The Differential Impact of SRC Expression on the Prognosis of Patients with Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Specimens
2.2. Immunohistochemical Analysis of Patient Samples
2.3. Statistical Analyses
3. Results
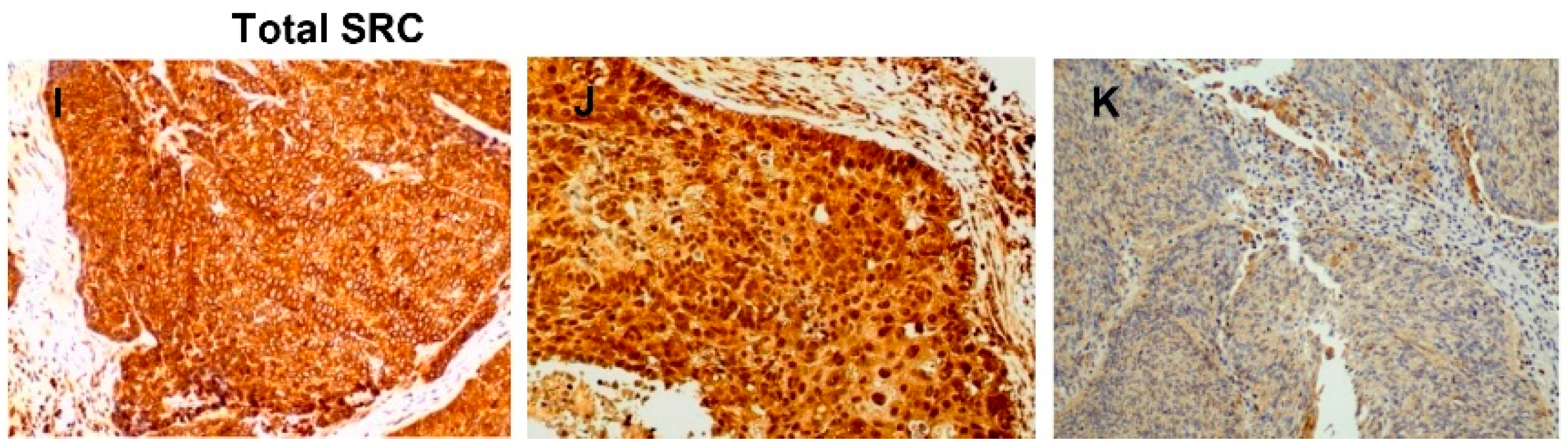
3.1. Detection of Active SRC in HNSCC Tissue Specimens
3.2. Correlations with Clinicopathological Parameters and Disease Outcome
3.3. Differential Clinical Significance of Active SRC Expression Depending on the Tumor Location
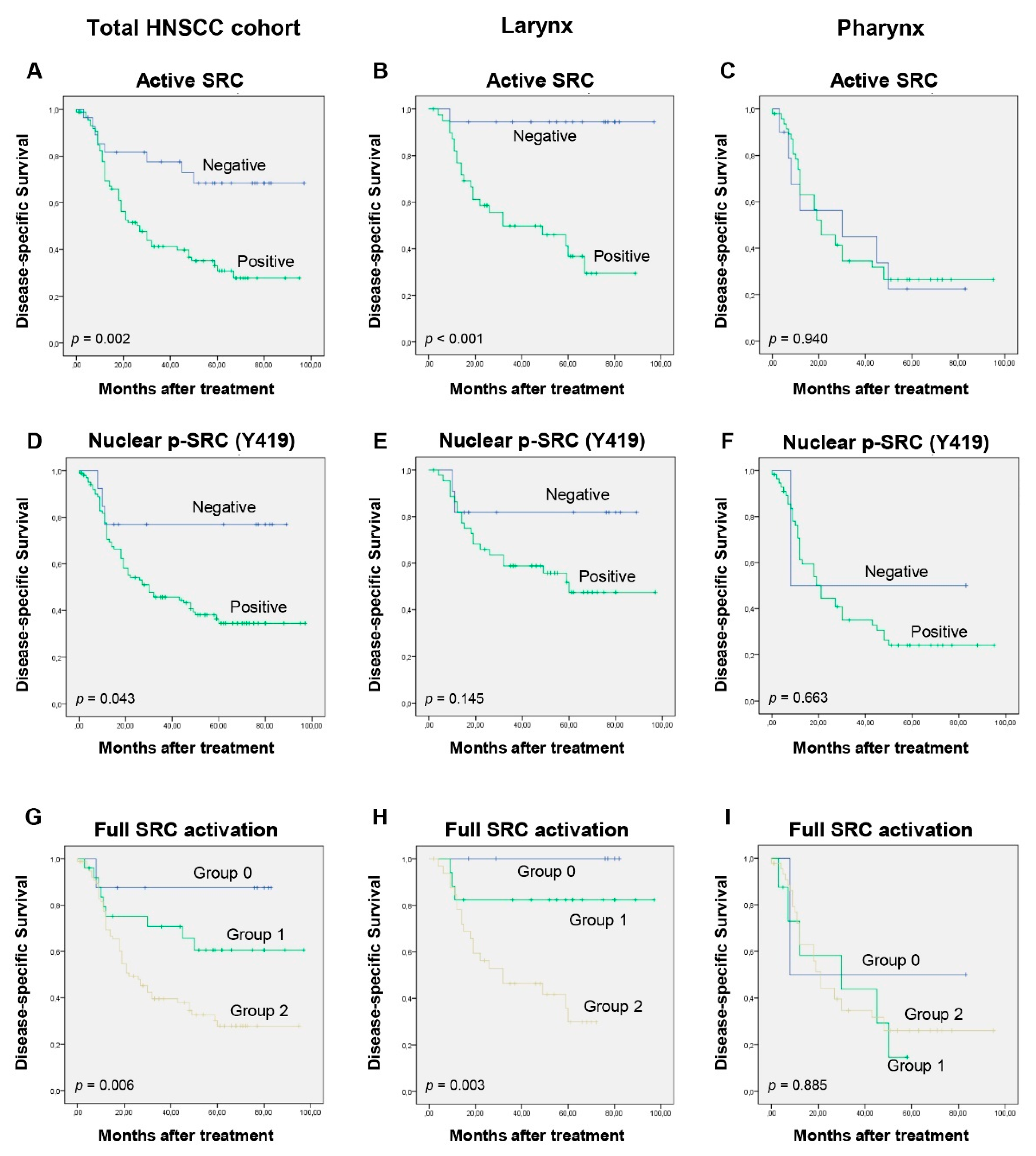
3.4. Impact of SRC Expression on Patients’ Survival
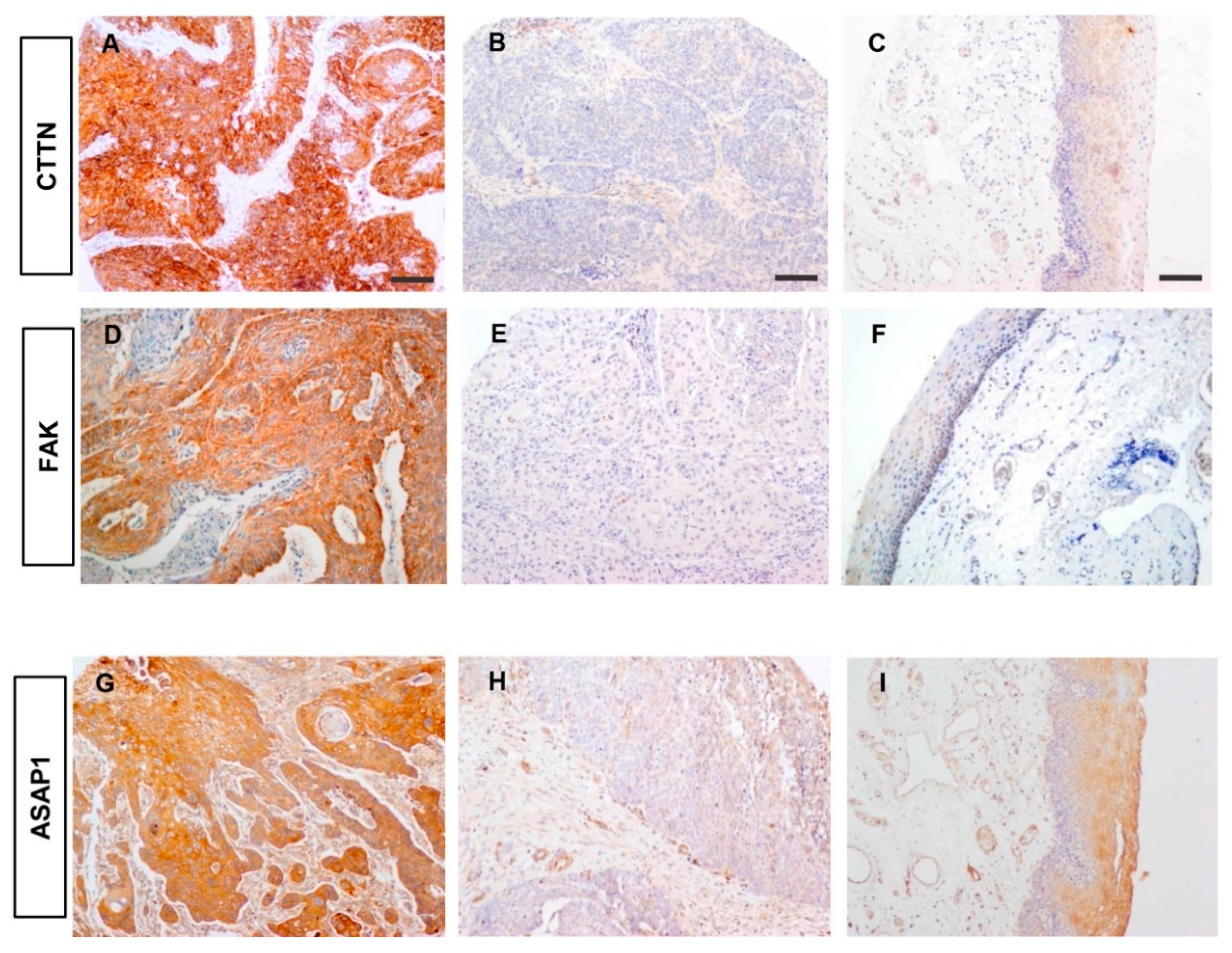
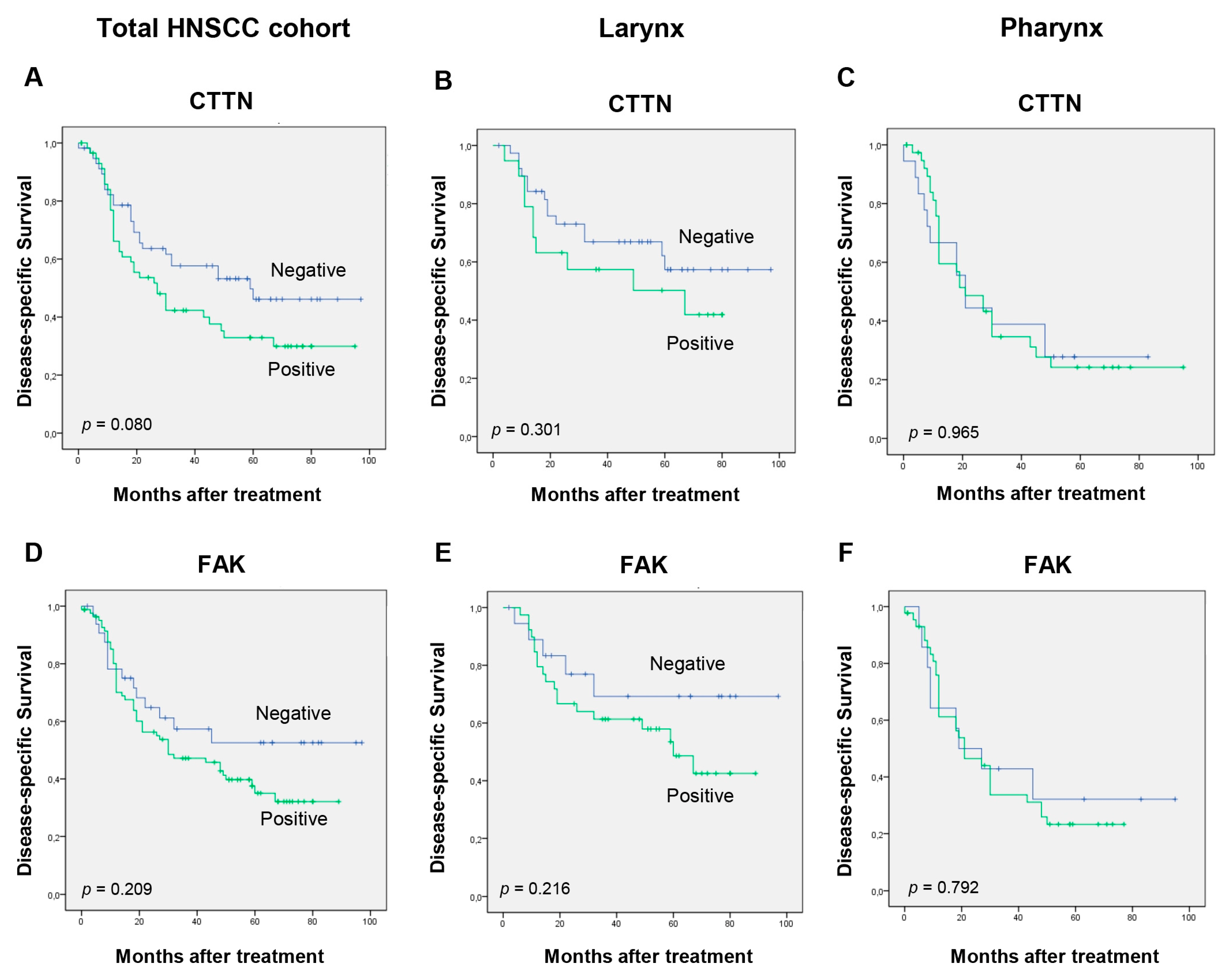
3.5. Correlations between the Expression of Active SRC and SRC-Related Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haddad, R.I.; Shin, D.M. Recent advances in head and neck cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, T.J. A renaissance for SRC. Nat. Rev. Cancer 2004, 4, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.C.; Song, L.; Haura, E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009, 6, 587–595. [Google Scholar] [CrossRef]
- Sen, B.; Johnson, F.M. Regulation of Src Family Kinases in Human Cancers. J. Signal Transduct. 2011, 2011, 865819. [Google Scholar] [CrossRef]
- Summy, J.M.; Gallick, G.E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003, 22, 337–358. [Google Scholar] [CrossRef]
- Parsons, S.J.; Parsons, J.T. Src family kinases, key regulators of signal transduction. Oncogene 2004, 23, 7906–7909. [Google Scholar] [CrossRef]
- Frame, M.C. Src in cancer: Deregulation and consequences for cell behaviour. Biochim. Biophys. Acta 2002, 1602, 114–130. [Google Scholar] [CrossRef]
- Irby, R.B.; Yeatman, T.J. Role of Src expression and activation in human cancer. Oncogene 2000, 19, 5636–5642. [Google Scholar] [CrossRef]
- Ishizawar, R.; Parsons, S.J. c-Src and cooperating partners in human cancer. Cancer Cell 2004, 6, 209–214. [Google Scholar] [CrossRef]
- Mandal, M.; Myers, J.N.; Lippman, S.M.; Johnson, F.M.; Williams, M.D.; Rayala, S.; Ohshiro, K.; Rosenthal, D.I.; Weber, R.S.; Gallick, G.E.; et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: Association of Src activation with E-cadherin downregulation, vimentin expression, and aggressive tumor features. Cancer 2008, 112, 2088–2100. [Google Scholar] [CrossRef] [PubMed]
