Clinical Significance of Various Drug-Sensitivity Markers in Patients with Surgically Resected Pulmonary Pleomorphic Carcinoma
Abstract
:1. Introduction
2. Results
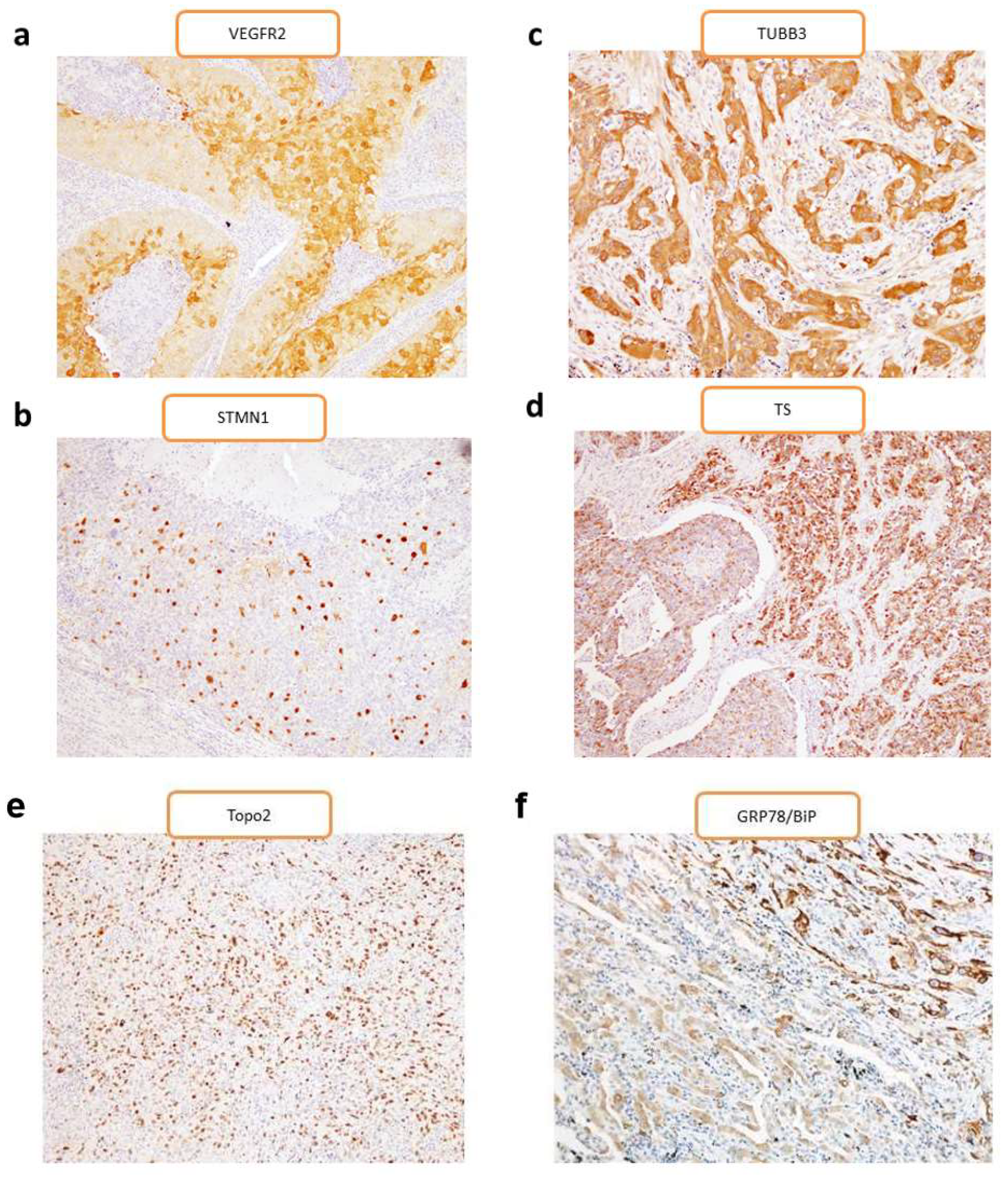
2.1. Patient Characteristics and Immunohistochemistry
2.2. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemical Staining
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, Y.L.; Lee, Y.C.; Shih, J.Y.; Wu, C.T. Pulmonary pleomorphic (spindle) cell carcinoma: Peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer 2001, 34, 91–97. [Google Scholar] [CrossRef]
- Fishback, N.F.; Travis, W.D.; Moran, C.A.; Guinee, D.G., Jr.; McCarthy, W.F.; Koss, M.N. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 1994, 73, 2936–2945. [Google Scholar] [CrossRef]
- Kerr, K.M.; Pelosi, G.; Austin, J.H.M.; Van Schil, P. Pleomorphic, spindle cell, and giant cell carcinoma. In World Health Organization Classification of Tumors; Travis, W.D., Brambilla, E., Müller-Hermelink, H.K., Harris, C.C., Eds.; IARC Press: Lyon, France, 2015; pp. 88–90. [Google Scholar]
- Bae, H.M.; Min, H.S.; Lee, S.H.; Kim, D.W.; Chung, D.H.; Lee, J.S.; Kim, Y.W.; Heo, D.S. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer 2007, 58, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Horie, Y.; Ayabe, E.; Murakami, H.; Takahashi, T.; Tsuya, A.; Nakamura, Y.; Naito, T.; Endo, M.; Kondo, H.; et al. Pulmonary pleomorphic carcinoma: A clinicopathological study including EGFR mutation analysis. J. Thorac. Oncol. 2010, 5, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Endo, M.; Abe, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Takahashi, T.; Murakami, H.; Tsuya, A.; Nakamura, Y.; et al. Biologic correlates of 18F-FDG uptake on PET in pulmonary pleomorphic carcinoma. Lung Cancer 2011, 71, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; Troiani, T.; Morelli, M.P.; Gridelli, C.; Ciardiello, F. Antiangiogenic drugs in non-small cell lung cancer treatment. Curr. Opin. Oncol. 2006, 18, 151–155. [Google Scholar] [CrossRef]
- Carrillo de Santa Pau, E.; Arias, F.C.; Caso Pelaez, E.; Munoz Molina, G.M.; Sanchez Hernandez, I.; Muguruza Trueba, I.; Moreno Balsalobre, R.; Sacristan Lopez, S.; Gomez Pinillos, A.; del Val Toledo Lobo, M. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer 2009, 115, 1701–1712. [Google Scholar] [CrossRef]
- Belmont, L.D.; Mitchison, T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 1996, 84, 623–631. [Google Scholar] [CrossRef]
