Radiomics Model Based on Non-Contrast CT Shows No Predictive Power for Complete Pathological Response in Locally Advanced Rectal Cancer
Abstract
1. Introduction
2. Results
2.1. Patients and Imaging
2.2. Machine Learning Classification
3. Discussion
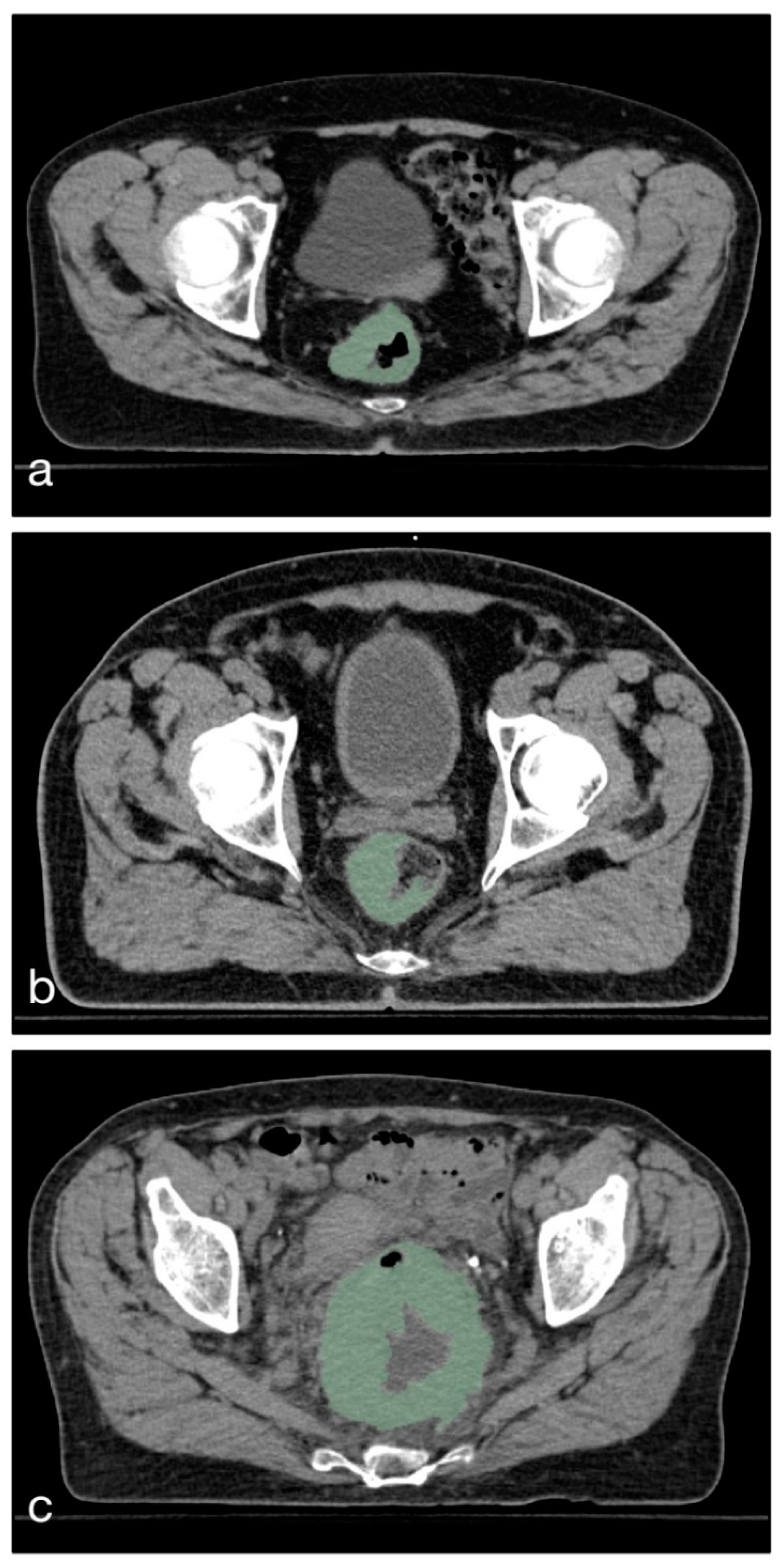
4. Materials and Methods
4.1. Ethical Statement
4.2. Dataset Composition
4.3. Algorithmic Modeling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Features |
|---|
| wavelet-HHH_firstorder_Skewness |
| wavelet-HHH_glszm_SizeZoneNonUniformityNormalized |
| lbp-3D-k_glszm_ZoneEntropy |
| wavelet-HHH_glrlm_HighGrayLevelRunEmphasis |
| wavelet-HHH_glszm_ZoneVariance |
| wavelet-HHH_glrlm_LowGrayLevelRunEmphasis |
| wavelet-LHH_firstorder_RootMeanSquared |
| log-sigma-2-0-mm-3D_glrlm_LongRunHighGrayLevelEmphasis |
| wavelet-HHL_glszm_ZoneVariance |
| wavelet-HHL_glszm_LargeAreaEmphasis |
| wavelet-HHL_gldm_DependenceVariance |
| original_shape_Maximum2DDiameterSlice |
| wavelet-LHH_firstorder_Mean |
| log-sigma-3-0-mm-3D_gldm_SmallDependenceLowGrayLevelEmphasis |
| log-sigma-2-0-mm-3D_firstorder_Skewness |
| wavelet-HHL_glszm_LargeAreaHighGrayLevelEmphasis |
| log-sigma-3-0-mm-3D_gldm_LargeDependenceHighGrayLevelEmphasis |
| log-sigma-2-0-mm-3D_glrlm_RunEntropy |
| wavelet-LHH_firstorder_Uniformity |
| original_shape_MinorAxisLength |
| wavelet-LHL_glszm_SmallAreaEmphasis |
| wavelet-HLL_glszm_LargeAreaHighGrayLevelEmphasis |
| wavelet-HLH_firstorder_Kurtosis |
| log-sigma-3-0-mm-3D_gldm_LowGrayLevelEmphasis |
| wavelet-LHH_firstorder_Median |
| wavelet-LHH_glrlm_HighGrayLevelRunEmphasis |
| wavelet-LHL_glszm_ZoneVariance |
| gradient_firstorder_Minimum |
| log-sigma-2-0-mm-3D_glszm_ZoneEntropy |