- Avizienyte, E.; Frame, M.C. Src and FAK signaling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 2005, 17, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Jones, K.C.; Hunter, T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: Summation of both c-Src and focal adhesion kinase initiated tyrosine phosphorylation events. Mol. Cell Biol. 1998, 18, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Gill, S.; Settleman, J.; Parsons, S.J. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 1995, 130, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Liao, K. Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 2011, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Hashimoto, S.; Hashimoto, A.; Morishige, M.; Mazaki, Y.; Yamada, A.; Ogawa, E.; Adachi, M.; Sakurai, T.; Manabe, T.; et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005, 24, 963–973. [Google Scholar] [CrossRef]
- Hashimoto, S.; Furukawa, S.; Hashimoto, A.; Tsutaho, A.; Fukao, A.; Sakamura, Y.; Parajuli, G.; Onodera, Y.; Otsuka, Y.; Handa, H.; et al. ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer. Proc. Natl Acad. Sci. USA 2019, 116, 17450–17459. [Google Scholar] [CrossRef]
- Masaki, T.; Igarashi, K.; Tokuda, M.; Yukimasa, S.; Han, F.; Jin, Y.J.; Li, J.Q.; Yoneyama, H.; Uchida, N.; Fujita, J.; et al. pp60c-src activation in lung adenocarcinoma. Eur. J. Cancer 2003, 39, 1447–1455. [Google Scholar] [CrossRef]
- Talamonti, M.S.; Roh, M.S.; Curley, S.A.; Gallick, G.E. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J. Clin. Invest. 1993, 91, 53–60. [Google Scholar] [CrossRef]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. The role of Src in solid and hematologic malignancies: Development of new-generation Src inhibitors. Cancer 2006, 107, 1918–1929. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol. Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Elsberger, B.; Stewart, B.; Tatarov, O.; Edwards, J. Is Src a viable target for treating solid tumours? Curr. Cancer Drug Targets 2010, 10, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Creedon, H.; Brunton, V.G. Src kinase inhibitors: Promising cancer therapeutics? Crit. Rev. Oncog. 2012, 17, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.M.; Bolejack, V.; Choy, E.; Ganjoo, K.N.; Staddon, A.P.; Chow, W.A.; Tawbi, H.A.; Samuels, B.L.; Patel, S.R.; von Mehren, M.; et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer 2017, 123, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Song, E.K.; Bagby, S.; Purkey, A.; McCarter, M.; Gajdos, C.; Quackenbush, K.S.; Cross, B.; Pitts, T.M.; Tan, A.C.; et al. Evaluation of the efficacy of dasatinib, a Src/Abl inhibitor, in colorectal cancer cell lines and explant mouse model. PLoS ONE 2017, 12, e0187173. [Google Scholar] [CrossRef] [PubMed]
