Galeterone and The Next Generation Galeterone Analogs, VNPP414 and VNPP433-3β Exert Potent Therapeutic Effects in Castration-/Drug-Resistant Prostate Cancer Preclinical Models In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
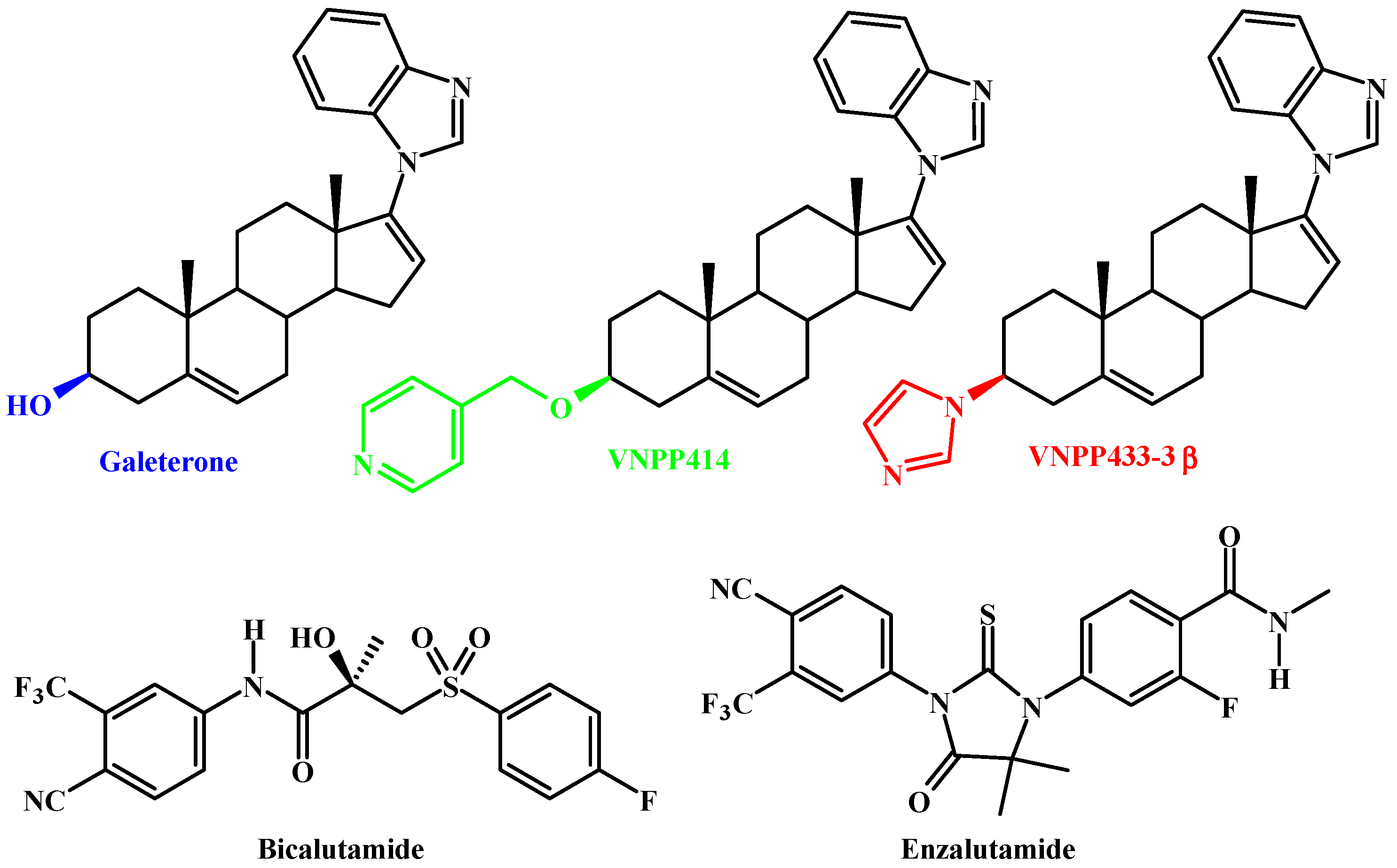
2.1. Unlike Enzalutamide, Galeterone and the Lead NGGAs Show Strong In Vitro Anti-Prostate Cancer Activities in Drug-Naïve and Drug-Resistant Prostate Cancer Cell Lines
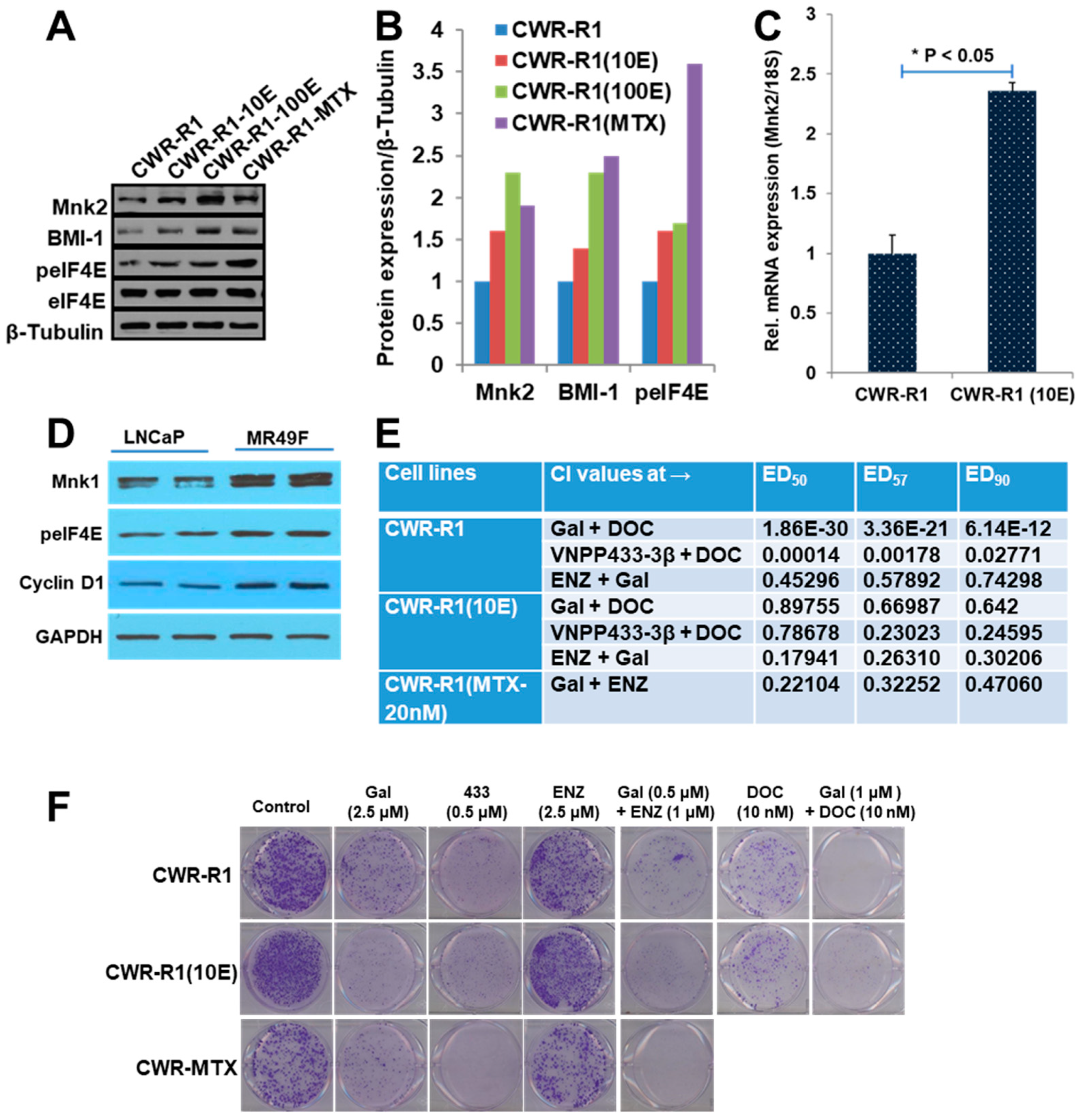
2.2. Gal and VNPP433-3β Synergize with Docetaxel (DOC) and Enzalutamide (ENZ)
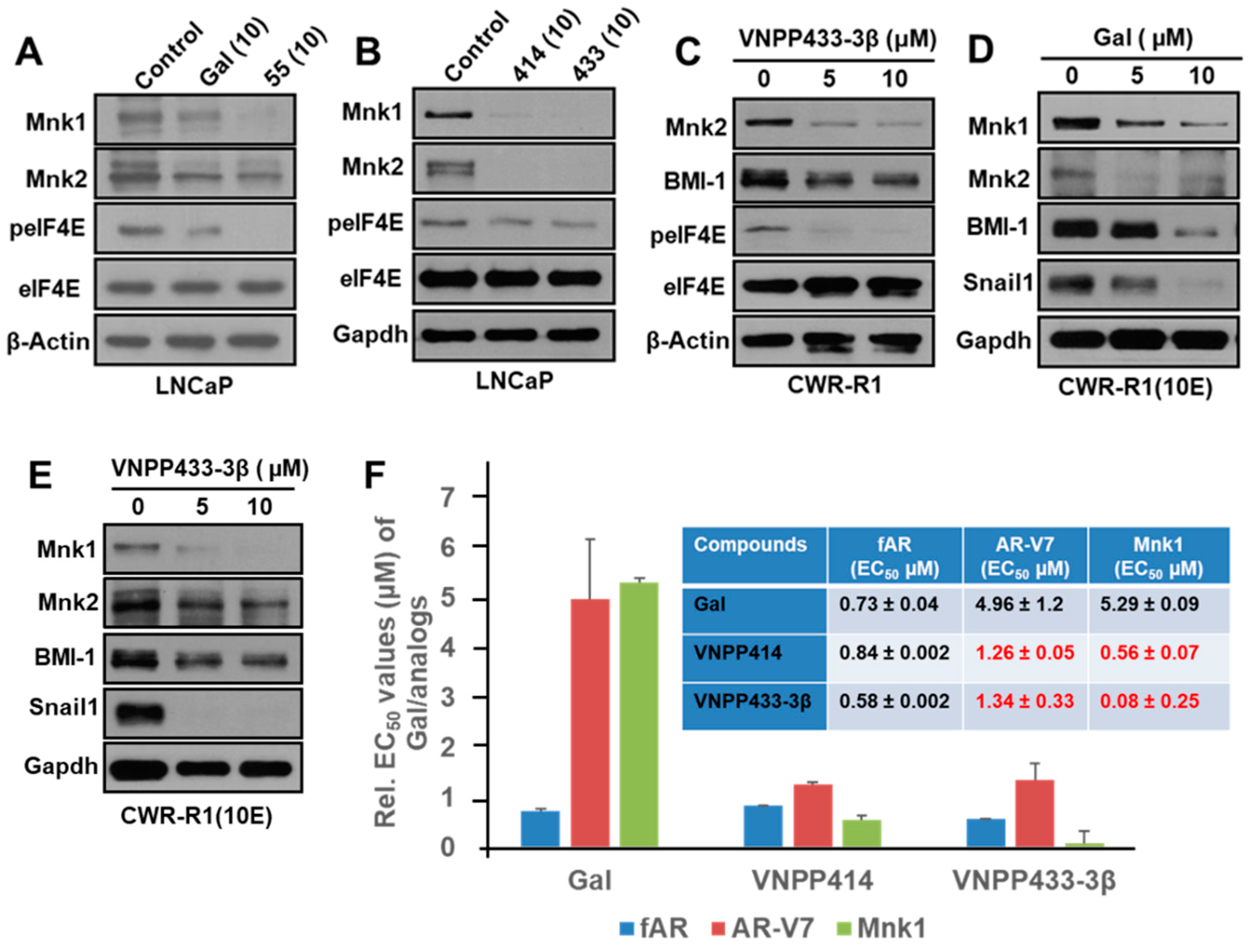
2.3. Gal and NGGAs Target AR/AR-V7 and Mnk1/2-eIF4E Signaling Pathways in Drug-Naïve and Drug-Resistant Prostate Cancer Cell Lines
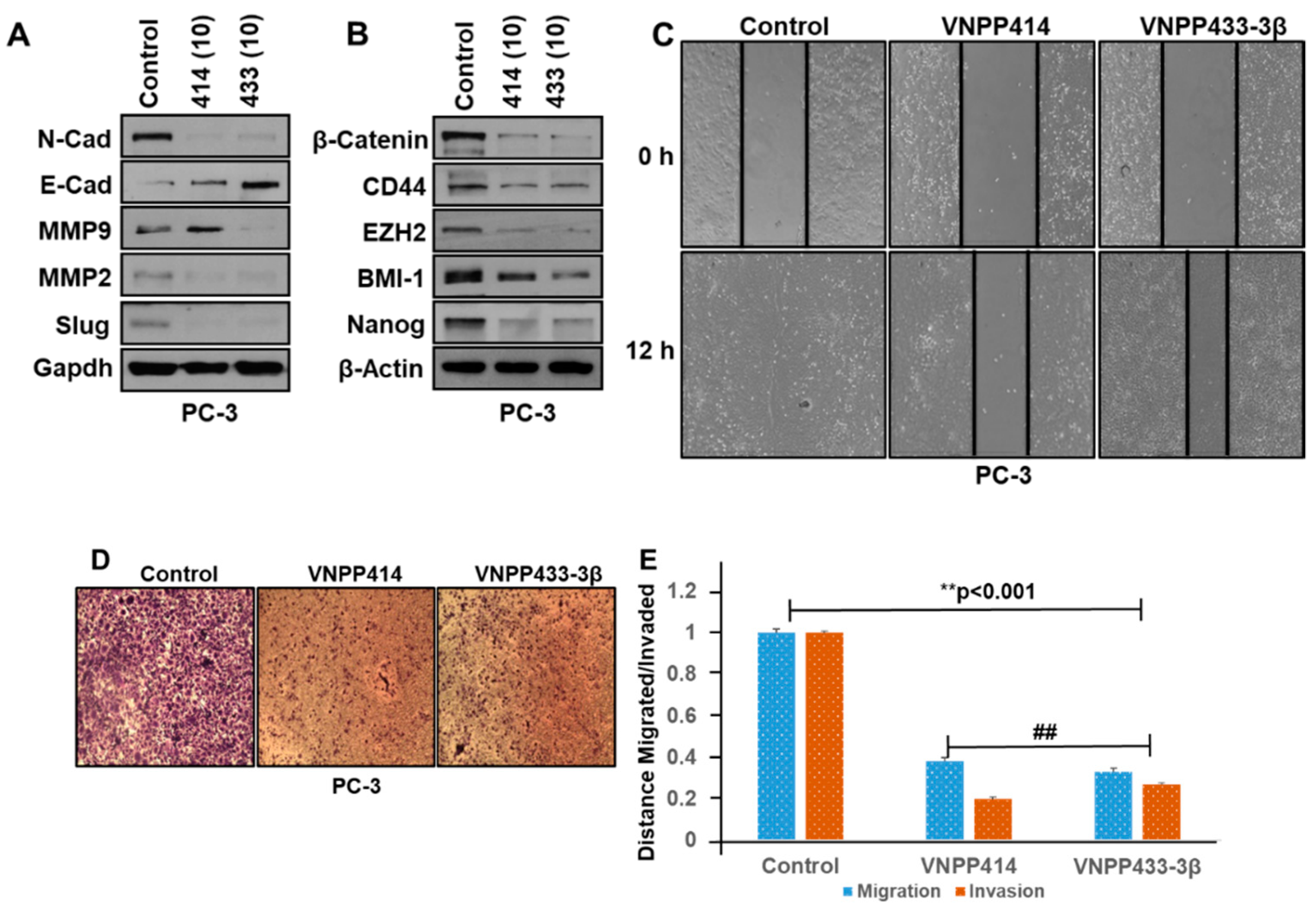
2.4. VNPP414 and VNPP433-3β Reverse EMT Activity, Deplete Stem Cell Like Factors and Inhibit Prostate Cancer Cells Migration and Invasion
2.5. Pharmacokinetic Parameters of VNPP414 and VNPP433-3β in Mouse are Superior to Those of Galeterone
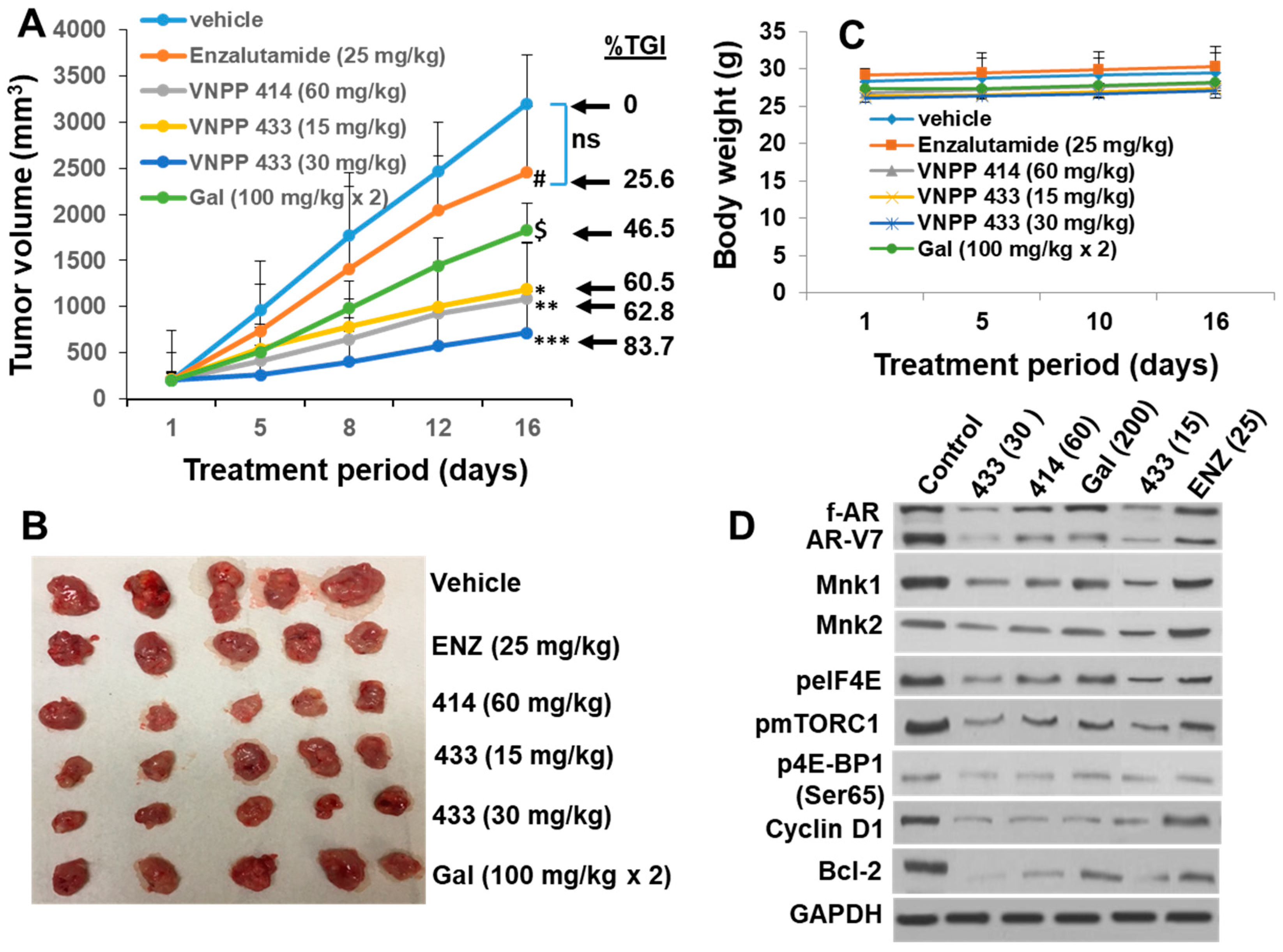
2.6. Galeterone and the NGGAs Are More Effective Than Enzalutamide (ENZ) in Castration-Resistant Prostate Cancer CWR22Rv1 Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Reagents, Compounds and Antibodies
4.2. Cell Culture
4.3. Western Blot Analysis
4.4. Cell Viability Assays MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium Bromide, Colorimetric Assay)
4.5. Combination Studies to Assess Synergy, Additivity or Antagonism
4.6. RNA Isolation and Real-Time Polymerase Chain Reaction Analysis
4.7. Cell Motility and Invasion Assay
4.8. Colony Formation Assay
4.9. Animal Study Approval
4.9.1. Pharmacokinetic Studies
4.9.2. In Vivo Anti-Tumor Studies in CWR22Rv1 CRPC Xenograft Model.
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Njar, V.C.; Brodie, A.M. Discovery and development of Galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancer. J. Med. Chem. 2015, 58, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Kwegyir-Afful, A.K.; Ramalingam, S.; Purushottamachar, P.; Ramamurthy, V.P.; Njar, V.C. Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo. Oncotarget 2015, 6, 27440–27460. [Google Scholar] [CrossRef] [PubMed]