| original_glszm_LargeAreaHighGrayLevelEmphasis |
| log-sigma-2-0-mm-3D_gldm_LargeDependenceHighGrayLevelEmphasis |
| log-sigma-2-0-mm-3D_glrlm_ShortRunLowGrayLevelEmphasis |
| wavelet-HHL_glszm_SizeZoneNonUniformityNormalized |
| log-sigma-2-0-mm-3D_glrlm_HighGrayLevelRunEmphasis |
| wavelet-LHH_glrlm_LowGrayLevelRunEmphasis |
| wavelet-HHH_glszm_SmallAreaHighGrayLevelEmphasis |
| log-sigma-2-0-mm-3D_glcm_Autocorrelation |
| log-sigma-3-0-mm-3D_glrlm_HighGrayLevelRunEmphasis |
| log-sigma-2-0-mm-3D_gldm_HighGrayLevelEmphasis |
| log-sigma-5-0-mm-3D_glcm_ClusterShade |
| log-sigma-2-0-mm-3D_glcm_JointAverage |
| log-sigma-5-0-mm-3D_glszm_ZoneEntropy |
| log-sigma-5-0-mm-3D_gldm_LowGrayLevelEmphasis |
| original_glszm_ZoneEntropy |
| log-sigma-5-0-mm-3D_glcm_Idmn |
| log-sigma-3-0-mm-3D_glcm_Idmn |
| wavelet-LHH_glszm_ZoneVariance |
| original_shape_LeastAxisLength |
| wavelet-LLL_glcm_Imc2 |
| original_firstorder_10Percentile |
| wavelet-LHH_firstorder_Variance |
| wavelet-HHH_firstorder_Variance |
| wavelet-LLH_firstorder_Skewness |
| wavelet-LLH_glszm_SizeZoneNonUniformityNormalized |
| wavelet-LHH_gldm_GrayLevelVariance |
| original_glcm_Imc1 |
| log-sigma-1-0-mm-3D_glrlm_RunLengthNonUniformity |
| log-sigma-5-0-mm-3D_glrlm_LowGrayLevelRunEmphasis |
| wavelet-LLL_glszm_LargeAreaHighGrayLevelEmphasis |
| wavelet-HHH_glszm_SmallAreaLowGrayLevelEmphasis |
| log-sigma-5-0-mm-3D_gldm_LargeDependenceLowGrayLevelEmphasis |
| wavelet-LHH_firstorder_MeanAbsoluteDeviation |
| wavelet-LHH_glszm_LargeAreaEmphasis |
References
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/ professionals/physician_gls/default.aspx#site (accessed on 3 May 2019).
