In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis
Abstract
1. Introduction
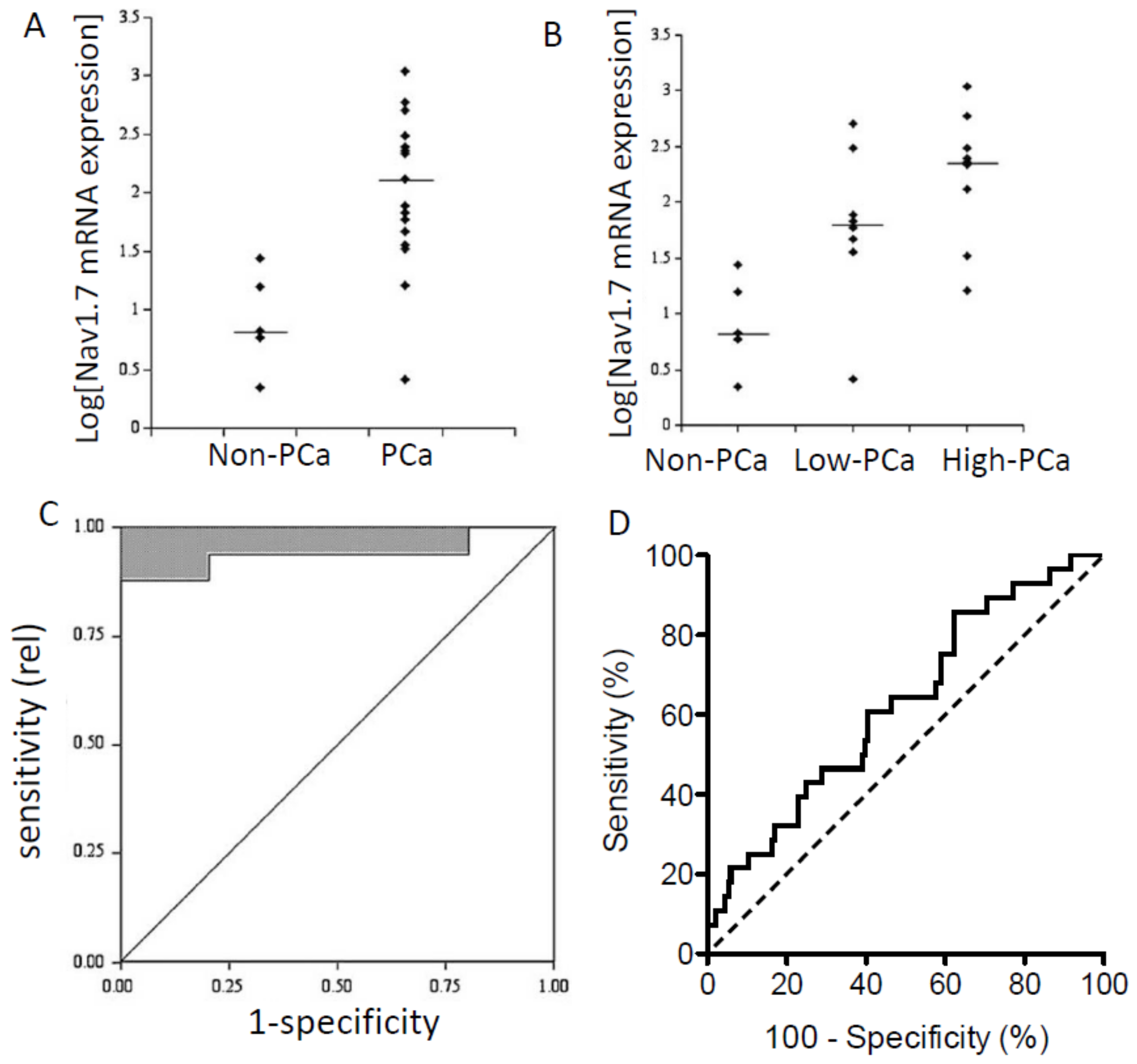
2. mRNA Level Studies
3. Protein Expression
4. Tissue Electrolytes
5. In Vivo Animal Tests
6. Clinical Studies
7. Conclusions and Future Perspective
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.A.; Coombes, R.C.; Schwab, A. Ion transport and cancer: From initiation to metastasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130092. [Google Scholar] [CrossRef] [PubMed]
- Lastraioli, E.; Iorio, J.; Arcangeli, A. Ion channel expression as promising cancer biomarker. Biochim. et Biophys. Acta 2015, 1848, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, J.; Tilli, T.M.; Levin, M. Ion channel and neurotransmitter modulators as electroceutical approaches to the control of cancer. Curr. Pharm. Des. 2017, 23, 4827–4841. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.A.; Fraser, S.P.; Stephens, G.J.; Downing, J.E.; Laniado, M.E.; Foster, C.S.; Abel, P.D.; Djamgoz, M.B.A. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: Contribution to invasiveness in vitro. FEBS Lett. 1995, 369, 290–294. [Google Scholar] [CrossRef]
- Laniado, M.E.; Lalani, E.-N.; Fraser, S.P.; Grimes, J.A.; Bhangal, G.; Djamgoz, M.B.A.; Abel, P.D. Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am. J. Pathol. 1997, 150, 1213–1221. [Google Scholar]
- Fraser, S.P.; Diss, J.K.J.; Chioni, A.M.; Mycielska, M.; Pan, H.; Yamaci, R.F.; Pani, F.; Siwy, Z.; Krasowska, M.; Grzywna, Z.; et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin. Cancer Res. 2005, 11, 5381–5389. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Chioni, A.M.; Diss, J.K.J.; Djamgoz, M.B.A. The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2007, 101, 149–160. [Google Scholar] [CrossRef]
- Nelson, M.; Yang, M.; Millican-Slater, R.; Brackenbury, W.J. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget 2015, 6, 32914–32929. [Google Scholar] [CrossRef] [PubMed]
- Driffort, V.; Gillet, L.; Bon, E.; Marionneau-Lambot, S.; Oullier, T.; Joulin, V.; Collin, C.; Pagès, J.C.; Jourdan, M.L.; Chevalier, S.; et al. Ranolazine inhibits Nav1.5-mediated breast cancer cell invasiveness and lung colonization. Mol. Cancer 2014, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Guzel, R.M.; Ogmen, K.; Ilieva, K.M.; Fraser, S.P.; Djamgoz, M.B.A. Colorectal cancer invasiveness in vitro: Predominant contribution of neonatal Nav1.5 under normoxia and hypoxia. J. Cell. Physiol. 2019, 234, 6582–6593. [Google Scholar] [CrossRef] [PubMed]
- House, C.D.; Vaske, C.J.; Schwartz, A.; Obias, V.; Frank, B.; Luu, T.; Sarvazyan, N.; Irby, R.; Strausberg, R.L.; Hales, T.G.; et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010, 70, 6957–6967. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Hon, D.T.; Robertson, F.M.; Robertson, G.B.; Owen, S.J.; Rogers, G.W.; Lydon, E.L.; Lee, N.H.; Hales, T.G. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and Nav1.5 channel function. Br. J. Anaesth. 2014, 113, i39–i48. [Google Scholar] [CrossRef] [PubMed]
- Diss, J.K.J.; Stewart, D.; Pani, F.; Foster, C.S.; Walker, M.M.; Patel, A.; Djamgoz, M.B.A. A potential novel marker for human prostate cancer: Voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005, 8, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Kubota, N.; Tsutsumi, T.; Oguri, A.; Imuta, H.; Jo, T.; Oonuma, H.; Soma, M.; Meguro, K.; Takano, H.; et al. Eicosapentaenoic acid inhibits voltage-gated sodium channels and invasiveness in prostate cancer cells. Br. J. Pharmacol. 2009, 156, 420–431. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, C.; Wang, Z.; Chen, Y.; Xie, H.; Li, S.; Liu, X.; Liu, Z.; Chen, P. Mechanistic insights into Nav1.7-dependent regulation of rat prostate cancer cell invasiveness revealed by toxin probes and proteomic analysis. FEBS J. 2019, 286, 2549–2561. [Google Scholar] [CrossRef]
- Campbell, T.M.; Main, M.J.; Fitzgerald, E.M. Functional expression of the voltage-gated Na+-channel Nav1.7 is necessary for EGF-mediated invasion in human non-small cell lung cancer cells. J. Cell Sci. 2013, 126, 4939–4949. [Google Scholar] [CrossRef]