- Purushottamachar, P.; Godbole, A.M.; Gediya, L.K.; Martin, M.S.; Vasaitis, T.S.; Kwegyir-Afful, A.K.; Ramalingam, S.; Ates-Alagoz, Z.; Njar, V.C. Systematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancer. J. Med. Chem. 2013, 56, 4880–4898. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Mamlouk, K.; Montgomery, B.; Taplin, M.E. Treatment with galeterone in an elderly man mith mastration-resistant prostate cancer: A case report. Clin. Genitourin. Cancer 2015, 13, e325–e328. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Werner, L.; Fiorillo, M.; Roberts, J.; Heath, E.I.; Bubley, G.J.; Montgomery, R.B.; Taplin, M.E. Efficacy of therapies after galeterone in patients with castration-resistant prostate cancer. Clin. Genitourin. Cancer 2017, 15, 463–471. [Google Scholar] [CrossRef]
- Montgomery, B.; Eisenberger, M.A.; Rettig, M.B.; Chu, F.; Pili, R.; Stephenson, J.J.; Vogelzang, N.J.; Koletsky, A.J.; Nordquist, L.T.; Edenfield, W.J.; et al. Androgen receptor modulation optimized for response (ARMOR) phase I and II studies: Galeterone for the treatment of castration-resistant prostate cancer. Clin. Cancer Res. 2016, 22, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Purushottamachar, P.R.; Ramalingam, S.; Njar, V.C.O. Development of benzimidazole compounds for cancer therapy. In Chemistry Applications Benzimidazole Derivatives; IntechOpen: London, UK, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Purushottamachar, P.; Kwegyir-Afful, A.K.; Martin, M.S.; Ramamurthy, V.P.; Ramalingam, S.; Njar, V.C.O. Identification of novel steroidal androgen receptor degrading agents inspired by galeterone 3β-Imidazole Carbamate. ACS Med. Chem. Lett. 2016, 7, 708–713. [Google Scholar] [CrossRef]
- Kwegyir-Afful, A.K.; Bruno, R.D.; Purushottamachar, P.; Murigi, F.N.; Njar, V.C. Galeterone and VNPT55 disrupt Mnk-eIF4E to inhibit prostate cancer cell migration and invasion. FEBS J. 2016, 283, 3898–3918. [Google Scholar] [CrossRef] [Green Version]
- Kwegyir-Afful, A.K.; Murigi, F.N.; Purushottamachar, P.; Ramamurthy, V.P.; Martin, M.S.; Njar, V.C.O. Galeterone and its analogs inhibit Mnk-eIF4E axis, synergize with gemcitabine, impede pancreatic cancer cell migration, invasion and proliferation and inhibit tumor growth in mice. Oncotarget 2017, 8, 52381–52402. [Google Scholar] [CrossRef]
- Ramamurthy, V.P.; Ramalingam, S.; Kwegyir-Afful, A.K.; Hussain, A.; Njar, V.C.O. Therapeutic targeting of protein translation as a new paradigm for prostate cancer. Curr. Opin. Oncol. 2017, 29, 210–220. [Google Scholar] [CrossRef]
- Scheper, G.C.; Morrice, N.A.; Kleijn, M.; Proud, C.G. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol. Cell Biol. 2001, 21, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Watanabe-Fukunaga, R.; Fukuyama, H.; Nagata, S.; Fukunaga, R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell Biol. 2004, 24, 6539–6549. [Google Scholar] [CrossRef]