- Gavet, O.; Ozon, S.; Manceau, V.; Lawler, S.; Curmi, P.; Sobel, A. The stathmin phosphoprotein family: Intracellular localization and effects on the microtubule network. J. Cell Sci. 1998, 111, 3333–3346. [Google Scholar]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Seve, P.; Reiman, T.; Dumontet, C. The role of betaIII tubulin in predicting chemoresistance in non-small cell lung cancer. Lung Cancer 2010, 67, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Isaac, S.; Souquet, P.J.; Bejui-Thivolet, F.; Pacheco, Y.; Peloux, N.; Frankfurter, A.; Luduena, R.; Perol, M. Expression of class III beta tubulin in non-small cell lung cancer is correlated with resistance to taxane chemotherapy. Bull. Cancer 2005, 92, E25–E30. [Google Scholar] [PubMed]
- Danenberg, P.V. Thymidylate synthetase—A target enzyme in cancer chemotherapy. Biochim. Biophys. Acta 1977, 473, 73–92. [Google Scholar] [CrossRef]
- Wada, H.; Hitomi, S.; Teramatsu, T. Adjuvant chemotherapy after complete resection in non-small-cell lung cancer. West Japan Study Group for Lung Cancer Surgery. J. Clin. Oncol. 1996, 14, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Nakano, J.; Huang, C.; Liu, D.; Masuya, D.; Nakashima, T.; Yokomise, H.; Ueno, M.; Wada, H.; Fukushima, M. Evaluations of biomarkers associated with 5-FU sensitivity for non-small-cell lung cancer patients postoperatively treated with UFT. Br. J. Cancer 2006, 95, 607–615. [Google Scholar] [CrossRef]
- Nakagawa, T.; Otake, Y.; Yanagihara, K.; Miyahara, R.; Ishikawa, S.; Fukushima, M.; Wada, H.; Tanaka, F. Expression of thymidylate synthase is correlated with proliferative activity in non-small cell lung cancer (NSCLC). Lung Cancer 2004, 43, 145–149. [Google Scholar] [CrossRef]
- Bartlett, J.M.; McConkey, C.C.; Munro, A.F.; Desmedt, C.; Dunn, J.A.; Larsimont, D.P.; O’Malley, F.P.; Cameron, D.A.; Earl, H.M.; Poole, C.J.; et al. Predicting Anthracycline Benefit: TOP2A and CEP17-Not Only but Also. J. Clin. Oncol. 2015, 33, 1680–1687. [Google Scholar] [CrossRef]
- Ceppi, P.; Longo, M.; Volante, M.; Novello, S.; Cappia, S.; Bacillo, E.; Selvaggi, G.; Saviozzi, S.; Calogero, R.; Papotti, M.; et al. Excision repair cross complementing-1 and topoisomerase IIalpha gene expression in small-cell lung cancer patients treated with platinum and etoposide: A retrospective study. J. Thorac. Oncol. 2008, 3, 583–589. [Google Scholar] [CrossRef]
