Computerized Cytological Features for Papillary Thyroid Cancer Diagnosis—Preliminary Report
Abstract
:1. Introduction
2. Results
2.1. Diagnosis of Papillary Thyroid Cancer (PTC) and Benign Follicular Lesions Other than Adenoma
2.2. Performance of the Logistic Model in Cytologically Indeterminate Categories
3. Discussion
4. Materials and Methods
4.1. Study Subjects
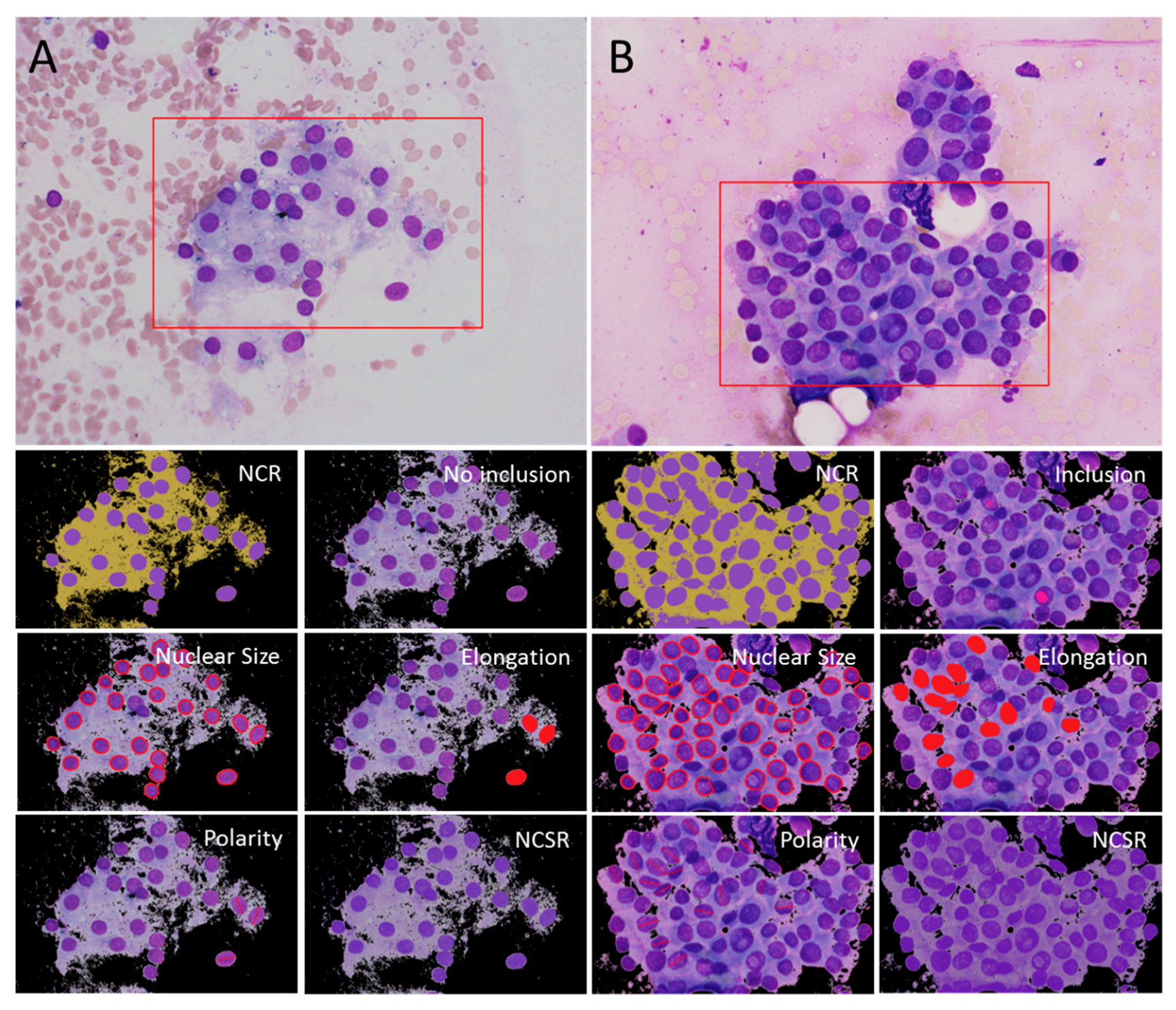
4.2. Computerized Analysis of Cytologic Features
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiovanni, M.; Spitale, A.; Faquin, W.C.; Mazzucchelli, L.; Baloch, Z.W. The Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis. Acta Cytol. 2012, 56, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.H.; Fischer, A.H.; Pisharodi, L.; Owens, C.L. Improved preoperative definitive diagnosis of papillary thyroid carcinoma in FNAs prepared with both ThinPrep and conventional smears compared with FNAs prepared with ThinPrep alone. Cancer Cytopathol. 2011, 119, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Ivanovic, M. The indeterminate thyroid fine-needle aspiration: Experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer 2009, 117, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Theoharis, C.G.; Schofield, K.M.; Hammers, L.; Udelsman, R.; Chhieng, D.C. The Bethesda Thyroid Fine-Needle Aspiration Classification System: Year 1 at an Academic Institution. Thyroid 2009, 19, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, S.R.; Chang, Y.C.; Li, H.Y.; Liau, J.Y.; Lee, C.Y.; Chen, C.M.; Chang, T.C. Preoperative prediction of papillary thyroid carcinoma prognosis with the assistance of computerized morphometry of cytology samples obtained by fine-needle aspiration: Preliminary report. Head Neck 2013, 35, 28–34. [Google Scholar] [CrossRef]
- Shih, S.-R.; Li, H.-Y.; Hsiao, Y.-L.; Chang, T.-C. Prognostic Significance of Cytologic Features in Fine-Needle Aspiration Cytology Samples of Papillary Thyroid Carcinoma: Preliminary Report. Thyroid 2006, 16, 775–780. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. Role of molecular markers in thyroid nodule management: Then and now. Endocr. Pract. 2017, 23, 979–988. [Google Scholar] [CrossRef]
