Mutation Induction in Humans and Mice: Where Are We Now?
Abstract
:1. Introduction
2. Mutation Induction and Transgenerational Effects
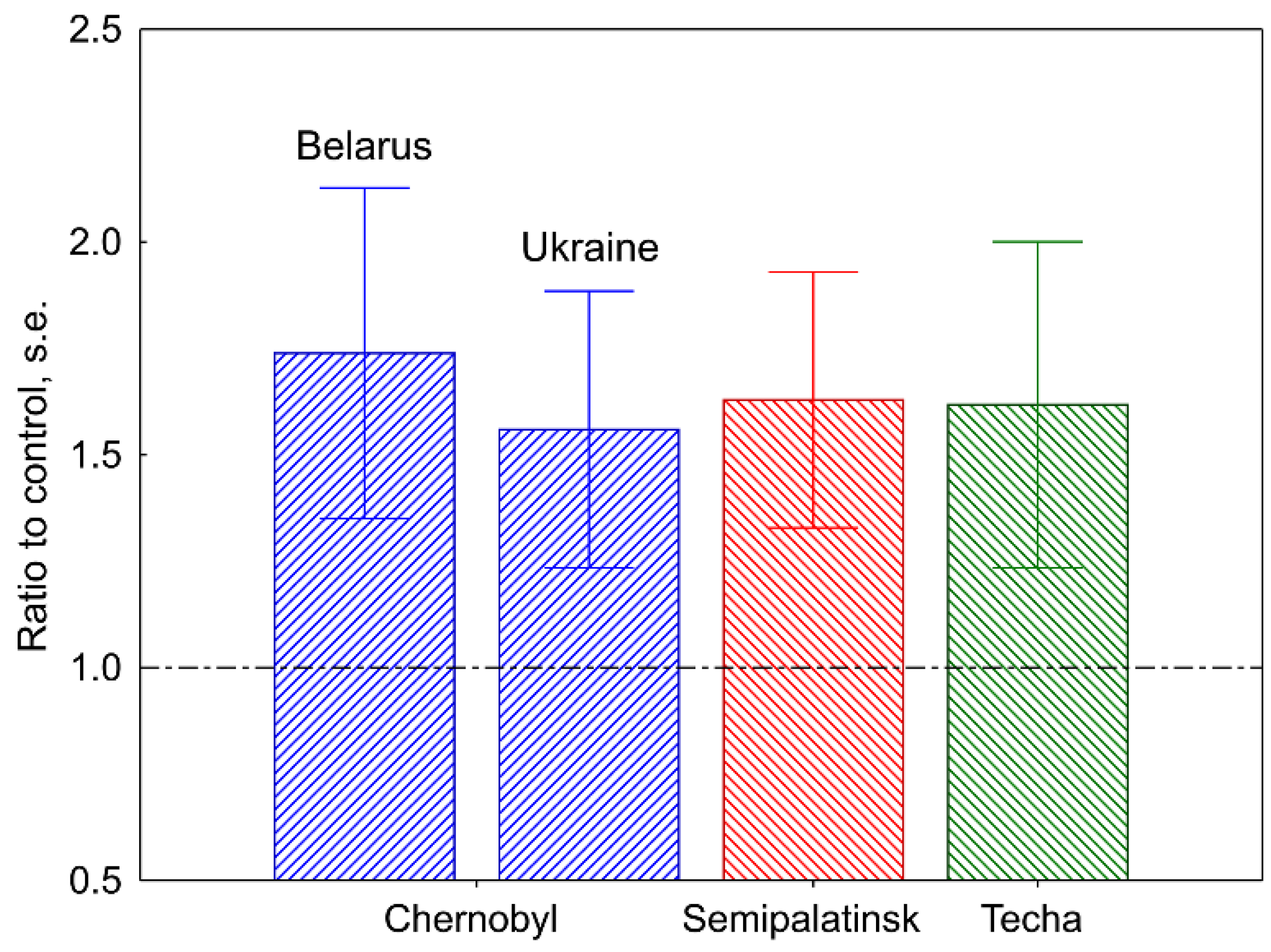
2.1. Mutation Induction in the Human and Mouse Germline
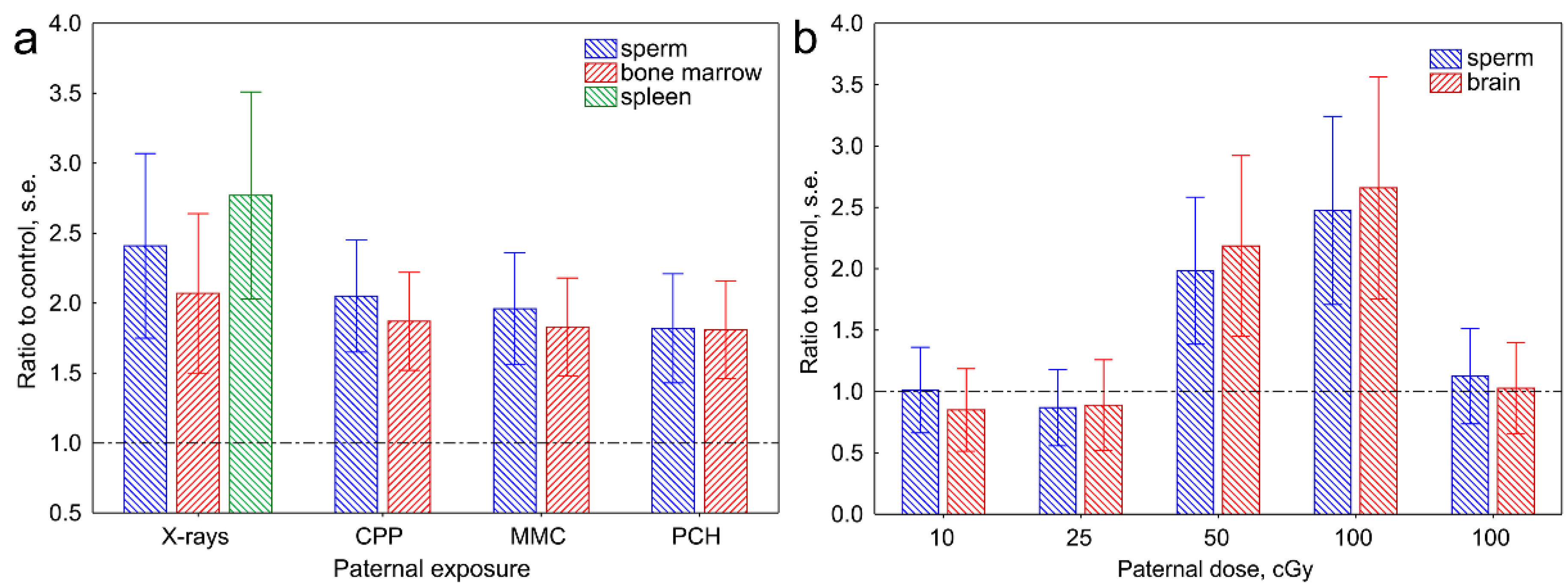
2.2. Transgenerational Effects of Parental Exposure to Mutagens
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- UNSCEAR. Hereditary Effects of Radiation; United Nations: New York, NY, USA, 2001; ISBN 92-1-142244-2. [Google Scholar]
- Nakamura, N.; Suyama, A.; Noda, A.; Kodama, Y. Radiation effects on human heredity. Ann. Rev. Genet. 2013, 47, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Speicher, M.R.; Antonarakis, S.E.; Motulsky, A.G. (Eds.) Vogel and Motulsky’s Human Genetics. Problems and Approaches, 4th ed.; Springer: Berlin, Germany, 2009; ISBN 978-3-540-37654-5. [Google Scholar]
- Neel, J.V.; Schull, W.J.; Awa, A.A.; Satoh, C.; Otake, M.; Kato, H.; Yoshimoto, Y. The children of parents exposed to atomic bombs: Estimates of the genetic doubling dose of radiation for humans. Am. J. Hum. Genet. 1990, 46, 1053–1072. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; Mulvihill, J.J.; Green, D.M.; Munro, H.M.; Stovall, M.; Weathers, R.E.; Mertens, A.C.; Whitton, J.A.; Robison, L.L.; Boice, J.D., Jr. Congenital anomalies in the children of cancer survivors: A report from the childhood cancer survivor study. J. Clin. Oncol. 2012, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, A.J.; Royle, N.J.; Wilson, V.; Wong, Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature 1988, 332, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Vergnaud, G.; Denoeud, F. Minisatellites: Mutability and genome architecture. Genome Res. 2000, 10, 899–907. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Nesterov, V.N.; Krouchinsky, N.G.; Ostapenko, V.A.; Neumann, R.; Neil, D.L.; Jeffreys, A.J. Human minisatellite mutation rate after the Chernobyl accident. Nature 1996, 380, 683–686. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Nesterov, V.N.; Krouchinsky, N.G.; Ostapenko, V.A.; Vergnaud, G.; Giraudeau, F.; Buard, J.; Jeffreys, A.J. Further evidence for elevated human minisatellite mutation rate in Belarus eight years after the Chernobyl accident. Mutat. Res. 1997, 381, 267–278. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Grant, G.; Chumak, A.A.; Stezhka, V.A.; Karakasian, A.N. Elevated minisatellite mutation rate in the post-Chernobyl families from Ukraine. Am. J. Hum. Genet. 2002, 71, 801–809. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Bersimbaev, R.I.; Djansugurova, L.B.; Tankimanova, M.K.; Mamyrbaeva, Z.Z.; Mustonen, R.; Lindholm, C.; Hultén, M.; Salomaa, S. Nuclear weapons tests and human germline mutation rate. Science 2002, 295, 1037. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Ploshchanskaya, O.G.; Kozionova, O.S.; Akleyev, A.V. Minisatellite germline mutation rate in the Techa River population. Mutat. Res. 2006, 602, 74–82. [Google Scholar] [CrossRef]
- Kodaira, M.; Izumi, S.; Takahashi, N.; Nakamura, N. No evidence of radiation effect on mutation rates at hypervariable minisatellite loci in the germ cells of atomic bomb survivors. Radiat. Res. 2004, 162, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, A.; Auvinen, A.; Luokkamäki, M.; Makkonen, K.; Veidebaum, T.; Tekkel, M.; Rahu, M.; Hakulinen, T.; Servomaa, K.; Rytömaa, T.; et al. Hereditary minisatellite mutations among the offspring of Estonian Chernobyl cleanup workers. Radiat. Res. 2003, 159, 651–655. [Google Scholar] [CrossRef]
- Livshits, L.A.; Malyarchuk, S.G.; Kravchenko, S.A.; Matsuka, G.H.; Lukyanova, E.M.; Antipkin, Y.G.; Arabskaya, L.P.; Petit, E.; Giraideau, F.; Vergnaud, G.; et al. Children of Chernobyl cleanup workers do not show elevated rates of mutations in minisatellite alleles. Radiat. Res. 2001, 155, 74–80. [Google Scholar] [CrossRef]
- May, C.A.; Tamaki, K.; Neumann, R.; Wilson, G.; Zagars, G.; Pollack, A.; Dubrova, Y.E.; Jeffreys, A.J.; Meistrich, M.L. Minisatellite mutation frequency in human sperm following radiotherapy. Mutat. Res. 2000, 453, 67–75. [Google Scholar] [CrossRef]
- Tawn, E.J.; Rees, G.S.; Leith, C.; Winther, J.F.; Curwen, G.B.; Stovall, M.; Olsen, J.H.; Rechnitzer, C.; Schroeder, H.; Guldberg, P.; et al. Germline minisatellite mutations in survivors of childhood and young adult cancer treated with radiation. Int. J. Radiat. Biol. 2011, 87, 330–340. [Google Scholar] [CrossRef]