- Miura, Y.; Kaira, K.; Sakurai, R.; Sunaga, N.; Saito, R.; Hisada, T.; Yamada, M. High expression of topoisomerase-II predicts favorable clinical outcomes in patients with relapsed small cell lung cancers receiving amrubicin. Lung Cancer 2018, 115, 42–48. [Google Scholar] [CrossRef]
- Hendershot, L.M. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 2004, 71, 289–297. [Google Scholar]
- Gazit, G.; Lu, J.; Lee, A.S. De-regulation of GRP stress protein expression in human breast cancer cell lines. Breast Cancer Res. Treat. 1999, 54, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, A.S. Stress induction of GRP78/BiP and its role in cancer. Curr. Mol. Med. 2006, 6, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Higashiyama, M.; Funai, H.; Imamura, F.; Uematsu, K.; Seki, N.; Eguchi, K.; Yamanaka, T.; Ichinose, Y. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer 2006, 53, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Dziadziuszko, R.; Chyczewski, L.; Jassem, E.; Jassem, J. Expression of vascular endothelial growth factor (VEGF) and its receptor FLK-1 in non-small cell lung cancer (NSCLC)—A preliminary report. Folia Histochem. Cytobiol. 2001, 39, 100–101. [Google Scholar]
- Koukourakis, M.I.; Giatromanolaki, A.; Thorpe, P.E.; Brekken, R.A.; Sivridis, E.; Kakolyris, S.; Georgoulias, V.; Gatter, K.C.; Harris, A.L. Vascular endothelial growth factor/KDR activated microvessel density versus CD31 standard microvessel density in non-small cell lung cancer. Cancer Res. 2000, 60, 3088–3095. [Google Scholar]
- Mineo, T.C.; Ambrogi, V.; Baldi, A.; Rabitti, C.; Bollero, P.; Vincenzi, B.; Tonini, G. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J. Clin. Pathol. 2004, 57, 591–597. [Google Scholar] [CrossRef]
- Uramoto, H.; Sugio, K.; Oyama, T.; Nakata, S.; Ono, K.; Yoshimastu, T.; Morita, M.; Yasumoto, K. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer 2005, 49, 55–62. [Google Scholar] [CrossRef]
- Sartori, G.; Cavazza, A.; Sgambato, A.; Marchioni, A.; Barbieri, F.; Longo, L.; Bavieri, M.; Murer, B.; Meschiari, E.; Tamberi, S.; et al. EGFR and K-ras mutations along the spectrum of pulmonary epithelial tumors of the lung and elaboration of a combined clinicopathologic and molecular scoring system to predict clinical responsiveness to EGFR inhibitors. Am. J. Clin. Pathol. 2009, 131, 478–489. [Google Scholar] [CrossRef]
- Kim, K.M.; Yu, T.K.; Chu, H.H.; Park, H.S.; Jang, K.Y.; Moon, W.S.; Kang, M.J.; Lee, D.G.; Kim, M.H.; Lee, J.H.; et al. Expression of ER stress and autophagy-related molecules in human non-small cell lung cancer and premalignant lesions. Int. J. Cancer 2012, 131, E362–E370. [Google Scholar] [CrossRef]