- Fulgenzi, G.; Graciotti, L.; Faronato, M.; Soldovieri, M.V.; Miceli, F.; Amoroso, S.; Annunziato, L.; Procopio, A.; Taglialatela, M. Human neoplastic mesothelial cells express voltage-gated sodium channels involved in cell motility. Int. J. Biochem. Cell Biol. 2006, 38, 1146–1159. [Google Scholar] [CrossRef]
- Diaz, D.; Delgadillo, D.M.; Hernandez-Gallegos, E.; Ramirez-Dominguez, M.E.; Hinojosa, L.M.; Ortiz, C.S.; Berumen, J.; Camacho, J.; Gomora, J.C. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J. Cell. Physiol. 2007, 210, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Plata, E.; Ortiz, C.S.; Marquina-Castillo, B.; Medina-Martinez, I.; Alfaro, A.; Berumen, J.; Rivera, M.; Gomora, J.C. Overexpression of Nav1.6 channels is associated with the invasion capacity of human cervical cancer. Int. J. Cancer 2012, 130, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Huang, N.; Huang, H.; Sun, L.; Dong, S.; Su, J.; Zhang, J.; Wang, L.; Lin, L.; Shi, M.; et al. Voltage-gated sodium channel Nav 1.7 promotes gastric cancer progression through MACC1-mediated upregulation of NHE1. Int. J. Cancer 2016, 139, 2553–2569. [Google Scholar] [CrossRef]
- Gao, R.; Shen, Y.; Cai, J.; Lei, M.; Wang, Z. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol. Rep. 2010, 23, 1293–1299. [Google Scholar] [PubMed]
- Gao, R.; Cao, T.; Chen, H.; Cai, J.; Lei, M.; Wang, Z. Nav1.5-E3 antibody inhibits cancer progression. Transl. Cancer Res. 2019, 8, 44–50. [Google Scholar] [CrossRef]
- Xie, A.; Gallant, B.; Guo, H.; Gonzalez, A.; Clark, M.; Madigan, A.; Feng, F.; Chen, H.D.; Cui, Y.; Dudley, S.C., Jr.; et al. Functional cardiac Na+ channels are expressed in human melanoma cells. Oncol. Lett. 2018, 16, 1689–1695. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, W.; Dai, Y.; Qian, C.; Dong, Y.; Chen, Z.; Meng, L.; Jiang, Z.; Huang, T.; Hu, J.; et al. Voltage-gated sodium channel Nav1.5 promotes proliferation, migration and invasion of oral squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2019, 51, 562–570. [Google Scholar] [CrossRef]
- Xing, D.; Wang, J.; Ou, S.; Wang, Y.; Qiu, B.; Ding, D.; Guo, F.; Gao, Q. Expression of neonatal Nav1.5 in human brain astrocytoma and its effect on proliferation, invasion and apoptosis of astrocytoma cells. Oncol. Rep. 2014, 31, 2692–2700. [Google Scholar] [CrossRef][Green Version]
- Ou, S.W.; Kameyama, A.; Hao, L.Y.; Horiuchi, M.; Minobe, E.; Wang, W.Y.; Makita, N.; Kameyama, M. Tetrodotoxin-resistant Na+ channels in human neuroblastoma cells are encoded by new variants of Nav1.5/SCN5A. Eur. J. Neurosci. 2005, 22, 793–801. [Google Scholar] [CrossRef]
- Liu, J.; Tan, H.; Yang, W.; Yao, S.; Hong, L. The voltage-gated sodium channel Nav1.7 associated with endometrial cancer. J. Cancer 2019, 10, 4954–4960. [Google Scholar] [CrossRef]
- Roger, S.; Rollin, J.; Barascu, A.; Besson, P.; Raynal, P.I.; Iochmann, S.; Lei, M.; Bougnoux, P.; Gruel, Y.; Le Guennec, J.Y. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int. J. Biochem. Cell Biol. 2007, 39, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.A. Biophysics of cancer: Cellular excitability (“CELEX”) hypothesis of metastasis. J. Clin. Exper. Oncol. 2014, S1, 005. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A. Bioelectricity of cancer: Voltage-gated ion channels and direct-current electric fields. In The Physiology of Bioelectricity in Development, Tissue Regeneration, and Cancer; Pullar, C., Ed.; Taylor & Francis: London, UK, 2011; pp. 269–294. [Google Scholar]
- Cabello, M.; Ge, H.; Aracil, C.; Moschou, D.; Estrela, P.; Manuel Quero, J.; Pascu, S.I.; Rocha, P.R.F. Extracellular electrophysiology in the prostate cancer cell model PC-3. Sensors 2019, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Yang, M.; Marrison, J.L.; James, A.D.; O’Toole, P.J.; Kaye, P.M.; Whittington, M.A.; Chawla, S.; Brackenbury, W.J. Metastatic breast cancer cells induce altered microglial morphology and electrical excitability in vivo. BioRxiv 2019. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Onkal, R. Persistent current blockers of voltage-gated sodium channels: A clinical opportunity for controlling metastatic disease. Recent Pat. Anticancer Drug Discov. 2013, 8, 66–84. [Google Scholar] [CrossRef]