- German Guideline Program in Oncology Evidenced-based Guideline for Colorectal Cancer. Available online: https://www.awmf.org/fileadmin/user_upload/Leitlinien/021_D_Ges_fuer_Verdauungs-_und_Stoffwechselkrankheiten/021-007OLe_S3_Colorectal_Cancer_2019-01.pdf (accessed on 3 May 2019).
- Fokas, E.; Fokas, E.; Liersch, T.; Liersch, T.; Fietkau, R.; Fietkau, R.; Hohenberger, W.; Hohenberger, W.; Beissbarth, T.; Beissbarth, T.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef]
- Nelemans, J.; Marañón, G.; Madrid, S.; Glynne-Jones, R.; Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.-J.J.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar]
- Hartley, A.; Ho, K.F.; McConkey, C.; Geh, J.I. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: Analysis of phase II/III trials. Br. J. Radiol. 2005, 78, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.T.; Heneghan, H.M.; Winter, D.C. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br. J. Surg. 2012, 99, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Dossa, F.; Chesney, T.R.; Acuna, S.A.; Baxter, N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef]
- Beets, G.L. What are we going to do with complete responses after chemoradiation of rectal cancer? Ann. Surg. Oncol. 2016, 23, 1801–1802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sclafani, F.; Brown, G.; Cunningham, D.; Wotherspoon, A.; Mendes, L.S.T.; Balyasnikova, S.; Evans, J.; Peckitt, C.; Begum, R.; Tait, D.; et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br. J. Cancer 2017, 117, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Lai, M.J.; Wu, C.C.; Jao, S.W.; Hsiao, C.W. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or “watch and wait”. Int. J. Colorectal Dis. 2016, 31, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Guillem, J.G.; Chessin, D.B.; Shia, J.; Moore, H.G.; Mazumdar, M.; Bernard, B.; Paty, P.B.; Saltz, L.; Minsky, B.D.; Weiser, M.R.; et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J. Clin. Oncol. 2005, 23, 3475–3479. [Google Scholar] [CrossRef]
- Hiotis, S.P.; Weber, S.M.; Cohen, A.M.; Minsky, B.D.; Paty, P.B.; Guillem, J.G.; Wagman, R.; Saltz, L.B.; Wong, W.D. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: An analysis of 488 patients. J. Am. Coll. Surg. 2002, 194, 131–135. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Bates, D.D.B.; Petkovska, I. Novel imaging techniques of rectal cancer: What do radiomics and radiogenomics have to offer? A literature review. Abdom. Radiol. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, e4006. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Dis. Prim. 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S.; Initiative for the I.B.S. Image biomarker standardisation initiative. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Bibault, J.E.; Giraud, P.; Durdux, C.; Taieb, J.; Berger, A.; Coriat, R.; Chaussade, S.; Dousset, B.; Nordlinger, B.; Burgun, A. Deep learning and radiomics predict complete response after neo-adjuvant chemoradiation for locally advanced rectal cancer. Sci. Rep. 2018, 8, e12611. [Google Scholar] [CrossRef]
- Colby, J.B. Radiomics approach fails to outperform null classifier on test data. Am. J. Neuroradiol. 2017, 38, 92–93. [Google Scholar] [CrossRef]