- Gore, L.; Kearns, P.R.; de Martino, M.L.; Lee; De Souza, C.A.; Bertrand, Y.; Hijiya, N.; Stork, L.C.; Chung, N.G.; Cardos, R.C.; et al. Dasatinib in Pediatric Patients With Chronic Myeloid Leukemia in Chronic Phase: Results From a Phase II Trial. J. Clin. Oncol. 2018, 36, 1330–1338. [Google Scholar] [CrossRef]
- Ocana, A.; Gil-Martin, M.; Martín, M.; Rojo, F.; Antolín, S.; Guerrero, Á.; Trigo, J.M.; Muñoz, M.; Pandiella, A.; Diego, N.G.; et al. A phase I study of the SRC kinase inhibitor dasatinib with trastuzumab and paclitaxel as first line therapy for patients with HER2-overexpressing advanced breast cancer. GEICAM/2010-04 study. Oncotarget 2017, 8, 73144–73153. [Google Scholar] [CrossRef]
- Montemurro, M.; Cioffi, A.; Dômont, J.; Rutkowski, P.; Roth, A.D.; von Moos, R.; Inauen, R.; Toulmonde, M.; Burkhard, R.O.; Knuesli, C.; et al. Long-term outcome of dasatinib first-line treatment in gastrointestinal stromal tumor: A multicenter, 2-stage phase 2 trial (Swiss Group for Clinical Cancer Research 56/07). Cancer 2018, 124, 1449–1454. [Google Scholar] [CrossRef]
- Kalinsky, K.; Lee, S.; Rubin, K.M.; Lawrence, D.P.; Iafrarte, A.J.; Borger, D.R.; Margolin, K.A.; Leitao, M.M., Jr.; Tarhini, A.A.; Koon, H.B.; et al. A phase 2 trial of dasatinib in patients with locally advanced or stage IV mucosal, acral, or vulvovaginal melanoma: A trial of the ECOG-ACRIN Cancer Research Group (E2607). Cancer 2017, 123, 2688–2697. [Google Scholar] [CrossRef]
- Parseghian, C.M.; Parikh, N.U.; Wu, J.Y.; Jiang, Z.Q.; Henderson, L.; Tian, F.; Pastor, B.; Ychou, M.; Raghav, K.; Dasari, A.; et al. Dual Inhibition of EGFR and c-Src by Cetuximab and Dasatinib Combined with FOLFOX Chemotherapy in Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2017, 23, 4146–4154. [Google Scholar] [CrossRef]
- Johnson, F.M.; Saigal, B.; Talpaz, M.; Donato, N.J. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin. Cancer Res. 2005, 11, 6924–6932. [Google Scholar] [CrossRef] [PubMed]