- Brisson, L.; Gillet, L.; Calaghan, S.; Besson, P.; Le Guennec, J.Y.; Roger, S.; Gore, J. Nav1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H+ efflux in caveolae. Oncogene 2011, 30, 2070–2076. [Google Scholar] [CrossRef]
- Brisson, L.; Driffort, V.; Benoist, L.; Poet, M.; Counillon, L.; Antelmi, E.; Rubino, R.; Besson, P.; Labbal, F.; Chevalier, S.; et al. Nav1.5 Na+ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013, 126, 4835–4842. [Google Scholar] [CrossRef]
- Leanza, L.; Managò, A.; Zoratti, M.; Gulbins, E.; Szabo, I. Pharmacological targeting of ion channels for cancer therapy: In vivo evidences. Biochim. Biophys. Acta 2016, 1863, 1385–1397. [Google Scholar] [CrossRef]
- Shan, B.; Dong, M.; Tang, H.; Wang, N.; Zhang, J.; Yan, C.; Jiao, X.; Zhang, H.; Wang, C. Voltage-gated sodium channels were differentially expressed in human normal prostate, benign prostatic hyperplasia and prostate cancer cells. Oncol. Lett. 2014, 8, 345–350. [Google Scholar] [CrossRef]
- Yang, M.; Kozminski, D.J.; Wold, L.A.; Modak, R.; Calhoun, J.D.; Isom, L.L.; Brackenbury, W.J. Therapeutic potential for phenytoin: Targeting Nav1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res. Treat. 2012, 134, 603–615. [Google Scholar] [CrossRef]
- Peng, J.; Ou, Q.; Wu, X.; Zhang, R.; Zhao, Q.; Jiang, W.; Lu, Z.; Wan, D.; Pan, Z.; Fang, Y. Expression of voltage-gated sodium channel Nav1.5 in non-metastatic colon cancer and its associations with estrogen receptor (ER)-β expression and clinical outcomes. Chin. J. Cancer 2017, 36, 89. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Charcas, O.; Espinosa, A.M.; Alfaro, A.; Herrera-Carrillo, Z.; Ramirez-Cordero, B.E.; Cortes-Reynosa, P.; Perez Salazar, E.; Berumen, J.; Gomora, J.C. The invasiveness of human cervical cancer associated to the function of Nav1.6 channels is mediated by MMP-2 activity. Sci. Rep. 2018, 8, 12995. [Google Scholar] [CrossRef] [PubMed]
- Igci, Y.Z.; Bozgeyik, E.; Borazan, E.; Pala, E.; Suner, A.; Ulasli, M.; Gurses, S.A.; Yumrutas, O.; Balik, A.A.; Igci, M. Expression profiling of SCN8A and NDUFC2 genes in colorectal carcinoma. Exp. Oncol. 2015, 37, 77–80. [Google Scholar] [CrossRef]
- Wu, W.K.; Li, G.R.; Wong, H.P.; Hui, M.K.; Tai, E.K.; Lam, E.K.; Shin, V.Y.; Ye, Y.N.; Li, P.; Yang, Y.H.; et al. Involvement of Kv1.1 and Nav1.5 in proliferation of gastric epithelial cells. J. Cell. Physiol. 2006, 207, 437–444. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Stock, C. Ion channels and transporters in cancer: Pathophysiology, regulation, and clinical potential. Cancer Res. 2013, 73, 1658–1661. [Google Scholar] [CrossRef]
- Martin, K.C.; Zukin, R.S. RNA trafficking and local protein synthesis in dendrites: An overview. J. Neurosci. 2006, 26, 7131–7134. [Google Scholar] [CrossRef]
- Pfeiffer, B.E.; Huber, K.M. Current advances in local protein synthesis and synaptic plasticity. J. Neurosci. 2006, 26, 7147–7150. [Google Scholar] [CrossRef]
- Schedel, J.; Distler, O.; Woenckhaus, M.; Gay, R.E.; Simmen, B.; Michel, B.A.; Müller-Ladner, U.; Gay, S. Discrepancy between mRNA and protein expression of tumour suppressor maspin in synovial tissue may contribute to synovial hyperplasia in rheumatoid arthritis. Ann. Rheum. Dis. 2004, 63, 1205–1211. [Google Scholar] [CrossRef]
- Bugan, I.; Kucuk, S.; Karagoz, Z.; Fraser, S.P.; Kaya, H.; Dodson, A.; Foster, C.S.; Altun, S.; Djamgoz, M.B.A. Anti-metastatic effect of ranolazine in an in vivo rat model of prostate cancer, and expression of voltage-gated sodium channel protein in human prostate. Prostate Cancer Prostatic Dis. 2019. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. Grading Committee. The 2014 International Society of Urological Pathology (ISUP) consensus Conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Suy, S.; Hansen, T.P.; Auto, H.D.; Kallakury, B.V.; Dailey, V.; Danner, M.; Macarthur, L.; Zhang, Y.; Miessau, M.J.; Collins, S.P.; et al. Expression of voltage-gated sodium channel Nav1.8 in human prostate cancer is associated with high histological grade. J. Clin. Exp. Oncol. 