- Waskiewicz, A.J.; Johnson, J.C.; Penn, B.; Mahalingam, M.; Kimball, S.R.; Cooper, J.A. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell Biol. 1999, 19, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Graff, J.; Ruggero, D.; Sonenberg, N. Targeting the eIF4F translation initiation complex: A critical nexus for cancer development. Cancer Res. 2015, 75, 250–263. [Google Scholar] [CrossRef]
- Proud, C.G. Mnks, eIF4E phosphorylation and cancer. Biochim. Biophys. Acta 2015, 1849, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Topisirovic, I.; Ruiz-Gutierrez, M.; Borden, K.L. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004, 64, 8639–8642. [Google Scholar] [CrossRef]
- Xie, J.; Merrett, J.E.; Jensen, K.B.; Proud, C.G. The MAP kinase-interacting kinases (MNKs) as targets in oncology. Expert Opin. Ther. Targets 2019, 23, 187–199. [Google Scholar] [CrossRef]
- D’Abronzo, L.S.; Bose, S.; Crapuchettes, M.E.; Beggs, R.E.; Vinall, R.L.; Tepper, C.G.; Siddiqui, S.; Mudryj, M.; Melgoza, F.U.; Durbin-Johnson, B.P.; et al. The androgen receptor is a negative regulator of eIF4E phosphorylation at S209: Implications for the use of mTOR inhibitors in advanced prostate cancer. Oncogene 2017, 36, 6359–6373. [Google Scholar] [CrossRef]
- D’Abronzo, L.S.; Ghosh, P.M. eIF4E Phosphorylation in Prostate Cancer. Neoplasia 2018, 20, 563–573. [Google Scholar] [CrossRef]
- Ramamurthy, V.P.; Ramalingam, S.; Gediya, L.; Kwegyir-Afful, A.K.; Njar, V.C. Simultaneous targeting of androgen receptor (AR) and MAPK-interacting kinases (MNKs) by novel retinamides inhibits growth of human prostate cancer cell lines. Oncotarget 2015, 6, 3195–3210. [Google Scholar] [CrossRef]
- Ramamurthy, V.P.; Ramalingam, S.; Gediya, L.K.; Njar, V.C.O. The retinamide VNLG-152 inhibits f-AR/AR-V7 and MNK-eIF4E signaling pathways to suppress EMT and castration-resistant prostate cancer xenograft growth. FEBS J. 2018, 285, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Robichaud, N.; Hulea, L.; Sonenberg, N.; Pelletier, J.; Topisirovic, I. Targeting the translation machinery in cancer. Nat. Rev. Drug. Discov. 