- Green, T.P.; Fennell, M.; Whittaker, R.; Curwen, J.; Jacobs, V.; Allen, J.; Logie, A.; Hargreaves, J.; Hickinson, D.M.; Wilkinson, R.W.; et al. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol. Oncol. 2009, 3, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.D.; Glisson, B.S.; Bekele, B.N.; Johnson, F.M.; Ginsberg, L.E.; El-Naggar, A.; Culotta, K.S.; Takebe, N.; Wright, J.; Tran, H.T.; et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer 2011, 117, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Fury, M.G.; Baxi, S.; Shen, R.; Kelly, K.W.; Lipson, B.L.; Carlson, D.; Stambuk, H.; Haque, S.; Pfister, D.G. Phase II study of saracatinib (AZD0530) for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). Anticancer Res. 2011, 31, 249–253. [Google Scholar]
- Kawakatsu, H.; Sakai, T.; Takagaki, Y.; Shinoda, Y.; Saito, M.; Owada, M.K.; Yano, J. A new monoclonal antibody which selectively recognizes the active form of Src tyrosine kinase. J. Biol. Chem. 1996, 271, 5680–5685. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Hermsen, M.A.; Fresno, M.F.; Brakenhoff, R.H.; García-Velasco, F.; Snijders, P.J.; Heideman, D.A.; García-Pedrero, J.M. Prevalence of human papillomavirus in laryngeal and hypopharyngeal squamous cell carcinomas in northern Spain. Cancer Epidemiol. 2015, 39, 37–41. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; García-Carracedo, D.; García, L.A.; Menéndez, S.; Allonca, E.; González, M.V.; Fresno, M.F.; Suárez, C.; García-Pedrero, J.M. Distinctive clinicopathological associations of amplification of the cortactin gene at 11q13 in head and neck squamous cell carcinomas. J. Pathol. 2009, 217, 516–523. [Google Scholar] [CrossRef]
- Canel, M.; Secades, P.; Garzón-Arango, M.; Allonca, E.; Suarez, C.; Serrels, A.; Frame, M.; Brunton, V.; Chiara, M.D. Involvement of focal adhesion kinase in cellular invasion of head and neck squamous cell carcinomas via regulation of MMP-2 expression. Br. J. Cancer 2008, 98, 1274–1284. [Google Scholar] [CrossRef]
- Sato, H.; Hatanaka, K.C.; Hatanaka, Y.; Hatakeyama, H.; Hashimoto, A.; Matsuno, Y.; Fukuda, S.; Sabe, H. High level expression of AMAP1 protein correlates with poor prognosis and survival after surgery of head and neck squamous cell carcinoma patients. Cell Commun. Signal 2014, 12, 17. [Google Scholar] [CrossRef]
- Becchetti, A.; Crescioli, S.; Zanieri, F.; Petroni, G.; Mercatelli, R.; Coppola, S.; Gasparoli, L.; D’Amico, M.; Pillozzi, S.; Crociani, O.; et al. The conformational state of hERG1 channels determines integrin association, downstream signaling, and cancer progression. Sci. Signal 2017, 10, eaaf3236. [Google Scholar] [CrossRef]
