf A1 | ATG3 siRNA, ATG7 siRNA | [292] |
| Tamoxifen (ER (estrogen receptor) inhibitor; anti-estrogenic analog) | Breast cancers | HCQ, Baf A1, 3-MA | ATG7 siRNA, BECN1 siRNA, LAMP3 shRNA, LC3B siRNA, ATG5 siRNA, BECN1 siRNA | [293,294,295,296,297,298,299] |
| Imatinib (BCR-ABL (breakpoint cluster region-abelson murine leukemia viral oncogene homolog 1) inhibitor) | Blood cancers, Gastrointestinal cancers, Colorectal cancers | Spautin-1, CQ, HCQ, Quinacrine, Vincristine | ATG7 siRNA, ATG12 siRNA, ATG4B shRNA, BECN1 shRNA, ATG5 shRNA, miR-30a (BECN1 and ATG5 knockdown) | [73,300,301,302,303,304,305,306] |
| HDIL-2 (high-dose interleukin-2 immunotherapy) | Liver cancers | CQ | - | [307,308] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.J.; Gorski, S.M. Molecular Mechanisms Underlying Autophagy-Mediated Treatment Resistance in Cancer. Cancers 2019, 11, 1775. https://doi.org/10.3390/cancers11111775
Ho CJ, Gorski SM. Molecular Mechanisms Underlying Autophagy-Mediated Treatment Resistance in Cancer. Cancers. 2019; 11(11):1775. https://doi.org/10.3390/cancers11111775
Chicago/Turabian StyleHo, Cally J., and Sharon M. Gorski. 2019. "Molecular Mechanisms Underlying Autophagy-Mediated Treatment Resistance in Cancer" Cancers 11, no. 11: 1775. https://doi.org/10.3390/cancers11111775
APA StyleHo, C. J., & Gorski, S. M. (2019). Molecular Mechanisms Underlying Autophagy-Mediated Treatment Resistance in Cancer. Cancers, 11(11), 1775. https://doi.org/10.3390/cancers11111775