2015, 14, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.; Qu, M.; Wan, H.; Cai, F.; Zhang, P. Inhibiting the MNK-eIF4E-beta-catenin axis increases the responsiveness of aggressive breast cancer cells to chemotherapy. Oncotarget 2017, 8, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Lineham, E.; Spencer, J.; Morley, S.J. Dual abrogation of MNK and mTOR: A novel therapeutic approach for the treatment of aggressive cancers. Future Med. Chem. 2017, 9, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Diez, C.; Garcia-Recio, E.M.; Perez-Morgado, M.I.; Garcia-Hernandez, M.; Sanz-Criado, L.; Sacristan, S.; Toledo-Lobo, M.V.; Perez-Mies, B.; Esteban-Rodriguez, I.; Pascual, A.; et al. Increased expression of MNK1b, the spliced isoform of MNK1, predicts poor prognosis and is associated with triple-negative breast cancer. Oncotarget 2018, 9, 13501–13516. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, N.; Sonenberg, N. Signalling to eIF4E in cancer. Biochem. Soc. Trans. 2015, 43, 763–772. [Google Scholar] [CrossRef]
- Bastos, D.A.; Antonarakis, E.S. Galeterone for the treatment of advanced prostate cancer: The evidence to date. Drug. Des. Devel Ther. 2016, 10, 2289–2297. [Google Scholar] [CrossRef]
- Boudadi, K.; Antonarakis, E.S. Resistance to Novel Antiandrogen Therapies in Metastatic Castration-Resistant Prostate Cancer. Clin. Med. Insights Oncol. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Kong, D.; Sethi, S.; Li, Y.; Chen, W.; Sakr, W.A.; Heath, E.; Sarkar, F.H. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate 2015, 75, 161–174. [Google Scholar] [CrossRef]
- Liu, C.; Armstrong, C.M.; Lou, W.; Lombard, A.P.; Cucchiara, V.; Gu, X.; Yang, J.C.; Nadiminty, N.; Pan, C.X.; Evans, C.P.; et al. Niclosamide and bicalutamide combination treatment overcomes enzalutamide- and bicalutamide-resistant prostate cancer. Mol. Cancer Ther. 2017, 16, 1521–1530. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Nakazawa, M.; Luo, J. Resistance to androgen-pathway drugs in prostate cancer. N. Engl.J. Med. 2014, 371, 2234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chan, S.C.; Brand, L.J.; Hwang, T.H.; Silverstein, K.A.; Dehm, S.M. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013, 73, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A.; Marck, B.T.; Plymate, S.R.; Vessella, R.L.; Balk, S.; Matsumoto, A.M.; Nelson, P.S.; Montgomery, R.B. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 2011, 17, 5913–5925. [Google Scholar] [CrossRef]
- Schrader, A.J.; Schrader, M.G.; Cronauer, M.V. Words of wisdom. Re: Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Eur. Urol. 2013, 64, 169–170. [Google Scholar] [CrossRef]
- Shapiro, D.; Tareen, B. Current and emerging treatments in the management of castration-resistant prostate cancer. Expert Rev. Anticancer Ther. 2012, 12, 951–964. [Google Scholar] [CrossRef]