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Altun, S.; Gumushan, H.; Patel, A.; Djamgoz, M.B.A. Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo. Cancer Lett. 2012, 323, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Onganer, P.U.; Seckl, M.J.; Djamgoz, M.B.A. Neuronal characteristics of small-cell lung cancer. Br. J. Cancer 2005, 93, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Onkal, R.; Mattis, J.H.; Fraser, S.P.; Diss, J.K.J.; Shao, D.; Okuse, K.; Djamgoz, M.B.A. Alternative splicing of Nav1.5: An electrophysiological comparison of ‘neonatal’ and ‘adult’ isoforms, and critical involvement of a lysine residue. J. Cell. Physiol. 2008, 216, 716–726. [Google Scholar] [CrossRef]
- Chioni, A.M.; Fraser, S.P.; Pani, F.; Foran, P.; Wilkin, G.P.; Diss, J.K.J.; Djamgoz, M.B.A. A novel polyclonal antibody specific for the Nav1.5 voltage-gated Na+ channel ‘neonatal’ splice form. J. Neurosci. Methods 2005, 147, 88–98. [Google Scholar] [CrossRef]
- Yamaci, R.F.; Fraser, S.P.; Battaloglu, E.; Kaya, H.; Erguler, K.; Foster, C.S.; Djamgoz, M.B.A. Neonatal Nav1.5 protein expression in normal adult human tissues and breast cancer. Pathol. Res. Prac. 2017, 213, 900–907. [Google Scholar] [CrossRef]
- Guzel, R.M. Studies of Ionic Mechanisms Associated with Human Cancers. Ph.D. Thesis, Imperial College, London, UK, 2012. [Google Scholar]
- Barshack, I.; Levite, M.; Lang, A.; Fudim, E.; Picard, O.; Ben Horin, S.; Chowers, Y. Functional voltage-gated sodium channels are expressed in human intestinal epithelial cells. Digestion 2008, 77, 108–117. [Google Scholar] [CrossRef]
- Chioni, A.M.; Shao, D.; Grose, R.; Djamgoz, M.B.A. Protein kinase A and regulation of neonatal Nav1.5 expression in human breast cancer cells: Activity-dependent positive feedback and cellular migration. Int. J. Biochem. Cell Bio. 2010, 42, 346–358. [Google Scholar] [CrossRef]
- Fraser, S.P.; Özerlat-Gunduz, I.; Brackenbury, W.J.; Fitzgerald, E.M.; Campbell, T.; Coombes, R.C.; Djamgoz, M.B.A. Regulation of voltage-gated sodium channel expression in cancer: Hormones, growth factors and auto-regulation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130105. [Google Scholar] [CrossRef]
- Zhang, C.; Bosch, M.A.; Qiu, J.; Rønnekleiv, O.K.; Kelly, M.J. 17β-Estradiol increases persistent Na+ current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol. Endocrinol. 2015, 29, 518–527. [Google Scholar] [CrossRef]
- Bi, R.Y.; Meng, Z.; Zhang, P.; Wang, X.D.; Ding, Y.; Gan, Y.H. Estradiol upregulates voltage-gated sodium channel 1.7 in trigeminal ganglion contributing to hyperalgesia of inflamed TMJ. PLoS ONE 2017, 12, e0178589. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, P.; Fang, J.; Li, Q.; Xiao, H.; Zhou, H.; Tang, B. Simultaneous quantitation of Na+ and K+ in single normal and cancer cells using a new near-infrared fluorescent probe. Anal. Chem. 2015, 87, 6057–6063. [Google Scholar] [CrossRef] [PubMed]
- Leslie, T.K.; James, A.D.; Zaccagna, F.; Grist, J.T.; Deen, S.; Kennerley, A.; Riemer, F.; Kaggie, J.D.; Gallagher, F.A.; Gilbert, F.J.; et al. Sodium homeostasis in the tumour microenvironment. Biochim. Biophys. Acta Rev. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, R.; Jacobs, M.A.; Macura, K.J.; Wolff, A.C.; Stearns, V.; Mezban, S.D.; Khouri, N.F.; Bluemke, D.A.; Bottomley, P.A. Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res. Treat. 2007, 106, 151–160. [Google Scholar] [CrossRef]
- Broeke, N.C.; Peterson, J.; Lee, J.; Martin, P.R.; Farag, A.; Gomez, J.A.; Moussa, M.; Gaed, M.; Chin, J.; Pautler, S.E.; et al. Characterization of clinical human prostate cancer lesions using 3.0-T sodium MRI registered to Gleason-graded whole-mount histopathology. J. Mag. Res. Imaging 2019, 49, 1409–1419. [Google Scholar] [CrossRef]