- Harrell, R.M.; Bimston, D.N. Surgical utility of Afirma: Effects of high cancer prevalence and oncocytic cell types in patients with indeterminate thyroid cytology. Endocr. Pract. 2014, 20, 364–369. [Google Scholar] [CrossRef]
- Marti, J.L.; Avadhani, V.; Donatelli, L.A.; Niyogi, S.; Wang, B.; Wong, R.J.; Shaha, A.R.; Ghossein, R.A.; Lin, O.; Morris, L.G.T.; et al. Wide Inter-institutional Variation in Performance of a Molecular Classifier for Indeterminate Thyroid Nodules. Ann. Surg. Oncol. 2015, 22, 3996–4001. [Google Scholar] [CrossRef] [PubMed]
- McIver, B.; Castro, M.R.; Morris, J.C.; Bernet, V.; Smallridge, R.; Henry, M.; Kosok, L.; Reddi, H. An Independent Study of a Gene Expression Classifier (Afirma) in the Evaluation of Cytologically Indeterminate Thyroid Nodules. J. Clin. Endocrinol. Metab. 2014, 99, 4069–4077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-L.; Wu, M.-T.; Yang, S.-F.; Chan, H.-M.; Chai, C.-Y. Computerized nuclear morphometry in thyroid follicular neoplasms. Pathol. Int. 2005, 55, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.-J.; Filetti, S.; Alexander, E.K.; Hay, I.D. Nontoxic diffuse goiter, nodular thyroid disorders, and thyroid malignancies. In Williams Textbook of Endocrinology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 449–488. [Google Scholar]
- Karbowniczek, M.; Chosia, M.; Domagała, W. Nuclear morphometry of MIB-1 positive and negative tumor cells in primary and metastatic malignant melanoma of the skin. Pol. J. Pathol. 1999, 50, 235–241. [Google Scholar]
- Kazanowska, B.; Jelen, M.; Reich, A.; Tarnawski, W.; Chybicka, A. The role of nuclear morphometry in prediction of prognosis for rhabdomyosarcoma in children. Histopathology 2004, 45, 352–359. [Google Scholar] [CrossRef]
- Paraskevakou, E.; Kavantzas, N.; Pavlopoulos, P.M.; Delibasis, A.; Yova, D.; Davaris, P. Computerized nuclear morphometry of renal cell carcinomas. Gen. Diagn. Pathol. 1996, 142, 101–104. [Google Scholar]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef]