- Kawahara, T.; Inoue, S.; Kashiwagi, E.; Chen, J.; Ide, H.; Mizushima, T.; Li, Y.; Zheng, Y.; Miyamoto, H. Enzalutamide as an androgen receptor inhibitor prevents urothelial tumorigenesis. Am. J. Cancer Res. 2017, 7, 2041–2050. [Google Scholar] [CrossRef]
- Furic, L.; Rong, L.; Larsson, O.; Koumakpayi, I.H.; Yoshida, K.; Brueschke, A.; Petroulakis, E.; Robichaud, N.; Pollak, M.; Gaboury, L.A.; et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc. Natl. Acad. Sci. USA 2010, 107, 14134–14139. [Google Scholar] [CrossRef] [Green Version]
- Graff, J.R.; Konicek, B.W.; Lynch, R.L.; Dumstorf, C.A.; Dowless, M.S.; McNulty, A.M.; Parsons, S.H.; Brail, L.H.; Colligan, B.M.; Koop, J.W.; et al. eIF4E activation is commonly elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Res. 2009, 69, 3866–3873. [Google Scholar] [CrossRef]
- Jia, Y.; Polunovsky, V.; Bitterman, P.B.; Wagner, C.R. Cap-dependent translation initiation factor eIF4E: An emerging anticancer drug target. Med. Res. Rev. 2012, 32, 786–814. [Google Scholar] [CrossRef]
- Hay, N. Mnk earmarks eIF4E for cancer therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 13975–13976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulholland, D.J.; Kobayashi, N.; Ruscetti, M.; Zhi, A.; Tran, L.M.; Huang, J.; Gleave, M.; Wu, H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012, 72, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Tasseff, R.; Nayak, S.; Salim, S.; Kaushik, P.; Rizvi, N.; Varner, J.D. Analysis of the molecular networks in androgen dependent and independent prostate cancer revealed fragile and robust subsystems. PLoS ONE 2010, 5, e8864. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef]
- Crea, F.; Duhagon Serrat, M.A.; Hurt, E.M.; Thomas, S.B.; Danesi, R.; Farrar, W.L. BMI1 silencing enhances docetaxel activity and impairs antioxidant response in prostate cancer. Int. J. Cancer 2011, 128, 1946–1954. [Google Scholar] [CrossRef]
- Kuruma, H.; Matsumoto, H.; Shiota, M.; Bishop, J.; Lamoureux, F.; Thomas, C.; Briere, D.; Los, G.; Gleave, M.; Fanjul, A.; et al. A novel antiandrogen, Compound 30, suppresses castration-resistant and MDV3100-resistant prostate cancer growth in vitro and in vivo. Mol. Cancer Ther. 2013, 12, 567–576. [Google Scholar] [CrossRef]
- Handratta, V.D.; Vasaitis, T.S.; Njar, V.C.; Gediya, L.K.; Kataria, R.; Chopra, P.; Newman, D., Jr.; Farquhar, R.; Guo, Z.; Qiu, Y.; et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: Synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J. Med. Chem. 2005, 48, 2972–2984. [Google Scholar] [CrossRef]