- Deen, S.S.; Riemer, F.; McLean, M.A.; Gill, A.B.; Kaggie, J.D.; Grist, J.T.; Crawford, R.; Latimer, J.; Baldwin, P.; Earl, H.M.; et al. Sodium MRI with 3D-cones as a measure of tumour cellularity in high grade serous ovarian cancer. Eur. J. Radiol. Open 2019, 6, 156–162. [Google Scholar] [CrossRef]
- Babsky, A.M.; Ju, S.; Bennett, S.; George, B.; McLennan, G.; Bansal, N. Effect of implantation site and growth of hepatocellular carcinoma on apparent diffusion coefficient of water and sodium MRI. NMR Biomed. 2012, 25, 312–321. [Google Scholar] [CrossRef]
- Babsky, A.M.; Hekmatyar, S.K.; Zhang, H.; Solomon, J.L.; Bansal, N. Application of 23Na MRI to monitor chemotherapeutic response in RIF-1 tumors. Neoplasia 2005, 7, 658–666. [Google Scholar] [CrossRef]
- Schepkin, V.D.; Chenevert, T.L.; Kuszpit, K.; Lee, K.C.; Meyer, C.R.; Johnson, T.D.; Rehemtulla, A.; Ross, B.D. Sodium and proton diffusion MRI as biomarkers for early therapeutic response in subcutaneous tumors. Magn. Reson. Imaging 2006, 24, 273–278. [Google Scholar] [CrossRef][Green Version]
- Schepkin, V.D.; Lee, K.C.; Kuszpit, K.; Muthuswami, M.; Johnson, T.D.; Chenevert, T.L.; Rehemtulla, A.; Ross, B.D. Proton and sodium MRI assessment of emerging tumor chemotherapeutic resistance. NMR Biomed. 2006, 19, 1035–1042. [Google Scholar] [CrossRef]
- Kline, R.P.; Wu, E.X.; Petrylak, D.P.; Szabolcs, M.; Alderson, P.O.; Weisfeldt, M.L.; Cannon, P.; Katz, J. Rapid in vivo monitoring of chemotherapeutic response using weighted sodium magnetic resonance imaging. Clin. Cancer Res. 2000, 6, 2146–2156. [Google Scholar] [PubMed]
- Jacobs, M.A.; Ouwerkerk, R.; Wolff, A.C.; Gabrielson, E.; Warzecha, H.; Jeter, S.; Bluemke, D.A.; Wahl, R.; Stearns, V. Monitoring of neoadjuvant chemotherapy using multiparametric, 23Na sodium MR, and multimodality (PET/CT/ MRI) imaging in locally advanced breast cancer. Breast Cancer Res. Treat. 2011, 128, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Near, J.; Romagnoli, C.; Bartha, R. Reduced power magnetic resonance spectroscopic imaging of the prostate at 4.0 Tesla. Magn. Reson. Med. 2009, 61, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Riemer, F.; McLean, M.A.; Kaggie, J.; Robb, F.; Tropp, J.S.; Warren, A.; Bratt, O.; Shah, N.; Gnanapragasam, V.J.; et al. Quantification of total and intracellular sodium concentration in primary prostate cancer and adjacent normal prostate tissue with magnetic resonance imaging. Invest. Radiol. 2018, 53, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sişman, A.R.; Sis, B.; Canda, T.; Onvural, B. Electrolytes and trace elements in human breast cyst fluid. Biol. Trace Element Res. 2009, 128, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Dalkin, A.C. Electrolyte disorders associated with cancer. Adv. Chronic Kidney Dis. 2014, 21, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Rinaldi, S.; Caramanti, M.; Grohè, C.; Santoni, M.; Morgese, F.; Torniai, M.; Savini, A.; Fiordoliva, I.; Cascinu, S. Hyponatremia in cancer patients: Time for a new approach. Crit. Rev. Oncol. Hematol. 2016, 102, 15–25. [Google Scholar] [CrossRef]
- Carter, B.C.; Bean, B.P. Sodium entry during action potentials of mammalian central neurons: Incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron 2009, 64, 898–909. [Google Scholar] [CrossRef]
- Lee, A.; Fraser, S.P.; Djamgoz, M.B.A. Propranolol inhibits neonatal Nav1.5 activity and invasiveness of MDA-MB-231 breast cancer cells: Effects of combination with ranolazine. J. Cell. Physiol. 2019. [Epub ahead of print]. [Google Scholar] [CrossRef]
- Batcioglu, K.; Uyumlu, A.B.; Satilmis, B.; Yildirim, B.; Yucel, N.; Demirtas, H.; Onkal, R.; Guzel, R.M.; Djamgoz, M.B.A. Oxidative stress in the in vivo DMBA rat model of breast cancer: Suppression by a voltage-gated sodium channel inhibitor (RS100642). Basic Clin. Pharmacol. Toxicol. 2012, 111, 137–141. [Google Scholar] [CrossRef]
- Sheets, M.F.; Fozzard, H.A.; Lipkind, G.M.; Hanck, D.A. Sodium channel molecular conformations and antiarrhythmic drug affinity. Trends Cardiovasc. Med. 2010, 20, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Yang, M.; Dowle, A.A.; Thomas, J.R.; Brackenbury, W.J. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol. Cancer 2015, 14, 13. [Google Scholar] [CrossRef]