- Tawn, E.J.; Curwen, G.B.; Rees, G.S.; Jonas, P. Germline minisatellite mutations in workers occupationally exposed to radiation at the Sellafield nuclear facility. J. Radiol. Prot. 2015, 35, 21–36. [Google Scholar] [CrossRef]
- Dubrova, Y.E. Germline mutation induction at human minisatellite loci. Swiss Med. Wkly. 2003, 133, 474–478. [Google Scholar]
- Bois, P.; Williamson, J.; Brown, J.; Dubrova, Y.E.; Jeffreys, A.J. A novel unstable mouse VNTR family expanded from SINE B1 element. Genomics 1998, 49, 122–128. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Jeffreys, A.J.; Malashenko, A.M. Mouse minisatellite mutations induced by ionizing radiation. Nat. Genet. 1993, 5, 92–94. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Plumb, M.; Brown, J.; Fennelly, J.; Bois, P.; Goodhead, D.; Jeffreys, A.J. Stage specificity, dose response, and doubling dose for mouse minisatellite germ-line mutation induced by acute radiation. Proc. Natl. Acad. Sci. USA 1998, 95, 6251–6255. [Google Scholar] [CrossRef] [Green Version]
- Dubrova, Y.E.; Plumb, M.; Brown, J.; Boulton, E.; Goodhead, D.; Jeffreys, A.J. Induction of minisatellite mutations in the mouse germline by low-dose chronic exposure to γ-radiation and fission neutrons. Mutat. Res. 2000, 453, 17–24. [Google Scholar] [CrossRef]
- Barber, R.C.; Hardwick, R.J.; Shanks, M.E.; Glen, C.D.; Mughal, S.K.; Voutounou, M.; Dubrova, Y.E. The effects of in utero irradiation on mutation induction and transgenerational instability in mice. Mutat. Res. 2009, 664, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.E.A.; Barber, R.C.; Dubrova, Y.E. The effects of maternal irradiation during adulthood on mutation induction and transgenerational instability in mice. Mutat. Res. 2012, 732, 21–25. [Google Scholar] [CrossRef]
- Vilarino-Guell, C.; Smith, A.G.; Dubrova, Y.E. Germline mutation induction at mouse repeat DNA loci by chemical mutagens. Mutat. Res. 2003, 526, 63–73. [Google Scholar] [CrossRef]
- Barber, R.; Plumb, M.A.; Smith, A.G.; Cesar, C.E.; Boulton, E.; Jeffreys, A.J.; Dubrova, Y.E. No correlation between germline mutation at repeat DNA and meiotic crossover in male mice exposed to X-rays or cisplatin. Mutat. Res. 2000, 457, 79–91. [Google Scholar] [CrossRef]