- Liu, C.; Armstrong, C.; Zhu, Y.; Lou, W.; Gao, A.C. Niclosamide enhances abiraterone treatment via inhibition of androgen receptor variants in castration resistant prostate cancer. Oncotarget 2016, 7, 32210–32220. [Google Scholar] [CrossRef]
- Gardelli, C.; Nizi, E.; Muraglia, E.; Crescenzi, B.; Ferrara, M.; Orvieto, F.; Pace, P.; Pescatore, G.; Poma, M.; Ferreira Mdel, R.; et al. Discovery and synthesis of HIV integrase inhibitors: Development of potent and orally bioavailable N-methyl pyrimidones. J. Med. Chem. 2007, 50, 4953–4975. [Google Scholar] [CrossRef]
- Godbole, A.M.; Ramalingam, S.; Ramamurthy, V.P.; Khandelwal, A.; Bruno, R.D.; Upreti, V.V.; Gediya, L.K.; Purushottamachar, P.; Mbatia, H.W.; Addya, S.; et al. VN/14-1 induces ER stress and autophagy in HP-LTLC human breast cancer cells and has excellent oral pharmacokinetic profile in female Sprague Dawley rats. Eur. J. Pharmacol. 2014, 734, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.H.; Tsai, H.J.; Liu, J.Y.; Morisseau, C.; Hammock, B.D. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J. Med. Chem. 2007, 50, 3825–3840. [Google Scholar] [CrossRef] [PubMed]
- Barrie, S.E.; Potter, G.A.; Goddard, P.M.; Haynes, B.P.; Dowsett, M.; Jarman, M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J. Steroid Biochem. Mol. Biol. 1994, 50, 267–273. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, J.; Jiang, Z.; Chen, J.; Gu, S.; Yu, S.; Zheng, K.; Jia, L. Drug enterohepatic circulation and disposition: Constituents of systems pharmacokinetics. Drug Discov. Today 2014, 19, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, M.; Li, Z.; Berk, M.; Li, J.; Tang, J.; Upadhyay, S.; Auchus, R.J.; Sharifi, N. Steroidogenic metabolism of galeterone reveals a diversity of biochemical activities. Cell Chem. Biol. 2017, 24, 825–832.e6. [Google Scholar] [CrossRef]
- Gunaydin, H.; Altman, M.D.; Ellis, J.M.; Fuller, P.; Johnson, S.A.; Lahue, B.; Lapointe, B. Strategy for extending half-life in drug design and its significance. ACS Med. Chem. Lett. 2018, 9, 528–533. [Google Scholar] [CrossRef]
- Brown, M.C.; Gromeier, M. MNK inversely regulates TELO2 vs. DEPTOR to control mTORC1 signaling. Mol. Cell Oncol. 2017, 4, e1306010. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.C.; Gromeier, M. MNK controls mTORC1: Substrate association through regulation of TELO2 binding with mTORC1. Cell Rep. 2017, 18, 1444–1457. [Google Scholar] [CrossRef]
