Size Matters in the Cytotoxicity of Polydopamine Nanoparticles in Different Types of Tumors
Abstract
:1. Introduction
2. Results
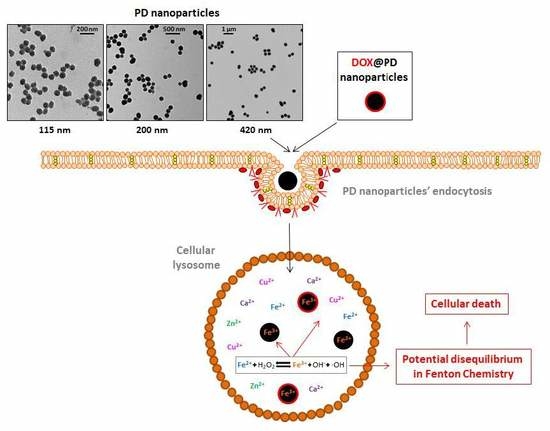
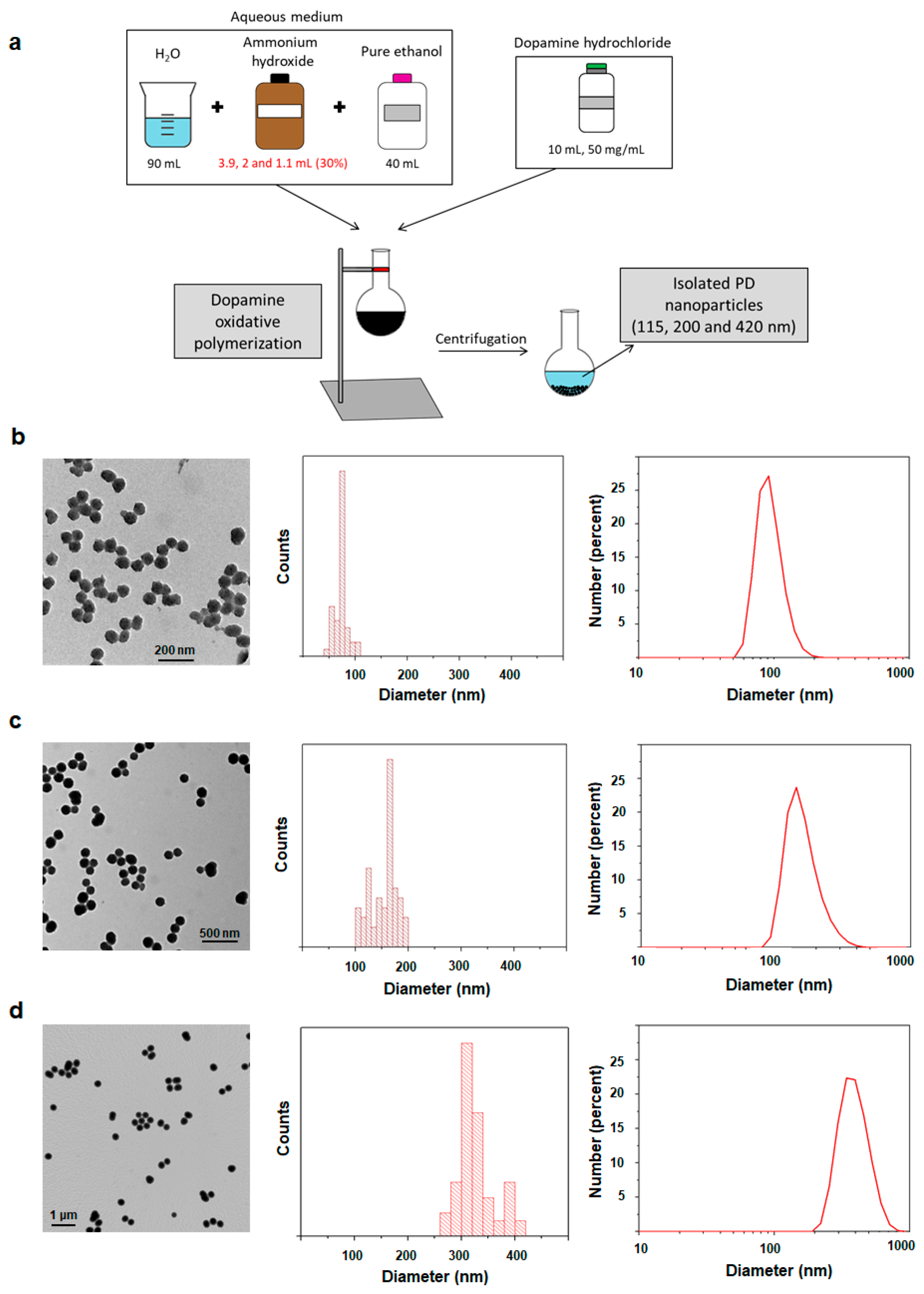
2.1. Synthesis and Characterization of 115, 200 and 420 nm PD NPs
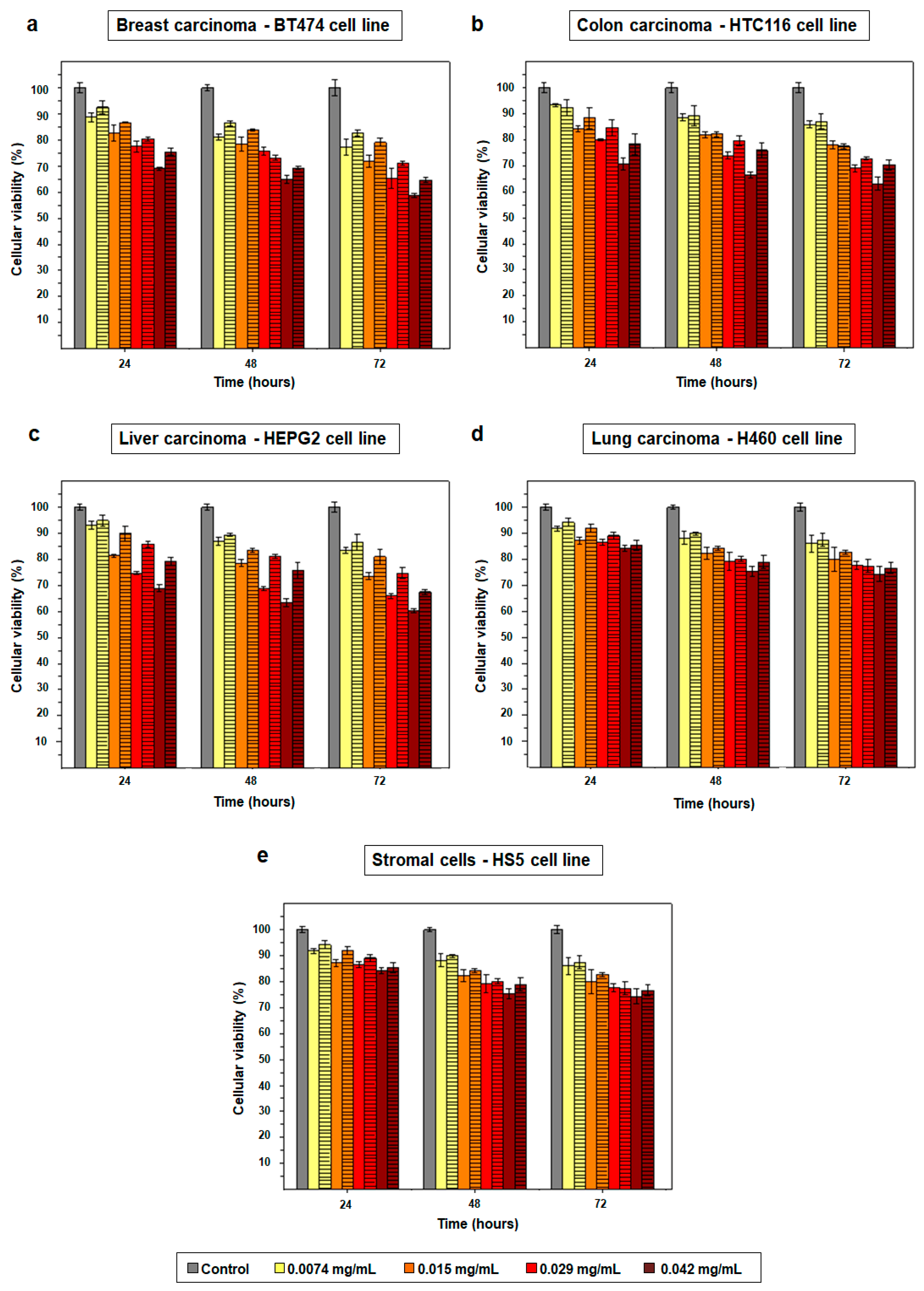
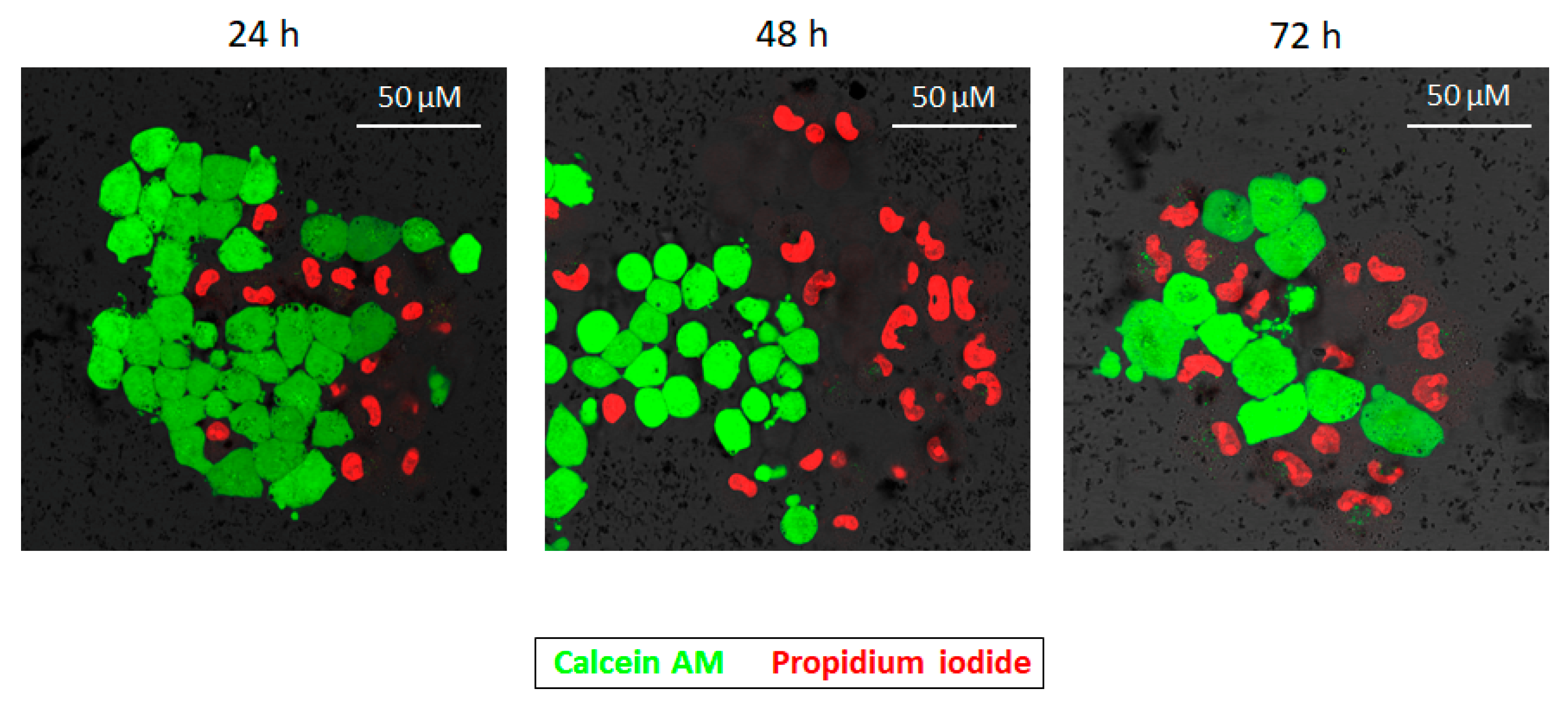
2.2. Cytotoxicity Effect of PD NPs Depends on Their Size
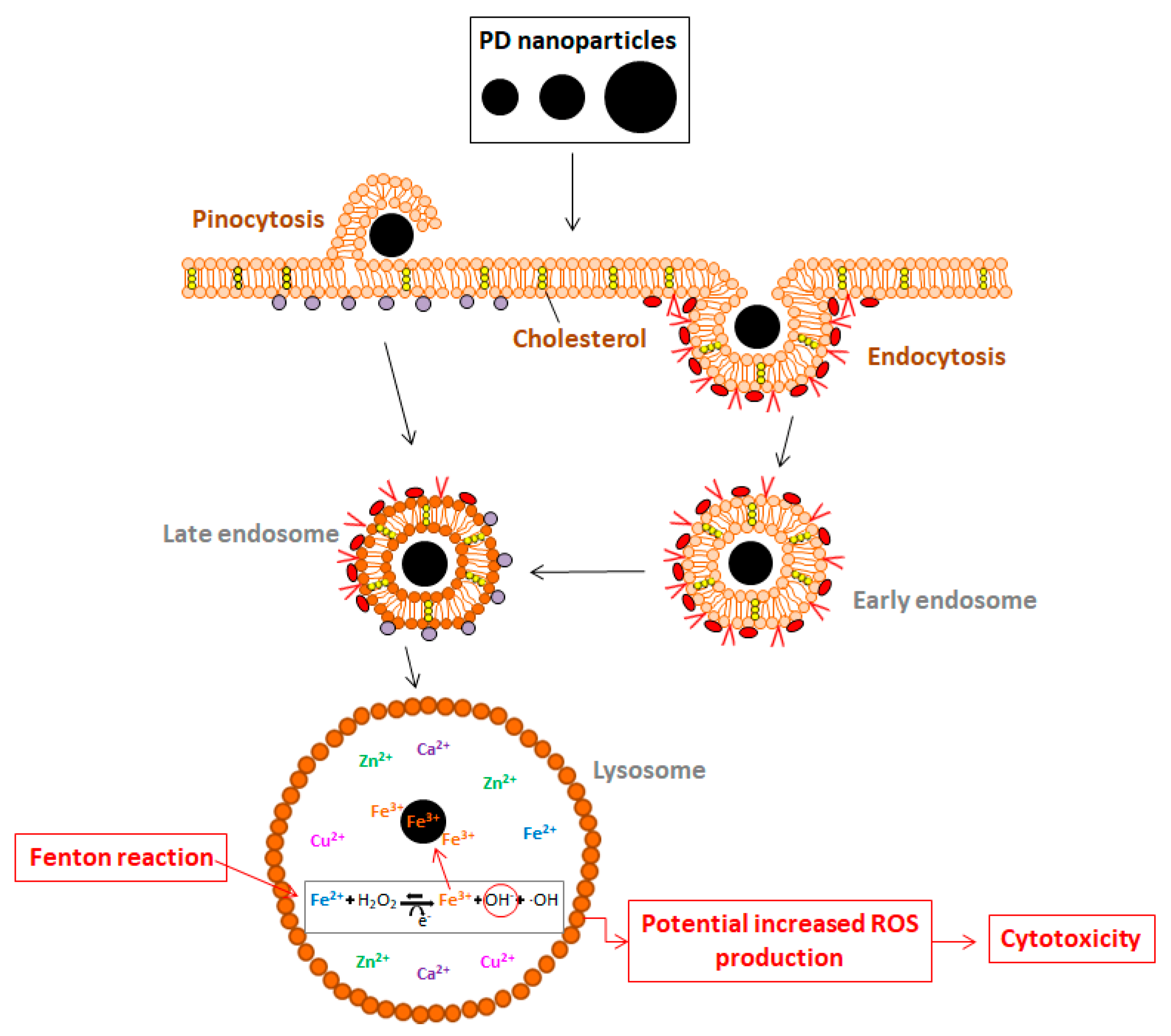
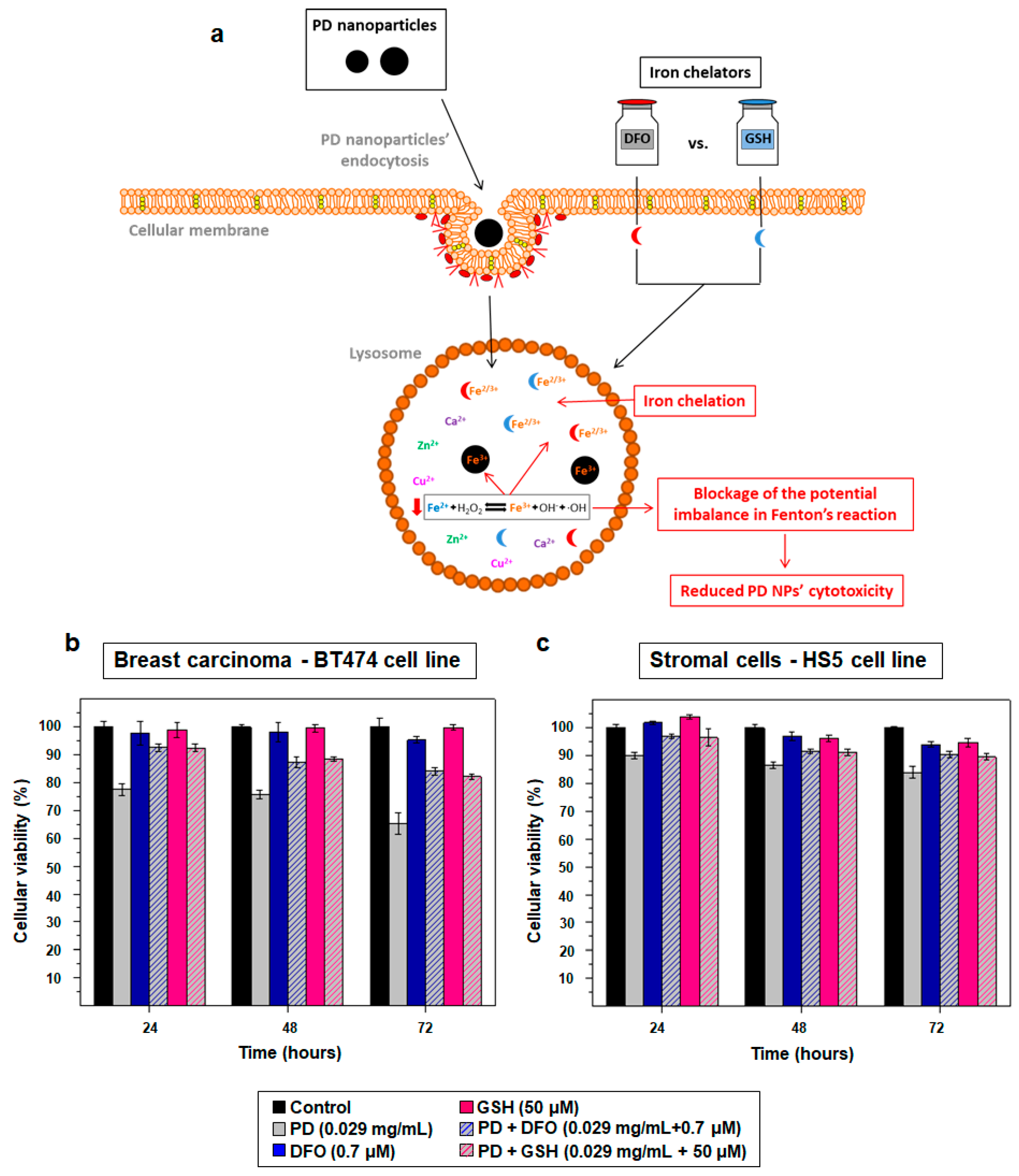
2.3. PD NPs Cytotoxicity in Tumor Cells Could be Related to Their Iron Affinity
2.4. DOX-Adsorbed PD NPs (DOX@PD NPs) Presented a Notable Antiproliferation Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis and Characterization of PD NPs of Different Sizes
4.3. Cell Culture
4.4. Size-Dependent Cytotoxicity Effect of PD NPs
4.5. PD Iron-Affinity could Explain the Cytotoxicity of PD NPs in Tumor Cells
4.6. DOX Adsorption onto PD NPs
4.7. Antiproliferative Activity of DOX@PD NPs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Awasthi, R.; Roseblade, A.; Hansbro, P.M.; Rathbone, M.J.; Dua, K.; Bebawy, M. Nanoparticles in Cancer Treatment: Opportunities and Obstacles. Curr. Drug Targets 2018, 19, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, Q.; Feng, L.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhacement. Nano Today 2018, 21, 55–73. [Google Scholar] [CrossRef]