- Celik, Z.E.; Altinay, S.; Kilinc, F.; Arslan, N.; Yilmaz, B.S.; Karabagli, P.; Ugurluoglu, C. Using Computerized Cytomorphometry to Distinguish between Benign and Malignant Cases in Thyroid Fine-Needle Aspiration Cytology. Diagn. Cytopathol. 2016, 44, 902–911. [Google Scholar] [CrossRef]
- Gilshtein, H.; Mekel, M.; Malkin, L.; Ben-Izhak, O.; Sabo, E. Computerized cytometry and wavelet analysis of follicular lesions for detecting malignancy: A pilot study in thyroid cytology. Surgery 2017, 161, 212–219. [Google Scholar] [CrossRef]
- Gupta, S.; Ajise, O.; Dultz, L.; Wang, B.; Nonaka, D.; Ogilvie, J.; Heller, K.S.; Patel, K.N. Follicular variant of papillary thyroid cancer: Encapsulated, nonencapsulated, and diffuse: Distinct biologic and clinical entities. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 227–233. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Faquin, W.; Giovanella, L.; Durante, C.; Kopp, P.; Trimboli, P. Impact of non-invasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP) on risk of malignancy in patients undergoing lobectomy/thyroidectomy for suspicious for malignancy or malignant fine-needle aspiration cytology findings: A systematic review and meta-analysis. Eur. J. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Riu, C.H. A new rapid method of staining thin blood film: First report. Taiwan I Hsueh Hui Tsa Chih. 1953, 52, 348–352. [Google Scholar]
- Wright, J.H. A Rapid Method for the Differential Staining of Blood Films and Malarial Parasites. J. Med. Res. 1902, 7, 138–144. [Google Scholar] [PubMed]
- Chen, A.; Hsiao, Y.H.; Chang, T.C.; Jan, I.S.; Shih, S.R.; Wang, H.M. Cytological Image Processing Device, and Method for Quantifying Characteristics of Cytological Image. Patent Number TWI630581B, 21 July 2018. [Google Scholar]


| Cytological Diagnosis (Bethesda Category) | Benign | Atypia of Undetermined Significance or Follicular Lesion of Undetermined Significance (AUS) | Follicular Neoplasm or Suspicious for a Follicular Neoplasm (FNSFN) | Suspicious for Malignancy (SUSP) | Malignant | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathologic Diagnosis | Benign | Malignant (%) | p | Benign | Malignant (%) | p | Benign | Malignant (%) | p | Benign | Malignant (%) | p | Malignant (%) |