- Chioni, A.M.; Brackenbury, W.J.; Calhoun, J.D.; Isom, L.L.; Djamgoz, M.B.A. A novel adhesion molecule in human breast cancer cells: Voltage-gated Na+ channel beta1 subunit. Int. J. Biochem. Cell Biol. 2009, 41, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Diss, J.K.J.; Fraser, S.P.; Walker, M.M.; Patel, A.; Latchman, D.S.; Djamgoz, M.B.A. Beta-subunits of voltage-gated sodium channels in human prostate cancer: Quantitative in vitro and in vivo analyses of mRNA expression. Prostate Cancer and Prostatic Dis. 2008, 11, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Millican-Slater, R.; Forrest, L.C.; Brackenbury, W.J. The sodium channel beta1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis. Int. J. Cancer 2014, 135, 2338–2351. [Google Scholar] [CrossRef]
- Jansson, K.H.; Lynch, J.E.; Lepori-Bui, N.; Czymmek, K.J.; Duncan, R.L.; Sikes, R.A. Overexpression of the VSSC-associated CAM, beta-2, enhances LNCaP cell metastasis associated behavior. Prostate 2012, 72, 1080–1092. [Google Scholar] [CrossRef]
- Adachi, K.; Toyota, M.; Sasaki, Y.; Yamashita, T.; Ishida, S.; Ohe-Toyota, M.; Maruyama, R.; Hinoda, Y.; Saito, T.; Imai, K.; et al. Identification of SCN3B as a novel p53-inducible proapoptotic gene. Oncogene 2004, 23, 7791–7798. [Google Scholar] [CrossRef]
- Bon, E.; Driffort, V.; Gradek, F.; Martinez-Caceres, C.; Anchelin, M.; Pelegrin, P.; Cayuela, M.L.; Marionneau-Lambot, S.; Oullier, T.; Guibon, R.; et al. SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nat. Commun. 2016, 7, 13648. [Google Scholar] [CrossRef]
- Sanchez-Sandoval, A.L.; Gomora, J.C. Contribution of voltage-gated sodium channel β-subunits to cervical cancer cells metastatic behavior. Cancer Cell Int. 2019, 19, 35. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, J.; Wu, W.; Liu, F.; Su, A.; Li, Z.; Zhu, J.; Wei, T. Preserved SCN4B expression is an independent indicator of favorable recurrence-free survival in classical papillary thyroid cancer. PLoS One 2018, 13, e0197007. [Google Scholar] [CrossRef]
- Martin, F.; Ufodiama, C.; Watt, I.; Bland, M.; Brackenbury, W.J. Therapeutic value of voltage-gated sodium channel inhibitors in breast, colorectal and prostate cancer: A systematic review. Front. Pharmacol. 2015, 6, 273. [Google Scholar] [CrossRef] [PubMed]
- Chamaraux-Tran, T.N.; Mathelin, C.; Aprahamian, M.; Joshi, G.P.; Tomasetto, C.; Diemunsch, P.; Akladios, C. Antitumor effects of lidocaine on human breast cancer cells: An in vitro and in vivo experimental trial. Anticancer Res. 2018, 38, 95–105. [Google Scholar] [PubMed]
- Johnson, M.Z.; Crowley, P.D.; Foley, A.G.; Xue, C.; Connolly, C.; Gallagher, H.C.; Buggy, D.J. Effect of perioperative lidocaine on metastasis after sevoflurane or ketamine-xylazine anaesthesia for breast tumour resection in a murine model. Br. J. Anaesth. 2018, 121, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Crowley, P.D.; Foley, A.G.; Gallagher, H.C.; Iwasaki, M.; Ma, D.; Buggy, D.J. Effect of perioperative lidocaine, propofol and steroids on pulmonary metastasis in a murine model of breast cancer surgery. Cancers 2019, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Crowley, P.D.; Foley, A.G.; Gallagher, H.C.; Iwasaki, M.; Ma, D.; Buggy, D.J. Effect of perioperative lidocaine and cisplatin on metastasis in a murine model of breast cancer surgery. Anticancer Res. 2018, 38, 5599–5606. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Chen, D.T.; Pan, J.H.; Chen, Y.H.; Yan, Y.; Li, Q.; Xue, R.F.; Yuan, Y.F.; Zeng, W.A. Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiology 2017, 126, 868–881. [Google Scholar] [CrossRef]
- Tada, M.; Imazeki, F.; Fukai, K.; Sakamoto, A.; Arai, M.; Mikata, R.; Tokuhisa, T.; Yokosuka, O. Procaine inhibits the proliferation and DNA methylation in human hepatoma cells. Hepatol. Int. 2007, 1, 355–364. [Google Scholar] [CrossRef]
- Fraser, S.P.; Foo, I.; Djamgoz, M.B.A. Local anaesthetic use in cancer surgery and disease recurrence: Role of voltage-gated sodium channels? Br. J. Anaesth. 2014, 113, 899–902. [Google Scholar] [CrossRef]