- Quader, S.; Kataoka, K. Nanomaterial-enabled cancer therapy. Mol. Ther. 2017, 25, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, J.; Zhang, T.; Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 2018, 286, 64–73. [Google Scholar] [CrossRef]
- Wu, H.; Hu, H.; Wan, J.; Li, Y.; Wu, Y.; Tang, Y.; Xiao, C.; Xu, H.; Yang, X.; Li, Z. Hydroxyethyl starch stabilized polydopamine nanoparticles for cancer chemotherapy. Chem. Eng. J. 2018, 349, 129–145. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging and antimicrobial activities. Acta Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-inspired polydopamine: A biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borestein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Gong, H.; Gao, M.; Zhu, W.; Sun, X.; Feng, L.; Fu, T.; Li, Y.; Liu, Z. Polydopamine nanoparticles as a versatile molecular loading platform to enable imaging-guided cancer combination therapy. Theranostics 2016, 6, 1031–1042. [Google Scholar] [CrossRef]
- Mrowczynski, R. Polydopamine-based multifunctional (nano)materials for cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 7541–7561. [Google Scholar] [CrossRef]
- Wang, L.; Dai, W.; Yang, M.; Wei, X.; Ma, K.; Song, B.; Jia, P.; Gong, Y.; Yang, J.; Zhao, J. Cell membrane mimetic copolymer coated polydopamine nanoparticles for combined pH-sensitive drug release and near-infrared photothermal therapeutic. Colloids