| Number | 78 | 12 (13.33%) | 17 | 23 (57.5%) | 22 | 10 (31.25%) | 3 | 50 (94.34%) | 25 (100%) | ||||
| Age (SD) | 52 (12) | 50 (19) | 0.525 | 46 (14) | 44 (11) | 0.564 | 55 (12) | 54 (20) | 0.817 | 50 (29) | 52 (11) | 0.868 | 51 (15) |
| Gender (Female: Male) | 69:9 | 10:2 | 0.629 | 13:4 | 16:7 | 0.645 | 18:4 | 6:4 | 0.215 | 3:0 | 38:12 | 0.355 | 22:3 |
| Pathologic Diagnosis | Papillary Thyroid Cancer and Benign Follicular Lesions other than Follicular Adenoma | Follicular Neoplasm | p * | ||
|---|---|---|---|---|---|
| Nodular Hyperplasia/Goiter, Adenomatous Hyperplasia/Goiter (Group 1) | Papillary Thyroid Cancer (Group 2) | Follicular Adenoma (Group 3) | Follicular Carcinoma Hurthle Cell Carcinoma (Group 4) | ||
| Number | 100 | 110 | 20 | 10 | |
| Age (year) | 53 (13) | 50 (13) | 48 (14) | 48 (19) | 0.358 |
| Gender (Female: Male) | 87:13 | 85:25 | 16:4 | 7:3 | 0.247 |
| Mean nuclear size (μm2) | 56.03 (43.46–74.93) | 75.07 (65.67–87.15) | 81.42 (60.69–95.13) | 77.60 (58.38–98.14) | <0.001 †,‡,§ |
| Standard deviation of nuclear size (μm2) | 8.02 (5.06–11.23) | 10.57 (8.16–14.36) | 12.92 (5.90–16.02) | 12.71 (8.95–20.16) | 0.007 † |
| Coefficient of variation of nuclear size | 0.150 (0.095–0.214) | 0.144 (0.114–0.192) | 0.161 (0.077–0.292) | 0.163 (0.137–0.180) | 0.927 |
| Mean nuclear circularity | 0.839 (0.819–0.851) | 0.832 (0.817–0.840) | 0.846 (0.831–0.861) | 0.839 (0.811–0.852) | 0.062 |
| Standard deviation of nuclear circularity | 0.019 (0.014–0.029) | 0.021 (0.015–0.031) | 0.014 (0.011–0.023) | 0.020 (0.015–0.024) | 0.632 |
| Coefficient of variation of nuclear circularity | 0.023 (0.016–0.036) | 0.025 (0.018–0.038) | 0.016 (0.013–0.027) | 0.024 (0.018–0.030) | 0.672 |
| Mean nuclear elongation | 0.505 (0.457–0.559) | 0.546 (0.503–0.569) | 0.478 (0.453–0.541) | 0.506 (0.486–0.572) | <0.001 †,|| |
| Standard deviation of nuclear elongation | 0.067 (0.049–0.080) | 0.062 (0.051–0.077) | 0.066 (0.046–0.098) | 0.069 (0.054–0.078) | 0.11 |