- Kaira, K.; Kawashima, O.; Endoh, H.; Imaizumi, K.; Goto, Y.; Kamiyoshihara, M.; Sugano, M.; Yamamoto, R.; Osaki, T.; Tanaka, S.; et al. Expression of amino acid transporter (LAT1 and 4F2hc) in pulmonary pleomorphic carcinoma. Hum. Pathol. 2019, 84, 142–149. [Google Scholar] [CrossRef]
- Altan, B.; Yokobori, T.; Mochiki, E.; Ohno, T.; Ogata, K.; Ogawa, A.; Yanai, M.; Kobayashi, T.; Luvsandagva, B.; Asao, T.; et al. Nuclear karyopherin-alpha2 expression in primary lesions and metastatic lymph nodes was associated with poor prognosis and progression in gastric cancer. Carcinogenesis 2013, 34, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Takahashi, T.; Murakami, H.; Shukuya, T.; Kenmotsu, H.; Ono, A.; Naito, T.; Tsuya, A.; Nakamura, Y.; Endo, M.; et al. The role of betaIII-tubulin in non-small cell lung cancer patients treated by taxane-based chemotherapy. Int. J. Clin. Oncol. 2013, 18, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Ohde, Y.; Nakagawa, K.; Okumura, T.; Murakami, H.; Takahashi, T.; Kondo, H.; Nakajima, T.; Endo, M.; Yamamoto, N. Thymidylate synthase expression is closely associated with outcome in patients with pulmonary adenocarcinoma. Med. Oncol. 2012, 29, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Kaira, K.; Sakurai, R.; Imai, H.; Tomizawa, Y.; Sunaga, N.; Minato, K.; Hisada, T.; Oyama, T.; Yamada, M. High expression of GRP78/BiP as a novel predictor of favorable outcomes in patients with advanced thymic carcinoma. Int. J. Clin. Oncol. 2017, 22, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Bonnesen, B.; Pappot, H.; Holmstav, J.; Skov, B.G. Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in non-small cell lung cancer patients: Relation to prognosis. Lung Cancer 2009, 66, 314–318. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Zheng, Z.; Chen, T.; Li, X.; Haura, E.; Sharma, A.; Bepler, G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. New Engl. J. Med. 2007, 356, 800–808. [Google Scholar] [CrossRef]

| Markers | VEGFR2 | TUBB3 | STMN1 | Topo-II | TS | GRP78/BiP |
|---|---|---|---|---|---|---|
| Average score | 2.86 | 3.44 | 2.14 | 1.42 | 3.13 | 3.36 |
| High-expression percentages | 33% (39/105) | 61% (64/105) | 35% (37/105) | 31% (33/105) | 51% (53/105) | 51% (53/105) |
| Variables | Total (n = 105) | VEGFR2 | TUBB3 | STMN1 | Topo-II | TS | GRP78/BiP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High (n = 39) | Low (n = 66) | p | High (n = 64) | Low (n = 41) | p | High (n = 37) | Low (n = 68) | p | High (n = 33) | Low (n = 72) | p | High (n = 53) | Low (n = 52) | p | High (n = 53) | Low (n = 52) | p | ||