- Eckerdt, F.; Beauchamp, E.; Bell, J.; Iqbal, A.; Su, B.; Fukunaga, R.; Lulla, R.R.; Goldman, S.; Platanias, L.C. Regulatory effects of a Mnk2-eIF4E feedback loop during mTORC1 targeting of human medulloblastoma cells. Oncotarget 2014, 5, 8442–8451. [Google Scholar] [CrossRef]
- Grzmil, M.; Huber, R.M.; Hess, D.; Frank, S.; Hynx, D.; Moncayo, G.; Klein, D.; Merlo, A.; Hemmings, B.A. MNK1 pathway activity maintains protein synthesis in rapalog-treated gliomas. J. Clin. Investig. 2014, 124, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Joubert, P.E.; Stapleford, K.; Guivel-Benhassine, F.; Vignuzzi, M.; Schwartz, O.; Albert, M.L. Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins via the Activation of the MnK/eIF4E Pathway. PLoS Pathog. 2015, 11, e1005091. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Ramamurthy, V.P.; Gediya, L.K.; Murigi, F.N.; Purushottamachar, P.; Huang, W.; Choi, E.Y.; Zhang, Y.; Vasaitis, T.S.; Kane, M.A.; et al. The Novel Mnk1/2 Degrader and Apoptosis Inducer VNLG-152 Potently Inhibits TNBC Tumor Growth and Metastasis. Cancers 2019, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.J.; van Royen, M.E.; de Morree, E.S.; Moll, J.M.; Teubel, W.; Wiemer, E.A.; Mathijssen, R.H.; de Wit, R.; van Weerden, W.M. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur. J. Cancer 2013, 49, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Teo, T.; Yu, M.; Yang, Y.; Gillam, T.; Lam, F.; Sykes, M.J.; Wang, S. Pharmacologic co-inhibition of Mnks and mTORC1 synergistically suppresses proliferation and perturbs cell cycle progression in blast crisis-chronic myeloid leukemia cells. Cancer Lett. 2015, 357, 612–623. [Google Scholar] [CrossRef]
- Purushottamachar, P.; Murigi, F.N.; Njar, V.C.O. Improved Procedures for gram-scale synthesis of galeterone 3β-Imidazole and galeterone 3β-Pyridine methoxylate, potent androgen receptor/Mnk degrading agents. Org. Process. Res. Dev. 2016, 20, 1654–1661. [Google Scholar] [CrossRef]
- Linn, D.E.; Yang, X.; Sun, F.; Xie, Y.; Chen, H.; Jiang, R.; Chen, H.; Chumsri, S.; Burger, A.M.; Qiu, Y. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer 2010, 1, 908–916. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, K.; Linn, D.E.; Yang, X.; Guo, Z.; Shimelis, H.; Nakanishi, T.; Ross, D.D.; Chen, H.; Fazli, L.; et al. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J. Biol. Chem. 2008, 283, 3349–3356. [Google Scholar] [CrossRef]
- Chou, T.C.; Tan, Q.H.; Sirotnak, F.M. Quantitation of the synergistic interaction of edatrexate and cisplatin in vitro. Cancer Chemother. Pharmacol. 1993, 31, 259–264. [Google Scholar] [CrossRef]