| Coefficient of variation of nuclear elongation | 0.134 (0.099–0.165) | 0.115 (0.092–0.141) | 0.133 (0.098–0.196) | 0.120 (0.108–0.154) | 0.012 † |
| Mean nuclear ellipticity | 0.872 (0.852–0.881) | 0.871 (0.860–0.879) | 0.880 (0.864–0.892) | 0.876 (0.861–0.889) | 0.017 ‡ |
| Standard deviation of nuclear ellipticity | 0.014 (0.010–0.021) | 0.015 (0.011–0.020) | 0.014 (0.008–0.019) | 0.014 (0.012–0.023) | 0.528 |
| Coefficient of variation of nuclear ellipticity | 0.015 (0.011–0.024) | 0.017 (0.012–0.024) | 0.015 (0.009–0.022) | 0.016 (0.014–0.027) | 0.556 |
| Nuclear-to-cytoplasmic hue ratio | 1.024 (1.011–1.043) | 1.036 (1.016–1.053) | 1.015 (0.998–1.026) | 1.027 (1.012–1.053) | 0.001 || |
| Standard deviation of Nuclear-to-cytoplasmic hue ratio | 0.985 (0.864–1.087) | 0.983 (0.885–1.116) | 0.901 (0.822–0.984) | 0.902 (0.830–0.990) | 0.082 |
| Nuclear-to-cytoplasmic saturation ratio | 1.944 (1.652–2.422) | 1.693 (1.570–1.979) | 2.232 (1.914–2.347) | 2.322 (1.731–2.566) | <0.001 †,|| |
| Standard deviation of Nuclear-to-cytoplasmic saturation ratio | 0.911 (0.797–1.109) | 0.908 (0.774–1.013) | 0.937 (0.813–1.152) | 0.920 (0.848–0.991) | 0.105 |
| Nuclear-to-cytoplasmic value ratio | 0.833 (0.791–0.872) | 0.835 (0.805–0.858) | 0.798 (0.756–0.863) | 0.864 (0.789–0.869) | 0.232 |
| Standard deviation of Nuclear-to-cytoplasmic value ratio | 1.086 (0.935–1.194) | 1.099 (0.986–1.286) | 0.987 (0.933–1.158) | 1.133 (0.945–1.227) | 0.506 |
| Nuclear-to-cytoplasmic ratio | 0.998 (0.740–1.373) | 1.230 (1.020–1.556) | 1.056 (0.755–1.806) | 0.941 (0.806–1.114) | 0.025 † |
| Nuclear polarity | 14.02 (5.04–23.49) | 22.48 (15.27–32.69) | 13.09 (4.19–18.26) | 12.75 (7.56–19.01) | <0.001 †,|| |
| Inclusion index | 0 (0.000–0.004) | 0.006 (0.002–0.012) | 0.004 (0.000–0.007) | 0.007 (0.005–0.018) | 0.011 † |
| Overlapping index | 0.436 (0.340–0.577) | 0.500 (0.432–0.589) | 0.473 (0.290–0.581) | 0.452 (0.374–0.596) | 0.221 |
| Variable | Coefficient | p |
|---|---|---|
| Mean nuclear size (μm2) | 0.06557 | <0.0001 |
| Mean nuclear elongation | 8.94005 | 0.0239 |
| Nuclear-to-cytoplasmic saturation ratio | −1.27953 | 0.0008 |