- Glen, C.D.; Smith, A.G.; Dubrova, Y.E. Single-molecule PCR analysis of germ line mutation induction by anticancer drugs in mice. Cancer Res. 2008, 68, 3630–3636. [Google Scholar] [CrossRef]
- Wilson, J.W.; Haines, J.; Sienkiewicz, Z.; Dubrova, Y.E. The effects of extremely low frequency magnetic fields on mutation induction in mice. Mutat. Res. 2015, 773, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Witt, K.L.; Bishop, J.B. Mutagenicity of anticancer drugs in mammalian germ cells. Mutat. Res. 1996, 355, 209–234. [Google Scholar] [CrossRef]
- Yauk, C.L.; Berndt, M.L.; Williams, A.; Rowan-Carroll, A.; Douglas, G.R.; Stämpfli, MR. Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res. 2007, 67, 5103–5106. [Google Scholar] [CrossRef]
- Somers, C.M.; Yauk, C.L.; White, P.A.; Parfett, C.L.; Quinn, J.S. Air pollution induces heritable DNA mutations. Proc. Natl. Acad. Sci. USA 2002, 99, 15904–15907. [Google Scholar] [CrossRef] [Green Version]
- Somers, C.M.; McCarry, B.E.; Malek, F.; Quinn, J.S. Reduction of particulate air pollution lowers the risk of heritable mutations in mice. Science 2004, 304, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Yauk, C.; Polyzos, A.; Rowan-Carroll, A.; Somers, C.M.; Godschalk, R.W.; Van Schooten, F.J.; Berndt, M.L.; Pogribny, I.P.; Koturbash, I.; Williams, A.; et al. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc. Natl. Acad. Sci. USA 2008, 105, 605–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, R.C.; Miccoli, L.; van Buul, P.P.W.; Burr, K.L.-A.; van Duyn-Goedhart, A.; Angulo, J.F.; Dubrova, Y.E. Germline mutation rates at tandem repeat loci in DNA-repair deficient mice. Mutat. Res. 2004, 554, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Burr, K.L.-A.; Smith, A.G.; Dubrova, Y.E. p53 deficiency does not affect mutation rate in the mouse germline. Oncogene 2005, 24, 4315–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burr, K.L.-A.; van Duyn-Goedhart, A.; Hickenbotham, P.; Monger, K.; van Buul, P.P.W.; Dubrova, Y.E. The effects of MSH2 deficiency on spontaneous and radiation-induced mutation rates in the mouse germline. Mutat. Res. 2007, 617, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Miccoli, L.; Burr, K.L.-A.; Hickenbotham, P.; Friedberg, E.C.; Angulo, J.F.; Dubrova, Y.E. The combined effects of xeroderma pigmentosum C deficiency and mutagens on mutation rates in the mouse germline. Cancer Res. 2007, 67, 4695–4699. [Google Scholar] [CrossRef]