- Menéndez, S.T.; Rodrigo, J.P.; Alvarez-Teijeiro, S.; Villaronga, M.Á.; Allonca, E.; Vallina, A.; Astudillo, A.; Barros, F.; Suárez, C.; García-Pedrero, J.M. Role of HERG1 potassium channel in both malignant transformation and disease progression in head and neck carcinomas. Mod. Pathol. 2012, 25, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Veracini, L.; Grall, D.; Schaub, S.; Beghelli-de la Forest Divonne, S.; Etienne-Grimaldi, M.C.; Milano, G.; Bozec, A.; Babin, E.; Sudaka, A.; Thariat, J.; et al. Elevated Src family kinase activity stabilizes E-cadherin-based junctions and collective movement of head and neck squamous cell carcinomas. Oncotarget 2015, 6, 7570–7583. [Google Scholar] [CrossRef] [PubMed]
- Koppikar, P.; Choi, S.H.; Egloff, A.M.; Cai, Q.; Suzuki, S.; Freilino, M.; Nozawa, H.; Thomas, S.M.; Gooding, W.E.; Siegfried, J.M.; et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4284–4291. [Google Scholar] [CrossRef] [PubMed]
- Arcaroli, J.J.; Touban, B.M.; Tan, A.C.; Varella-Garcia, M.; Powell, R.W.; Eckhardt, S.G.; Elvin, P.; Gao, D.; Messersmith, W.A. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin. Cancer Res. 2010, 16, 4165–4177. [Google Scholar] [CrossRef]
- Nagaraj, N.S.; Smith, J.J.; Revetta, F.; Washington, M.K.; Merchant, N.B. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol. Cancer Ther. 2010, 9, 2322–2332. [Google Scholar] [CrossRef]
- Suwaki, N.; Vanhecke, E.; Atkins, K.M.; Graf, M.; Swabey, K.; Huang, P.; Schraml, P.; Moch, H.; Cassidy, A.M.; Brewer, D.; et al. A HIF-regulated VHL-PTP1B-Src signaling axis identifies a therapeutic target in renal cell carcinoma. Sci. Transl. Med. 2011, 3, 85ra47. [Google Scholar] [CrossRef]
- Zou, D.; Yoon, H.S.; Anjomshoaa, A.; Perez, D.; Fukuzawa, R.; Guilford, P.; Humar, B. Increased levels of active c-Src distinguish invasive from in situ lobular lesions. Breast Cancer Res. 2009, 11, R45. [Google Scholar] [CrossRef]
- Cheng, S.J.; Kok, S.H.; Lee, J.J.; Yen-Ping Kuo, M.; Cheng, S.L.; Huang, Y.L.; Chen, H.M.; Chang, H.H.; Chiang, C.P. Significant association of SRC protein expression with the progression, recurrence, and prognosis of oral squamous cell carcinoma in Taiwan. Head Neck 2012, 34, 1340–1345. [Google Scholar] [CrossRef]
- Ke, L.; Xiang, Y.; Guo, X.; Lu, J.; Xia, W.; Yu, Y.; Peng, Y.; Wang, L.; Wang, G.; Ye, Y.; et al. c-Src activation promotes nasopharyngeal carcinoma metastasis by inducing the epithelial-mesenchymal transition via PI3K/Akt signaling pathway: A new and promising target for NPC. Oncotarget 2016, 7, 28340–28355. [Google Scholar] [CrossRef]