| Nuclear-to-cytoplasmic ratio | 1.89500 | <0.0001 |
| Nuclear polarity | 0.038098 | 0.0259 |
| Inclusion index | 32.19870 | 0.0473 |
| Constant | −9.71158 | 0.0001 |
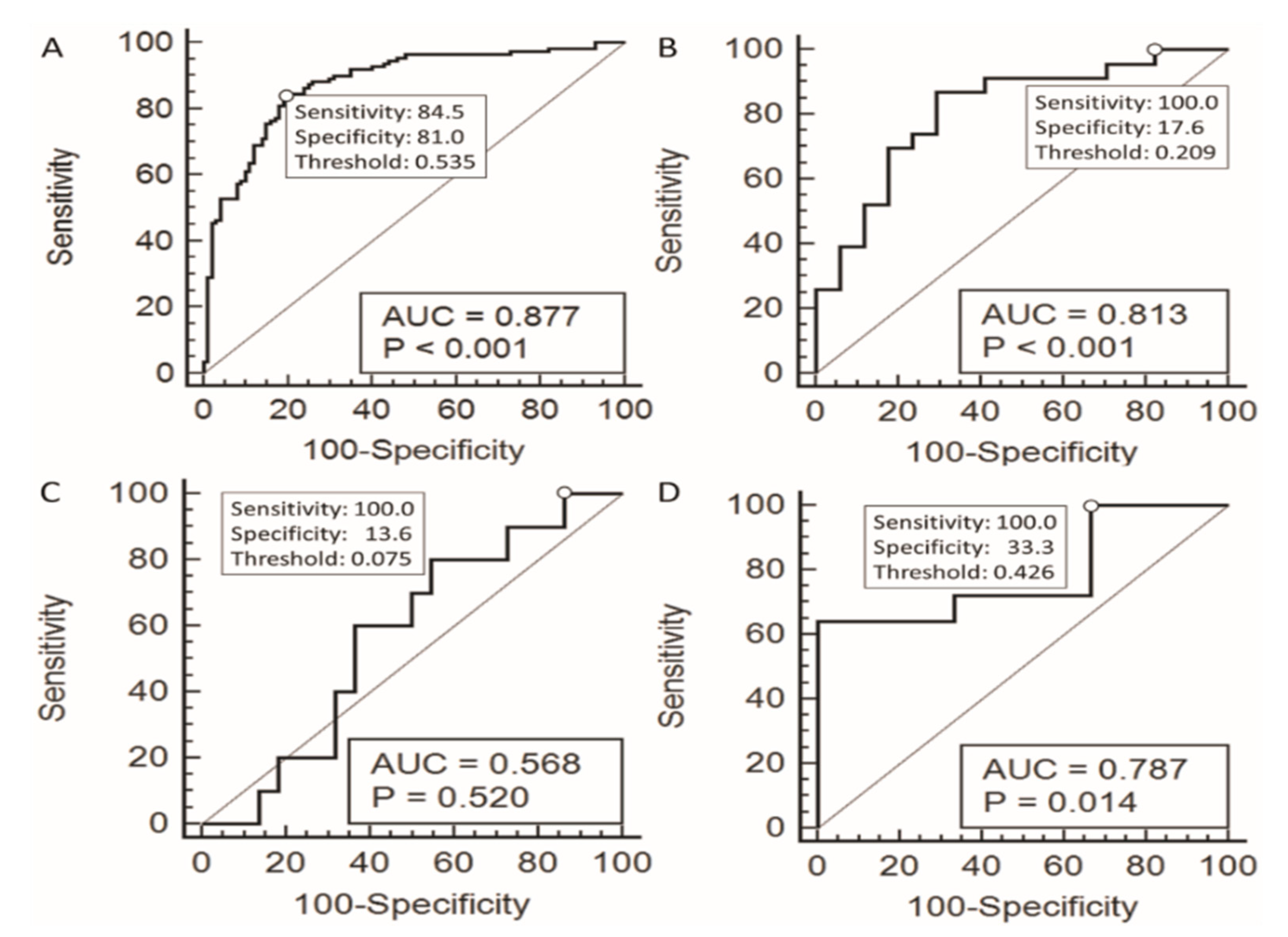
| Cytological Diagnosis | AUROC (p-Value) | Model 1: Threshold Value was Set at the Sensitivity that was 100% with the Specificity as High as Possible | Model 2: Threshold Value was Set at the Cut-off Point on the ROC Curve that was Closest to (0,1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Threshold Value | Sensitivity | Specificity | PPV | NPV | Threshold Value | ||
| AUS | 0.813 (<0.001) | 100.00% | 17.60% | 62.20% | 100.00% | 0.209 | 87.00% | 70.60% | 80.00% | 80.00% | 0.523 |
| FN/SFN | 0.568 (0.520) | 100.00% | 13.60% | 34.50% | 100.00% | 0.075 | 80.00% | 45.50% | 40.00% | 83.30% | 0.304 |
| SUSP | 0.787 (0.014) | 100.00% | 33.30% | 96.20% | 100.00% | 0.426 | 64.00% | 100.00% | 100.00% | 14.30% | 0.733 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, S.-R.; Jan, I.-S.; Chen, K.-Y.; Chuang, W.-Y.; Wang, C.-Y.; Hsiao, Y.-L.; Chang, T.-C.; Chen, A. Computerized Cytological Features for Papillary Thyroid Cancer Diagnosis—Preliminary Report. Cancers 2019, 11, 1645. https://doi.org/10.3390/cancers11111645
Shih S-R, Jan I-S, Chen K-Y, Chuang W-Y, Wang C-Y, Hsiao Y-L, Chang T-C, Chen A. Computerized Cytological Features for Papillary Thyroid Cancer Diagnosis—Preliminary Report. Cancers. 2019; 11(11):1645. https://doi.org/10.3390/cancers11111645
Chicago/Turabian StyleShih, Shyang-Rong, I-Shiow Jan, Kuen-Yuan Chen, Wan-Yu Chuang, Chih-Yuan Wang, Yung-Lien Hsiao, Tien-Chun Chang, and Argon Chen. 2019. "Computerized Cytological Features for Papillary Thyroid Cancer Diagnosis—Preliminary Report" Cancers 11, no. 11: 1645. https://doi.org/10.3390/cancers11111645
APA StyleShih, S.-R., Jan, I.-S., Chen, K.-Y., Chuang, W.-Y., Wang, C.-Y., Hsiao, Y.-L., Chang, T.-C., & Chen, A. (2019). Computerized Cytological Features for Papillary Thyroid Cancer Diagnosis—Preliminary Report. Cancers, 11(11), 1645. https://doi.org/10.3390/cancers11111645