- Mughal, S.K.; Myazin, A.E.; Zhavoronkov, L.P.; Rubanovich, A.V.; Dubrova, Y.E. The dose and dose-rate effects of paternal irradiation on transgenerational instability in mice. A radiotherapy connection. PLoS ONE 2012, 7, e41300. [Google Scholar] [CrossRef]
- Jeffreys, A.J.; Neil, D.L.; Neumann, R. Repeat instability at human minisatellites arising from meiotic recombination. EMBO J. 1998, 17, 4147–4157. [Google Scholar] [CrossRef] [Green Version]
- Frankenberg-Schwager, M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat. Environ. Biophys. 1990, 290, 273–292. [Google Scholar] [CrossRef]
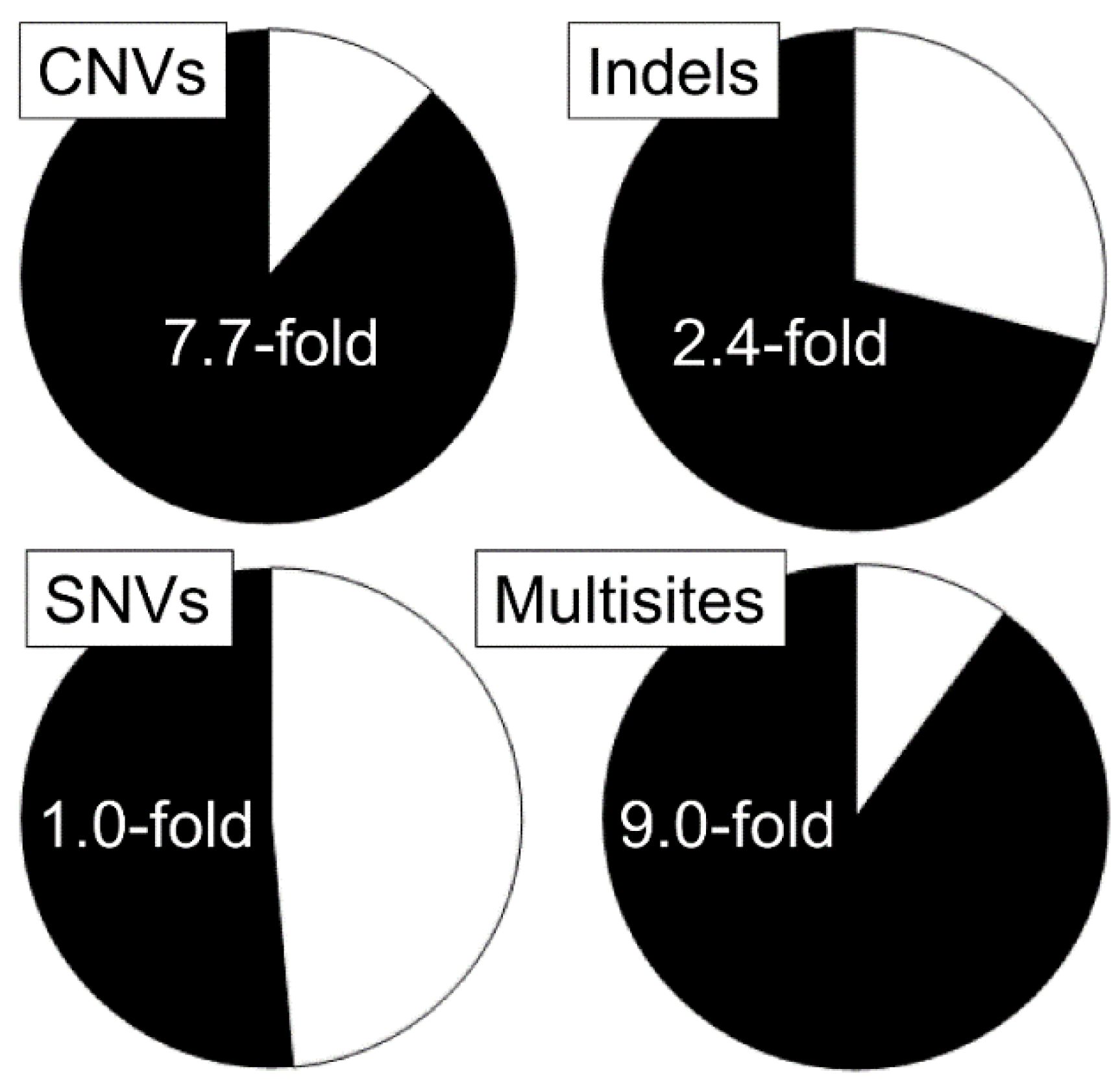
- Adewoye, A.B.; Lindsay, S.J.; Dubrova, Y.E.; Hurles, M.E. The genome-wide effects of ionising radiation on mutation induction in the mammalian germline. Nat. Commun. 2015, 6, 6684. [Google Scholar] [CrossRef]
- Georgakillas, A.G.; O’Neill, P.; Stewart, R. Induction and repair of clustered DNA lesions: What do we know so far? Radiat. Res. 2013, 180, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.A.; Meier, M.J.; Williams, A.; Rowan-Carroll, A.; Gagné, R.; Lindsay, S.J.; Fitzgerald, T.; Hurles, M.E.; Marchetti, F.; Yauk, C.L. Paternal exposure to benzo(a)pyrene induces genome-wide mutations in mouse offspring. Commun. Biol. 2019, 2, 228. [Google Scholar] [CrossRef] [PubMed]
- Jónsson, H.; Sulem, P.; Kehr, B.; Kristmundsdottir, S.; Zink, F.; Hjartarson, E.; Hardarson, M.T.; Hjorleifsson, K.E.; Eggertsson, H.P.; Gudjonsson, S.A.; et al. Parental influence on human germline de novo mutations in 1548 trios from Iceland. Nature 2017, 579, 519–522. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Lin, K.; Zhu, L.; Werling, D.M.; Dong, S.; Brand, H.; Wang, H.Z.; Zhao, X.; Schwartz, G.B.; Collins, R.L.; et al. Genome-wide de novo risk score implicates promoter variation in autism spectrum disorder. Science 2018, 362, eaat6576. [Google Scholar] [CrossRef]