Surf. B Biointerfaces 2019, 176, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Mengying, Z.; Dongdong, B.; Xiaoyu, Q.; Yifei, G.; Xiangtao, W.; Meihua, H. Alendronate-modified polydopamine-coated paclitaxel nanoparticles for osteosarcoma-targeted therapy. J. Drug Deliv. Sci. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Huang, R.; Cang, H.; Cai, Z.; Sun, B. pH-sensitive prodrug conjugated polydopamine for NIR-triggered synergistic chemo-photothermal therapy. Eur. J. Pharm. Biopharm. 2018, 128, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, Y.; Hou, S.; Upputuri, P.K.; Wu, D.; Li, J.; Wang, P.; Zhen, X.; Pramanik, M.; Pu, K.; et al. Compact plasmonic blackbody for cancer theranosis in the near-infrared II window. ACS Nano 2018, 12, 2643–2651. [Google Scholar] [CrossRef]
- Kim, S.E.; Zhang, L.; Ma, K.; Riegman, M.; Chen, F.; Ingold, I.; Conrad, M.; Turker, M.Z.; Gao, M.; Jiang, X.; et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 2016, 11, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread. Sci. Rep. 2019, 9, 9280–9289. [Google Scholar] [CrossRef]
- Vega, M.A.; Nieto, C.; Marcelo, G.; Martín del Valle, E.M. Cytotoxicity of paramagnetic cations-loaded polydopamine nanoparticles. Colloids Surf. B Biointerfaces 2018, 167, 284–290. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Martín del Valle, E.