- Kim, R. Anesthetic technique and cancer recurrence in oncologic surgery: Unraveling the puzzle. Cancer Metastasis Rev. 2017, 36, 159–177. [Google Scholar] [CrossRef]
- Grandhi, R.K.; Perona, B. Mechanisms of action by which local anesthetics reduce cancer recurrence: A systematic review. Pain Medicine 2019. [Epub ahead of print]. [Google Scholar] [CrossRef]
- Exadaktylos, A.K.; Buggy, D.J.; Moriarty, D.C.; Mascha, E.; Sessler, D.I. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006, 105, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Biki, B.; Mascha, E.; Moriarty, D.C.; Fitzpatrick, J.M.; Sessler, D.I.; Buggy, D.J. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology 2008, 109, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.T.; Huang, C.; Lin, H.C.; Huang, C.Y. Antiarrhythmic drug usage and prostate cancer: A population-based cohort study. Asian J. Androl. 2018, 20, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, C.; Watt, I.; Martin, F.; Bland, M.; Brackenbury, W.J. Sodium channel-inhibiting drugs and survival of breast, colon and prostate cancer: A population-based study. Sci. Rep. 2015, 5, 16758. [Google Scholar] [CrossRef]
- Fairhurst, C.; Martin, F.; Watt, I.; Doran, T.; Bland, M.; Brackenbury, W.J. Sodium channel-inhibiting drugs and cancer survival: Protocol for a cohort study using the CPRD primary care database. BMJ Open 2016, 6, e011661. [Google Scholar] [CrossRef]
- Takada, M.; Fujimoto, M.; Motomura, H.; Hosomi, K. Inverse association between sodium channel-blocking antiepileptic drug use and cancer: Data mining of spontaneous reporting and claims databases. Int. J. Med. Sci. 2016, 13, 48–59. [Google Scholar] [CrossRef]
- Walker, A.J.; Card, T.; Bates, T.E.; Muir, K. Tricyclic antidepressants and the incidence of certain cancers: A study using the GPRD. Br. J. Cancer 2011, 104, 193–197. [Google Scholar] [CrossRef]
- Reddy, J.P.; Dawood, S.; Mitchell, M.; Debeb, B.G.; Bloom, E.; Gonzalez-Angulo, A.M.; Sulman, E.P.; Buchholz, T.A.; Woodward, W.A. Antiepileptic drug use improves overall survival in breast cancer patients with brain metastases in the setting of whole brain radiotherapy. Radiother. Oncol. 2015, 117, 308–314. [Google Scholar] [CrossRef]
- Minotti, G. Pharmacology at work for cardio-oncology: Ranolazine to treat early cardiotoxicity induced by antitumor drugs. J. Pharmacol. Exp. Ther. 2013, 346, 343–349. [Google Scholar] [CrossRef]
- Menna, P.; Calabrese, V.; Armento, G.; Annibali, O.; Greco, C.; Salvatorelli, E.; Marchesi, F.; Reggiardo, G.; Minotti, G. Pharmacology of cardio-oncology: Chronotropic and lusitropic effects of B-type natriuretic peptide in cancer patients with early diastolic dysfunction induced by anthracycline or nonanthracycline chemotherapy. J. Pharmacol. Exp. Ther. 2018, 366, 158–168. [Google Scholar] [CrossRef]
- Benhaim, L.; Gerger, A.; Bohanes, P.; Paez, D.; Wakatsuki, T.; Yang, D. Gender-specific profiling in SCN1A polymorphisms and time-to-recurrence in patients with stage II/III colorectal cancer treated with adjuvant 5-fluoruracil chemotherapy. Pharmacogenomics J. 2014, 14, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. Voltage-gated sodium channel as a target for metastatic risk reduction with re-purposed drugs. F1000Research 2015, 4, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Wu, C.; Li, Y.; Kong, Y.; Zhou, R.; Shi, K.; Guo, J.; Li, N.; Liu, J.; et al. Evaluation of the anticancer and anti-metastasis effects of novel synthetic sodium channel blockers in prostate cancer cells in vitro and in vivo. Prostate 2019, 79, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Brackenbury, W.J.; Djamgoz, M.B.A. Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line. J. Physiol. 2006, 573, 343–356. [Google Scholar] [CrossRef] [PubMed]





| Carcinoma | VGSC Subtype(s) | Comment(s) | Reference(s) |
|---|---|---|---|
| Breast | nNav1.5 (and (n)Nav1.7) | Dominant at mRNA level and functional contribution to invasiveness/metastasis verified in vitro and in vivo | [9,10,11,12] |