- Kadhim, M.; Salomaa, S.; Wright, E.; Hildebrandt, G.; Belyakov, O.V.; Price, K.M.; Little, M.P. Non-targeted effects of ionising radiation—Implication for low dose risk. Mutat. Res. 2013, 752, 84–98. [Google Scholar] [CrossRef]
- Luning, K.G.; Frolen, H.; Nilsson, A. Genetic effects of 239Pu salt injections in male mice. Mutat. Res. 1976, 34, 539–542. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Plumb, M.; Gutierrez, B.; Boulton, E.; Jeffreys, A.J. Transgenerational mutation by radiation. Nature 2000, 405, 37. [Google Scholar] [CrossRef]
- Barber, R.; Plumb, M.A.; Boulton, E.; Roux, I.; Dubrova, Y.E. Elevated mutation rates in the germline of first-and second-generation offspring of irradiated male mice. Proc. Natl. Acad. Sci. USA 2002, 99, 6877–6882. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.C.; Hickenbotham, P.; Hatch, T.; Kelly, D.; Topchiy, N.; Almeida, G.; Jones, G.G.D.; Johnson, G.E.; Parry, J.M.; Rothkamm, K.; et al. Radiation-induced transgenerational alterations in genome stability and DNA damage. Oncogene 2006, 25, 7336–7342. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, K.; Shimura, T.; Taga, M.; Uematsu, N.; Gondo, Y.; Ohtaki, M.; Kominami, R.; Niwa, O. Persistent induction of somatic reversions of the pink-eyed unstable mutation in F-1 mice born to fathers irradiated at the spermatozoa stage. Radiat. Res. 2002, 157, 661–667. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Hickenbotham, P.; Glen, C.D.; Monger, K.; Wong, H.-P.; Barber, R.C. Paternal exposure to ethylnitrosourea results in transgenerational genomic instability in mice. Environ. Mol. Mutagen. 2008, 49, 308–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glen, C.D.; Dubrova, Y.E. Exposure to anticancer drugs can result in transgenerational genomic instability in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 2984–2988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorobtsova, I.E.; Aliyakparova, L.M.; Anisimov, V.N. Promotion of skin tumors by 12-O-tetradecanoylphorbol-13-acetate in two generations of descendants of male mice exposed to X-ray irradiation. Mutat. Res. 1993, 287, 207–216. [Google Scholar] [CrossRef]
- Lord, B.I.; Woolford, L.B.; Wang, L.; Stones, V.A.; McDonald, D.; Lorimore, S.A.; Papworth, D.; Wright, E.G.; Scott, D. Tumour induction by methyl-nitroso-urea following preconceptional paternal contamination with plutonium-239. Br. J. Cancer 1998, 78, 301–311. [Google Scholar] [CrossRef]