- Hermida-Prado, F.; Villaronga, M.A.; Granda-Díaz, R.; del-Río-Ibisate, N.; Santos, L.; Hermosilla, M.A.; Oro, P.; Allonca, E.; Agorreta, J.; Garmendia, I.; et al. The SRC Inhibitor Dasatinib Induces Stem Cell-Like Properties in Head and Neck Cancer Cells that are Effectively Counteracted by the Mithralog EC-8042. J. Clin. Med. 2019, 8, 1157. [Google Scholar] [CrossRef]
- Baro, M.; de Llobet, L.I.; Figueras, A.; Skvortsova, I.; Mesia, R.; Balart, J. Dasatinib worsens the effect of cetuximab in combination with fractionated radiotherapy in FaDu- and A431-derived xenografted tumours. Br. J. Cancer 2014, 111, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Ammer, A.G.; Kelley, L.C.; Hayes, K.E.; Evans, J.V.; Lopez-Skinner, L.A.; Martin, K.H.; Frederick, B.; Rothschild, B.L.; Raben, D.; Elvin, P.; et al. Saracatinib Impairs Head and Neck Squamous Cell Carcinoma Invasion by Disrupting Invadopodia Function. J. Cancer Sci. Ther. 2009, 1, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Bauman, J.E.; Duvvuri, U.; Gooding, W.E.; Rath, T.J.; Gross, N.D.; Song, J.; Jimeno, A.; Yarbrough, W.G.; Johnson, F.M.; Wang, L.; et al. Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI Insight 2017, 2, e90449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, W.C.; Li, P.; Guo, H.; Poh, S.B.; Brady, S.W.; Xiong, Y.; Tseng, L.M.; Li, S.H.; Ding, Z.; et al. Combating trastuzumab resistance by targeting SRC, a common nodedownstream of multiple resistance pathways. Nat. Med. 2011, 17, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Rexer, B.N.; Ham, A.J.; Rinehart, C.; Hill, S.; Granja-Ingram Nde, M.; González-Angulo, A.M.; Mills, G.B.; Dave, B.; Chang, J.C.; Liebler, D.C.; et al. Phosphoproteomic mass spectrometry profiling links Src family kinases to escape from HER2 tyrosine kinase inhibition. Oncogene 2011, 30, 4163–4174. [Google Scholar] [CrossRef]
- Bertotti, A.; Bracco, C.; Girolami, F.; Torti, D.; Gastaldi, S.; Galimi, F.; Medico, E.; Elvin, P.; Comoglio, P.M.; Trusolino, L. Inhibition of Src impairs the growth of met-addicted gastric tumors. Clin. Cancer Res. 2010, 16, 3933–3943. [Google Scholar] [CrossRef]
- Sen, B.; Peng, S.; Saigal, B.; Williams, M.D.; Johnson, F.M. Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin. Cancer Res. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Song, N.; Qu, X.; Liu, S.; Zhang, S.; Liu, J.; Qu, J.; Zheng, H.; Liu, Y.; Che, X. Dual inhibition of MET and SRC kinase activity as a combined targeting strategy for colon cancer. Exp. Ther. Med. 2017, 14, 1357–1366. [Google Scholar] [CrossRef][Green Version]