| Age | |||||||||||||||||||
| <69/≥69 (y) | 54/51 | 25/14 | 29/37 | 0.06 | 34/30 | 20/21 | 0.69 | 21/16 | 33/35 | 0.54 | 23/10 | 31/41 | 0.01* | 30/23 | 24/28 | 0.33 | 29/34 | 25/27 | 0.85 |
| Gender | |||||||||||||||||||
| Male/Female | 79/26 | 28/11 | 51/15 | 0.64 | 44/35 | 35/6 | <0.01* | 26/11 | 53/15 | 0.47 | 26/7 | 53/19 | 0.63 | 38/15 | 41/11 | 0.49 | 43/10 | 36/17 | 0.12 |
| T factor | |||||||||||||||||||
| T1-2/T3-4 | 65/40 | 25/14 | 40/26 | 0.83 | 37/28 | 28/13 | 0.31 | 21/16 | 44/24 | 0.52 | 12/21 | 53/19 | <0.01* | 29/24 | 36/16 | 0.16 | 39/14 | 26/16 | 0.29 |
| N factor | |||||||||||||||||||
| Absent/Present | 72/33 | 27/12 | 45/21 | >0.99 | 46/24 | 26/15 | 0.84 | 25/12 | 47/21 | >0.99 | 21/12 | 51/21 | 0.50 | 35/18 | 37/15 | 0.67 | 40/13 | 32/1 | 0.01* |
| Pathological stage | |||||||||||||||||||
| I-II/III-IV | 69/36 | 24/15 | 45/21 | >0.99 | 41/28 | 28/13 | 0.42 | 20/17 | 49/19 | 0.08 | 25/8 | 44/28 | 0.18 | 34/19 | 35/17 | 0.83 | 41/12 | 28/24 | 0.01* |
| Smoking | |||||||||||||||||||
| Yes/No | 84/21 | 28/11 | 56/10 | 0.13 | 51/13 | 33/8 | >0.99 | 27/10 | 57/11 | 0.21 | 27/5 | 57/15 | 0.75 | 44/9 | 40/12 | 0.47 | 46/7 | 38/14 | 0.09 |
| Lymphatic permeation | |||||||||||||||||||
| Absent/Present | 41/64 | 12/27 | 29/37 | 0.21 | 25/16 | 16/25 | 0.07 | 13/24 | 28/40 | 0.67 | 14/19 | 27/45 | 0.67 | 20/33 | 21/31 | 0.84 | 19/34 | 22/30 | 0.55 |
| Vascular invasion | |||||||||||||||||||
| Absent/Present | 31/74 | 11/28 | 20/46 | >0.99 | 20/11 | 11/30 | <0.01* | 10/27 | 21/47 | 0.82 | 10/23 | 21/51 | >0.99 | 16/37 | 15/37 | >0.99 | 15/38 | 16/36 | 0.83 |
| Pleural invasion | |||||||||||||||||||
| Absent/Present | 49/56 | 17/22 | 32/34 | 0.68 | 30/19 | 19/22 | 0.20 | 18/19 | 31/37 | 0.84 | 17/16 | 35/40 | 0.68 | 27/26 | 22/30 | 0.43 | 22/31 | 27/25 | 0.33 |
| Adjuvant chemotherapy | |||||||||||||||||||
| Absent /Present | 77/28 | 27/12 | 50/16 | 0.49 | 43/34 | 34/7 | 0.68 | 26/11 | 51/17 | 0.64 | 25/8 | 52/20 | 0.81 | 35/18 | 42/10 | 0.12 | 39/14 | 38/14 | >0.99 |
| VEGFR2 | |||||||||||||||||||
| High/Low | — | — | — | — | 25/39 | 14/27 | 0.52 | 19/18 | 20/48 | 0.03* | 14/19 | 25/47 | 0.51 | 24/29 | 15/37 | 0.11 | 21/32 | 18/34 | 0.68 |
| TUBB3 | |||||||||||||||||||
| High/Low | 64/41 | 25/14 | 39/27 | 0.52 | — | — | — | 17/10 | 37/31 | 0.49 | 18/15 | 46/26 | 0.39 | 41/12 | 23/29 | <0.01* | 36/17 | 28/24 | 0.16 |