| Colon | nNav1.5 | Dominant at mRNA level and expressed early in invasiveness; functional contribution to invasiveness verified in vitro and in vivo | [13,14,15] |
| Prostate | (n)Nav1.7 (and Nav1.6) | Dominant at mRNA level; specific functional contribution to invasiveness tested using peptide toxins | [8,16,17,18] |
| Non-small cell lung carcinoma (NSCLC) | Nav1.7 | Dominant at mRNA level; potentiation of invasiveness demonstrated by use of siRNA | [19] |
| Mesothelioma | Nav1.2, Nav1.6, and Nav1.7 | VGSC activity shown to promote migration in vitro, but the subtype(s) responsible not determined | [20] |
| Cervical | (n)Nav1.6 | Dominant at mRNA level; over-expression potentiated invasiveness | [21,22] |
| Stomach | Nav1.7 | Dominant at mRNA level; silencing suppressed tumour growth in mouse model in vivo | [23] |
| Ovary | Nav1.5 | Dominant at mRNA level; E3 antibody suppressed in vivo growth and in vitro invasiveness | [24,25] |
| Melanoma | Nav1.5 | Expression induced membrane potential depolarization and inhibited Ca2+ uptake | [26] |
| Oral squamous cell carcinoma | Nav1.5 | siRNA confirmed in vitro potentiation of proliferation and invasiveness | [27] |
| Astrocytoma | nNav1.5 | siRNA confirmed in vitro potentiation of proliferation and invasiveness | [28] |
| Neuroblastoma | nNav1.5 | Where ‘neonatal’ Nav1.5 splicing was first described, but role in cancer not investigated | [29] |
| Endometrium | Nav1.7 | Channel block attenuated in vitro cell invasion; expression was associated (i) positively with tumour size and local lymph node metastasis, and (ii) negatively with survival (5–10 years) | [30] |
| VGSC Property | Clinical Consequence(s) | Reference(s) |
|---|---|---|
| Expression much higher in strongly vs. weakly metastatic cancers (by up to several orders of magnitude at mRNA level) | Potential functional diagnostic molecular biomarker | e.g., [9,16,40] |
| Expression is early and upstream of the genes driving invasiveness | Potential functional ‘early’ marker, ideal for diagnostics | [14] |
| Upregulation maintained at protein and functional (signalling) levels | Diagnostics can be extended to conventional immunohistochemistry and even clinical imaging of tissue sodium possibly resulting from channel activity (e.g., 23Na-MRI); expression can be used to determine treatment strategy and efficacy | [9,16,57,65,66] |
| Gene/protein expressed in neonatal splice form in several carcinomas; targetable by antibody | Diagnostics can be made even more specific; antibody can also be used as a drug (i) to block channel activity/metastasis and/or (ii) kill tumour cells (in antibody-drug conjugate mode) | [9,10,56] |
| Activity-dependent regulation (positive feedback)—VGSC blockers suppress both channel activity and expression | VGSC blockage would suppress both activity and expression of the channel, thereby providing long-term benefit to cancer patients taking VGSC drugs | [60,116] |
| Promotes a range of cellular behaviours integral to the metastatic cascade in vitro, as well as metastasis per se in vivo | Therapeutic potential—possible ‘repurposing’ of existing VGSC drugs, INaP blockers (as well as novel antibody), possibly with minimal side effects | [11,12,36,114] |
| Functional expression under the control of steroid hormones and growth factors | Therapeutic potential extended to ‘combination’ treatments | [19,61] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djamgoz, M.B.A.; Fraser, S.P.; Brackenbury, W.J. In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers 2019, 11, 1675. https://doi.org/10.3390/cancers11111675
Djamgoz MBA, Fraser SP, Brackenbury WJ. In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers. 2019; 11(11):1675. https://doi.org/10.3390/cancers11111675
Chicago/Turabian StyleDjamgoz, Mustafa B. A., Scott P. Fraser, and William J. Brackenbury. 2019. "In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis" Cancers 11, no. 11: 1675. https://doi.org/10.3390/cancers11111675
APA StyleDjamgoz, M. B. A., Fraser, S. P., & Brackenbury, W. J. (2019). In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers, 11(11), 1675. https://doi.org/10.3390/cancers11111675