| Characteristic | No. | Active SRC Expression (%) | P |
|---|---|---|---|
| pT classification | |||
| T1, T2 | 32 | 21 (66) | 0.145 † |
| T3, T4 | 84 | 67 (79) | |
| pN classification | |||
| N0 | 39 | 24 (62) | 0.020 † |
| N1–3 | 77 | 64 (83) | |
| Disease stage | |||
| I, II | 15 | 8 (53) | 0.048 † |
| III, IV | 101 | 80 (79) | |
| Pathological grade | |||
| Well differentiated | 33 | 22 (67) | 0.286 # |
| Moderately differentiated | 53 | 41 (77) | |
| Poorly differentiated | 30 | 25 (83) | |
| Site | |||
| Pharynx | 58 | 48 (83) | 0.128 † |
| Larynx | 58 | 40 (69) | |
| Tumor Recurrence ‡ | |||
| No | 26 | 14 (54) | 0.002 † |
| Yes | 68 | 58 (85) | |
| Disease status | |||
| Alive without disease | 30 | 16 (53) | 0.001 † |
| Dead of index cancer | 64 | 56 (88) | |
| ___ | |||
| Died of other causes | 22 | 16 (73) | |
| Total Cases | 116 | 88 (76) | |
| Characteristic | Pharynx (No. = 58) | Larynx (No. = 58) | ||||
|---|---|---|---|---|---|---|
| No. | Active SRC (%) | P | No. | Active SRC (%) | p | |
| pT classification | ||||||
| T1, T2 | 17 | 13 (76) | 15 | 8 (53) | ||
| T3, T4 | 41 | 35 (85) | 0.458 † | 43 | 32 (74) | 0.194 † |
| pN classification | ||||||
| N0 | 10 | 7 (70) | 29 | 17 (59) | 0.155 † | |
| N1–3 | 48 | 41 (85) | 0.353 † | 29 | 23 (79) | |
| Disease stage | ||||||
| I, II | 5 | 4 (80) | 1.000 † | 10 | 4 (40) | 0.055 † |
| III, IV | 53 | 44 (83) | 48 | 36 (75) | ||
| Pathological grade | ||||||
| Well differentiated | 12 | 10 (83) | 21 | 12 (57) | ||
| Moderately differentiated | 26 | 22 (85) | 0.917 # | 27 | 19 (70) | 0.177 # |
| Poorly differentiated | 20 | 16 (80) | 10 | 9 (90) | ||
| Tumor Recurrence ‡ | ||||||
| No | 8 | 7 (88) | 1.000 † | 18 | 7 (39) | <0.001 † |
| Yes | 42 | 34 (81) | 26 | 24 (92) | ||
| Disease status | ||||||
| Alive without disease | 10 | 8 (80) | 1.000 † | 20 | 8 (40) | <0.001 † |
| Dead of index cancer | 40 | 33 (83) | 24 | 23 (96) | ||
| ___ | ||||||
| Died of other causes | 8 | 7 (88) | 14 | 9 (64) | ||
| Molecular Feature | No. | Active SRC (%) | p# |
|---|---|---|---|
| CTTN protein expression | (0.050) | ||
| Negative | 57 | 42 (74) | 0.594 |
| Positive (scores 2–3) | 59 | 46 (78) | |
| FAK protein expression | (0.225) | ||
| Negative | 33 | 20 (61) | 0.015 |
| Positive (scores 2–3) | 83 | 68 (82) | |
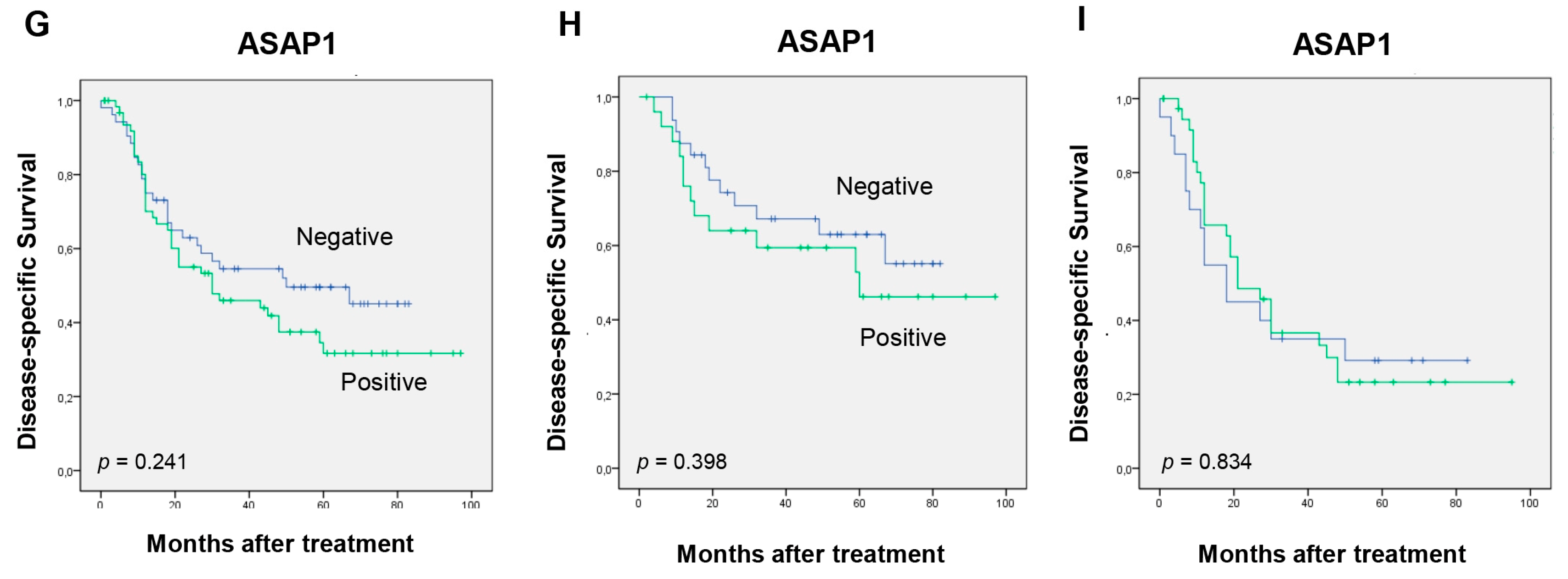