- Boughorbel, S.; Jarray, F.; El-Anbari, M. Optimal classifier for imbalanced data using matthews correlation coefficient metric. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Jurman, G.; Riccadonna, S.; Furlanello, C. A Comparison of MCC and CEN error measures in multi-class prediction. PLoS ONE 2012, 7, e41882. [Google Scholar] [CrossRef]
- Vandendorpe, B.; Durot, C.; Lebellec, L.; Le Deley, M.C.; Sylla, D.; Bimbai, A.M.; Amroun, K.; Ramiandrisoa, F.; Cordoba, A.; Mirabel, X.; et al. Prognostic value of the texture analysis parameters of the initial computed tomographic scan for response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. Radiother. Oncol. 2019, 135, 153–160. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, Z.; Huang, Y.; Liang, C.; He, L.; Ma, Z.; Chen, X.; Wu, X.; Li, Y.; Liang, C.; et al. CT-based radiomics signature to discriminate high-grade from low-grade colorectal adenocarcinoma. Acad. Radiol. 2018, 25, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.G.; Kim, Y.H.; Lee, K.H.; Lee, Y.J.; Park, J.H.; Lee, H.S.; Ahn, S.; Kim, B. CT texture analysis in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: A potential imaging biomarker for treatment response and prognosis. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, S.; Rosman, A.S.; Desiato, V.; Hindman, N.M.; Newman, E.; Berman, R.; Pachter, H.L.; Melis, M. MRI-based apparent diffusion coefficient for predicting pathologic response of rectal cancer after neoadjuvant therapy: Systematic review and meta-analysis. Am. J. Roentgenol. 2018, 211, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Sun, Y.; Gollub, M.J.; Peng, W.; Cai, S.; Zhang, Z.; Gu, Y. Dynamic contrast-enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J. Magn. Reson. Imaging 2015, 42, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2017, 8, 59492–59499. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.Y.; Shi, Y.J.; Wang, L.; Zhu, H.T.; Tang, Z.; Wang, S.; Li, X.T.; Tian, J.; Sun, Y.S. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin. Cancer Res. 2017, 23, 7253–7262. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Wang, L.; Luo, R.; Li, J.; Zheng, H.; Yin, Q.; Zhang, Z.; Duan, S.; Li, X.; et al. MRI radiomics analysis for predicting preoperative synchronous distant metastasis in patients with rectal cancer. Eur. Radiol. 2019, 29, 4418–4426. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, P.; Wang, J.; Shen, L.; Xia, F.; Qing, G.; Hu, W.; Zhang, Z.; Xin, C.; Peng, W.; et al. Radiomic features of pretreatment MRI could identify T stage in patients with rectal cancer: Preliminary findings. J. Magn. Reson. Imaging 2018, 48, 615–621. [Google Scholar] [CrossRef]
- Berenguer, R.; Pastor-Juan, M.D.R.; Canales-Vázquez, J.; Castro-García, M.; Villas, M.V.; Mansilla Legorburo, F.; Sabater, S. Radiomics of CT features may be nonreproducible and redundant: Influence of CT acquisition parameters. Radiology 2018, 288, 407–415. [Google Scholar] [CrossRef]
- Parmar, C.; Grossmann, P.; Bussink, J.; Lambin, P.; Aerts, H.J.W.L. Machine learning methods for quantitative radiomic biomarkers. Sci. Rep. 2015, 5, e13087. [Google Scholar] [CrossRef]