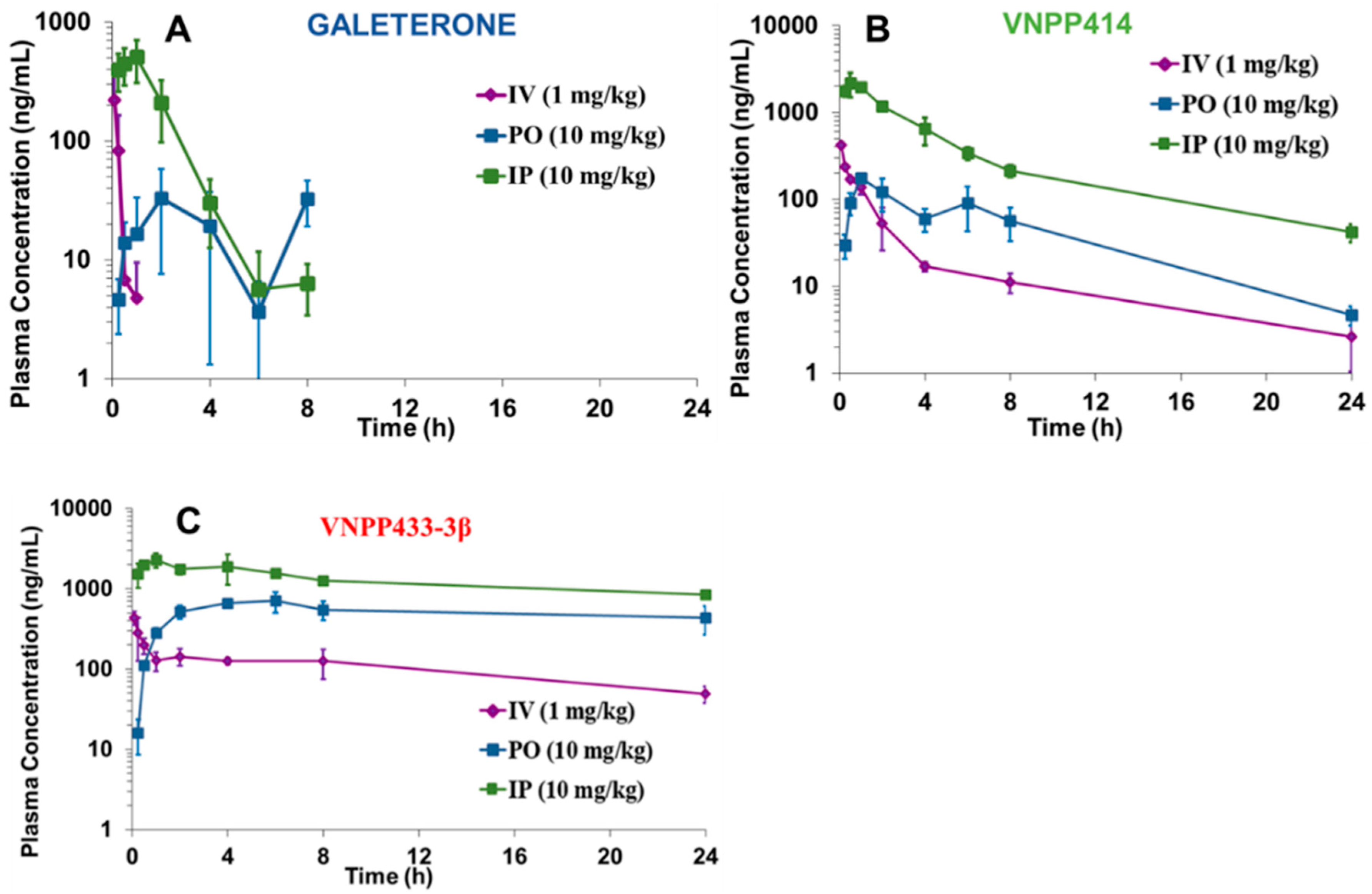
| Compounds | Dosing | AUC (0–∞) (ng.h/mL) | Cmax (ng/mL) | T1/2 (h) | F (%) |
|---|---|---|---|---|---|
| Galeterone | IV (1 mg/kg) | 57.90 | - | 0.17 | - |
| IP (10 mg/kg) | 111.29 | 506.59 | 1.24 | 168.81 | |
| PO (10 mg/kg) | 969.07 | 32.8 | 1.26 | 18.44 | |
| VNPP414 | IV (1 mg/kg) | 547.43 | - | 7.48 | - |
| IP (10 mg/kg) | 8601.34 | 2174.37 | 6.25 | 157.12 | |
| PO (10 mg/kg) | 1030.00 | 175.40 | 4.30 | 19.35 | |
| VNPP433-3β | IV (1 mg/kg) | 3469.23 | - | 14.26 | - |
| IP (10 mg/kg) | 57290.45 | 2294.19 | 22.38 | 123.20 | |
| PO (10 mg/kg) | 31755.01 | 706.27 | 31.19 | 49.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwegyir-Afful, A.K.; Ramalingam, S.; Ramamurthy, V.P.; Purushottamachar, P.; Murigi, F.N.; Vasaitis, T.S.; Huang, W.; Kane, M.A.; Zhang, Y.; Ambulos, N.; et al. Galeterone and The Next Generation Galeterone Analogs, VNPP414 and VNPP433-3β Exert Potent Therapeutic Effects in Castration-/Drug-Resistant Prostate Cancer Preclinical Models In Vitro and In Vivo. Cancers 2019, 11, 1637. https://doi.org/10.3390/cancers11111637
Kwegyir-Afful AK, Ramalingam S, Ramamurthy VP, Purushottamachar P, Murigi FN, Vasaitis TS, Huang W, Kane MA, Zhang Y, Ambulos N, et al. Galeterone and The Next Generation Galeterone Analogs, VNPP414 and VNPP433-3β Exert Potent Therapeutic Effects in Castration-/Drug-Resistant Prostate Cancer Preclinical Models In Vitro and In Vivo. Cancers. 2019; 11(11):1637. https://doi.org/10.3390/cancers11111637
Chicago/Turabian StyleKwegyir-Afful, Andrew K., Senthilmurugan Ramalingam, Vidya P. Ramamurthy, Puranik Purushottamachar, Francis N. Murigi, Tadas S. Vasaitis, Weiliang Huang, Maureen A. Kane, Yuji Zhang, Nicholas Ambulos, and et al. 2019. "Galeterone and The Next Generation Galeterone Analogs, VNPP414 and VNPP433-3β Exert Potent Therapeutic Effects in Castration-/Drug-Resistant Prostate Cancer Preclinical Models In Vitro and In Vivo" Cancers 11, no. 11: 1637. https://doi.org/10.3390/cancers11111637
APA StyleKwegyir-Afful, A. K., Ramalingam, S., Ramamurthy, V. P., Purushottamachar, P., Murigi, F. N., Vasaitis, T. S., Huang, W., Kane, M. A., Zhang, Y., Ambulos, N., Tiwari, S., Srivastava, P., Nnane, I. P., Hussain, A., Qiu, Y., Weber, D. J., & Njar, V. C. O. (2019). Galeterone and The Next Generation Galeterone Analogs, VNPP414 and VNPP433-3β Exert Potent Therapeutic Effects in Castration-/Drug-Resistant Prostate Cancer Preclinical Models In Vitro and In Vivo. Cancers, 11(11), 1637. https://doi.org/10.3390/cancers11111637