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; He, K.; Song, H.; Ma, Z.; Yin, W.; Xu, L.X. Deferoxamine-induced increase in the cellular iron levels in highly aggressive breast cancer cells leads to increased cell migration by enhancing TFN-α dependent NF-κB signaling and TGF-β signaling. J. Inorg. Biochem. 2016, 160, 40–48. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Ball, V.; Bour, J.; Michel, M. Step-by-step deposition of synthetic dopamine-eumelanin and metal cations. J. Colloid Interface Sci. 2013, 405, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, W.; Kim, D.H. Silica-coated metal chelting-melanin nanoparticles as a dual-modal contrast enhacement imaging and therapeutic agent. ACS Appl. Mater. Interfaces 2017, 9, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Lin, M.; Li, X.; Liu, S.; Wang, W.; Li, S.; Zhang, Z.; Liu, Y.; Liu, L.; Shi, F.; et al. Cu2+-loaded polydopamine nanoparticles for magnetic resonance imaging-guided pH- and near-infrared-light-stimulated thermochemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 19706–19716. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Eaton, J.W.; Brunk, U.T. The role of lysosomes in iron metabolism and recycling. Int. J. Biochem. Cell. Biol. 2011, 43, 1686–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.Z.; Yang, Y.; Sun, X.; Dong, X.