- Aghajanyan, A.; Kuzmina, N.; Sipyagyna, A.; Baleva, L.; Suskov, I. Analysis of genomic instability in the offspring of fathers exposed to low doses of ionizing radiation. Environ. Mol. Mutagen. 2011, 52, 538–546. [Google Scholar] [CrossRef]
- Tawn, E.J.; Whitehouse, C.A.; Winther, J.F.; Curwen, G.B.; Rees, G.S.; Stovall, M.; Olsen, J.H.; Guldberg, P.; Rechnitzer, C.; Schrøder, H.; et al. Chromosomal analysis in childhood cancer survivors and their offspring—No evidence for radiotherapy-induced persistent genomic instability. Mutat. Res. 2005, 583, 198–206. [Google Scholar] [CrossRef]
| Mutagen, Reference | Strains | Doses | Results 1 |
|---|---|---|---|
| Ionizing radiation: | |||
| Acute X-rays [22] | CBA/H | 0.5–1 Gy | + |
| Acute γ-rays [39] | BALB/c | 0.1–1 Gy | + |
| Chronic γ-rays [23,39] | CBA/H | 0.5–1 Gy | + |
| Fission neutrons [23] | CBA/H | + | |
| Anti-cancer drugs: | |||
| Cisplatin [27] | F1(C57BL/6JxCBA/Ca) | 10 mg.kg | - |
| Bleomycin [28] | F1(C57BL/6JxCBA/Ca) | 15–30 mg/kg | + |
| Cyclophosphamide [28] | F1(C57BL/6JxCBA/Ca) | 40–80 mg/kg | + |
| Mitomycin C [28] | F1(C57BL/6JxCBA/Ca) | 2.5–5 mg/kg | + |
| Procarbazine [28] | F1(C57BL/6JxCBA/Ca) | 50–100 mg/kg | + |
| Etoposide [26] | CBA/Ca | 80 mg/kg | + |
| Chemical mutagens: | |||
| Ethylnitrosourea [26] | CBA/Ca | 12.5–75 mg/kg | + |
| Isopropyl methanesulfonate [26] | CBA/Ca | 12.5–37.5 mg/kg | + |
| Extremely low frequency magnetic fields [29] | F1(BALB/cxCBA/Ca) | 10–300 μT | - |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubrova, Y. Mutation Induction in Humans and Mice: Where Are We Now? Cancers 2019, 11, 1708. https://doi.org/10.3390/cancers11111708
Dubrova Y. Mutation Induction in Humans and Mice: Where Are We Now? Cancers. 2019; 11(11):1708. https://doi.org/10.3390/cancers11111708
Chicago/Turabian StyleDubrova, Yuri. 2019. "Mutation Induction in Humans and Mice: Where Are We Now?" Cancers 11, no. 11: 1708. https://doi.org/10.3390/cancers11111708
APA StyleDubrova, Y. (2019). Mutation Induction in Humans and Mice: Where Are We Now? Cancers, 11(11), 1708. https://doi.org/10.3390/cancers11111708