| ASAP1 protein expression | (0.221) | ||
| Negative | 52 | 34 (65) | 0.017 |
| Positive (scores 2–3) | 64 | 54 (84) | |
| HERG1 protein expression | (0.240) | ||
| Negative | 14 | 7 (50) | 0.011 |
| Positive (scores 2–3) | 98 | 79 (81) |
| Molecular Feature | Pharynx (No. = 58) | Larynx (No. = 58) | ||||
|---|---|---|---|---|---|---|
| No. | Active SRC (%) | p# | No. | Active SRC (%) | p# | |
| FAK protein expression | (0.326) | |||||
| Negative | 14 | 11 (79) | (0.063) | 19 | 9 (47) | 0.013 |
| Positive (scores 2–3) | 44 | 37 (85) | 0.641 | 39 | 31 (79) | |
| ASAP1 protein expression | (0.230) | |||||
| Negative | 20 | 15 (75) | (0.149) | 32 | 19 (59) | 0.082 |
| Positive (scores 2–3) | 38 | 33 (87) | 0.264 | 26 | 21 (81) | |
| HERG1 protein expression | (0.366) | |||||
| Negative | 5 | 4 (80) | (0.02) | 9 | 3 (33) | 0.006 |
| Positive (scores 2–3) | 52 | 43 (83) | 0.882 | 46 | 36 (80) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermida-Prado, F.; Granda-Díaz, R.; del-Río-Ibisate, N.; Villaronga, M.Á.; Allonca, E.; Garmendia, I.; Montuenga, L.M.; Rodríguez, R.; Vallina, A.; Alvarez-Marcos, C.; et al. The Differential Impact of SRC Expression on the Prognosis of Patients with Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 1644. https://doi.org/10.3390/cancers11111644
Hermida-Prado F, Granda-Díaz R, del-Río-Ibisate N, Villaronga MÁ, Allonca E, Garmendia I, Montuenga LM, Rodríguez R, Vallina A, Alvarez-Marcos C, et al. The Differential Impact of SRC Expression on the Prognosis of Patients with Head and Neck Squamous Cell Carcinoma. Cancers. 2019; 11(11):1644. https://doi.org/10.3390/cancers11111644
Chicago/Turabian StyleHermida-Prado, Francisco, Rocío Granda-Díaz, Nagore del-Río-Ibisate, M. Ángeles Villaronga, Eva Allonca, Irati Garmendia, Luis M. Montuenga, René Rodríguez, Aitana Vallina, César Alvarez-Marcos, and et al. 2019. "The Differential Impact of SRC Expression on the Prognosis of Patients with Head and Neck Squamous Cell Carcinoma" Cancers 11, no. 11: 1644. https://doi.org/10.3390/cancers11111644
APA StyleHermida-Prado, F., Granda-Díaz, R., del-Río-Ibisate, N., Villaronga, M. Á., Allonca, E., Garmendia, I., Montuenga, L. M., Rodríguez, R., Vallina, A., Alvarez-Marcos, C., Rodrigo, J. P., & García-Pedrero, J. M. (2019). The Differential Impact of SRC Expression on the Prognosis of Patients with Head and Neck Squamous Cell Carcinoma. Cancers, 11(11), 1644. https://doi.org/10.3390/cancers11111644