-P. Methods for monitoring Ca2+ and ion channels in the lysosome. Cell Calcium 2017, 64, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Jung., M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a central player and promising target in cancer progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and cancer: Recent insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef]
- Gaument, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge and shape determine therapeutic effects of nanoparticles in brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Hamai, A.; Hienzsch, A.; Cañeque, T.; Müller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin kills cancer stem cells by sequestering iron in lysososmes. Nat. Chem. 2017, 9, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Corcé, V.; Gouin, S.G.; Renaud, S.; Gaboriau, F.; Deniaud, D. Recent advances in cancer treatment by iron chelators. Bioorg. Med. Chem. Lett. 2016, 26, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, H.; Yang, J.; Qiu, H.; Cheng, Y.; Liu, M. Pharmacokinetics and cardiotoxicity of doxorubicin and its secondary alcohol metabolites in rats. Biomed. Pharmacother. 2019, 116, 108964. [Google Scholar] [CrossRef]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized phospholipids in doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Boulos, L.; Prévost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD® BaclightTM: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 34, 77–86. [Google Scholar] [CrossRef]
- Li, Y.; Khuu, N.; Gevorkian, A.; Sarfinsky, S.; Therien-Aubin, H.; Wang, Y.; Cho, S.; Kumacheva, E. Supramolecular nanofibrillar thermoreversible hydrogel for growth and release of cancer spheroids. Angew. Chem. Int. 2016, 55, 1–6. [Google Scholar]
- Chung, S.; Nguyen, V.; Lin, Y.L.; Kamen, L.; Song, A. Thaw-and-use target cells pre-labeled with calcein AM for antibody-dependent cell-mediated cytotoxicity assays. J. Immunol. Methods 2017, 447, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhu, X.; Wang, Y.; Bingyang, S.; Ling, X.; Chen, H.; Nan, W.; Barrett, A.; Guo, Z.; Tao, W.; et al. Intracellular fate of nanoparticles with polydopamine surface engineering and a novel strategy for exocytosis-inhibiting, lysosome impairment-based cancer therapy. Nano Lett. 2017, 17, 8790–8801. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, S.; Zahavy, E.; Wrasdilo, W.; Berns, M.; Chan, M.; Esener, S. A novel doxorubicin prodrug with controllable photolysis activation for cancer therapy. Pharm. Res. 2010, 27, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wan, Y.; Wang, Y.; Zhang, H.; Jiao, Z. Anticancer efficacy enhacement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int. J. Nanomed. 2011, 6, 2321–2326. [Google Scholar]
- Bi, D.; Zhao, L.; Li, H.; Guo, Y.; Wang, X.; Han, M. A comparative study of polydopamine modified and conventional chemical synthesis method in DOX liposomes form the aspect of tumor targeted therapy. Int. J. Pharm. 2019, 559, 76–85. [Google Scholar] [CrossRef]