- Kikinis, R.; Pieper, S.D.; Vosburgh, K.G. 3D slicer: A platform for subject-specific image analysis, visualization, and clinical support. In Intraoperative Imaging and Image-Guided Therapy; Springer: New York, NY, USA, 2013; pp. 277–289. ISBN 978-1-4614-7656-6. [Google Scholar]
- Hosny, A.; van Griethuysen, J.J.M.; Parmar, C.; Aerts, H.J.W.L.; Fedorov, A.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Aucoin, N.; Narayan, V.; Pieper, S. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, 104–107. [Google Scholar]
- Rietveld, D.; Aerts, H.J.W.L.; Lambin, P.; Rietbergen, M.M.; Grossmann, P.; Haibe-Kains, B.; Bussink, J.; Parmar, C.; Leijenaar, R.T.H.; Rios Velazquez, E. Radiomic feature clusters and prognostic signatures specific for lung and head & neck cancer. Sci. Rep. 2015, 5, 1–10. [Google Scholar]
- Criminisi, A. Decision forests: A unified framework for classification, regression, density estimation, manifold learning and semi-supervised learning. Found. Trends® Comput. Graph. Vis. 2012, 7, 81–227. [Google Scholar] [CrossRef]
- Millman, K.J.; Aivazis, M. Python for scientists and engineers. Comput. Sci. Eng. 2011, 13, 9–12. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]

| Items | Value | Range/Percent |
|---|---|---|
| Age (mean, range) | 56 years | (41–90) |
| Sex | ||
| Male | 126 | 75% |
| Female | 43 | 25% |
| T stage | ||
| 2 | 11 | 7% |
| 3 | 136 | 80% |
| 4 | 22 | 13% |
| N stage | ||
| 0 | 31 | 18% |
| 1 | 89 | 53% |
| 2 | 49 | 29% |
| Tumor volume (mean, range) | 45.3 cm3 | (3.3–483.6) |
| WHO Tumor Grading | ||
| Grade 1 | 4 | 2% |
| Grade 2 | 128 | 76% |
| Grade 3 | 37 | 22% |
| Treatment | ||
| Delivered Dose (mean, range) | 50.4 Gy | (45–52.2) |
| Days to surgery (mean, range) | 46.7 days | (9–124) |
| Follow-up (mean, range) | 34 months | (2–95) |
| Outcome | ||
| pCR (male/female) | 22 (13/9) | 13% |
| non-pCR (male/female) | 147 (113/34) | 87% |
| Item | Value |
|---|---|
| Scanner | Siemens Emotion (16 Slices) |
| Acquisition matrix | 512 × 512 |
| Voxel size | 0.98 × 0.98 × 3 mm |
| Dose Modulation | None |
| Convolution Kernel | B40s |
| Contrast Agent | Non-contrast |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamerla, G.; Meyer, H.-J.; Hambsch, P.; Wolf, U.; Kuhnt, T.; Hoffmann, K.-T.; Surov, A. Radiomics Model Based on Non-Contrast CT Shows No Predictive Power for Complete Pathological Response in Locally Advanced Rectal Cancer. Cancers 2019, 11, 1680. https://doi.org/10.3390/cancers11111680
Hamerla G, Meyer H-J, Hambsch P, Wolf U, Kuhnt T, Hoffmann K-T, Surov A. Radiomics Model Based on Non-Contrast CT Shows No Predictive Power for Complete Pathological Response in Locally Advanced Rectal Cancer. Cancers. 2019; 11(11):1680. https://doi.org/10.3390/cancers11111680
Chicago/Turabian StyleHamerla, Gordian, Hans-Jonas Meyer, Peter Hambsch, Ulrich Wolf, Thomas Kuhnt, Karl-Titus Hoffmann, and Alexey Surov. 2019. "Radiomics Model Based on Non-Contrast CT Shows No Predictive Power for Complete Pathological Response in Locally Advanced Rectal Cancer" Cancers 11, no. 11: 1680. https://doi.org/10.3390/cancers11111680
APA StyleHamerla, G., Meyer, H.-J., Hambsch, P., Wolf, U., Kuhnt, T., Hoffmann, K.-T., & Surov, A. (2019). Radiomics Model Based on Non-Contrast CT Shows No Predictive Power for Complete Pathological Response in Locally Advanced Rectal Cancer. Cancers, 11(11), 1680. https://doi.org/10.3390/cancers11111680