- Ji, F.; Sun, H.; Qin, Z.; Zhang, E.; Cui, J.; Wang, J.; Li, S.; Yao, F. Engineering polyzwitterion and polydopamine decorated doxorubicin-loaded mesoporous silica nanoparticles as a pH-sensitive drug delivery. Polymers 2018, 10, 326. [Google Scholar] [CrossRef]
- Win, K.-Y.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, C.; Vega, M.A.; Enrique, J.; Marcelo, G.; Martín del Valle, E.M. Size Matters in the Cytotoxicity of Polydopamine Nanoparticles in Different Types of Tumors. Cancers 2019, 11, 1679. https://doi.org/10.3390/cancers11111679
Nieto C, Vega MA, Enrique J, Marcelo G, Martín del Valle EM. Size Matters in the Cytotoxicity of Polydopamine Nanoparticles in Different Types of Tumors. Cancers. 2019; 11(11):1679. https://doi.org/10.3390/cancers11111679
Chicago/Turabian StyleNieto, Celia, Milena A. Vega, Jesús Enrique, Gema Marcelo, and Eva M. Martín del Valle. 2019. "Size Matters in the Cytotoxicity of Polydopamine Nanoparticles in Different Types of Tumors" Cancers 11, no. 11: 1679. https://doi.org/10.3390/cancers11111679
APA StyleNieto, C., Vega, M. A., Enrique, J., Marcelo, G., & Martín del Valle, E. M. (2019). Size Matters in the Cytotoxicity of Polydopamine Nanoparticles in Different Types of Tumors. Cancers, 11(11), 1679. https://doi.org/10.3390/cancers11111679