| Stathmin 1 | |||||||||||||||||||
| High/Low | 37/68 | 19/20 | 18/48 | 0.03* | 27/37 | 10/31 | 0.49 | — | — | — | 12/21 | 25/47 | >0.99 | 31/22 | 6/46 | <0.01* | 21/32 | 16/36 | 0.41 |
| Topo 2 | |||||||||||||||||||
| High/Low | 33/72 | 14/25 | 19/47 | 0.51 | 18/46 | 15/26 | 0.39 | 12/25 | 21/47 | >0.99 | — | — | — | 18/35 | 15/37 | 0.67 | 19/34 | 14/38 | 0.40 |
| TS | |||||||||||||||||||
| High/Low | 53/52 | 24/29 | 15/37 | 0.11 | 41/12 | 23/29 | <0.01* | 31/22 | 6/46 | <0.01* | 18/35 | 15/37 | 0.67 | - | - | - | 30/23 | 23/29 | 0.24 |
| GRP78/BiP | |||||||||||||||||||
| High/Low | 53/52 | 21/32 | 18/34 | 0.68 | 36/17 | 28/24 | 0.16 | 36/17 | 28/24 | 0.16 | 21/32 | 16/36 | 0.41 | 30/13 | 23/29 | 0.01* | - | - | - |
| OS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | All Patients | Patients with AC Component | Patients with Non-AC Component | |||||||||
| MST | HR | 95% CI | p Value | MST | HR | 95% CI | p Value | MST | HR | 95% CI | p Value | |
| Age, ≤69/>69 (y) | 361/1047 | 0.82 | 0.49–1.37 | 0.52 | 157/1038 | 1.34 | 0.59–3.04 | 0.48 | 352/1068 | 1.10 | 0.56–2.16 | 0.77 |
| Gender (female/male) | 1038/654 | 0.83 | 0.46–1.51 | 0.55 | 1038/1009 | 1.06 | 0.44–2.54 | 0.89 | 881/147 | 0.63 | 0.27–1.49 | 0.30 |
| p-stage (I-II/III-IV) | 1338/228 | 0.21 | 0.11–0.93 | <0.01 | 1839/615 | 0.33 | 0.13–0.83 | 0.01 | 1265/145 | 0.07 | 0.02–0.18 | <0.01 |
| Ly (present/absent) | 624/1209 | 1.47 | 0.85–2.39 | 0.17 | 1009/1939 | 1.22 | 0.52–2.83 | 0.64 | 288/1047 | 1.56 | 0.78–3.11 | 0.20 |
| v (present/absent) | 640/1265 | 1.41 | 0.82–2.41 | 0.21 | 1038/1839 | 1.16 | 0.49–2.72 | 0.72 | 353/1265 | 1.51 | 0.73–3.10 | 0.26 |
| Pl (present/absent) | 615/1202 | 1.56 | 0.93–2.61 | 0.09 | 654/3665 | 1.95 | 0.86–4.41 | 0.11 | 336/1068 | 1.30 | 0.66–2.56 | 0.57 |
| VEGFR2 (high/low) | 352/1209 | 2.23 | 1.28–3.83 | <0.01 | 615/1839 | 2.23 | 0.98–5.02 | 0.05 | 187/1068 | 2.71 | 1.26–5.82 | 0.01 |
| TUBB3 (high/low) | 361/2010 | 2.15 | 1.27–3.62 | <0.01 | 640/NR | 2.07 | 0.91–4.68 | 0.08 | 244/1546 | 2.26 | 1.12–4.42 | 0.02 |
| STMN1 (high/low) | 359/1202 | 2.34 | 1.34–4.08 | <0.01 | 291/2010 | 4.34 | 1.55–12.1 | <0.01 | 507586 | 1.71 | 0.85–3.41 | 0.12 |
| Topo-II (high/low) | 1338/615 | 0.57 | 0.33–0.99 | 0.04 | 1089/1039 | 0.81 | 0.33–1.95 | 0.63 | 1338/336 | 0.52 | 0.25–1.06 | 0.07 |
| TS (high/low) | 361/NR | 2.15 | 1.29–3.57 | <0.01 | 624/NR | 2.84 | 1.25–6.41 | 0.01 | 352/1047 | 1.55 | 0.77–3.14 | 0.21 |
| GRP78/BiP (high/low) | 1047/5.7 | 0.59 | 0.35–0.99 | 0.04 | 1657/615 | 0.53 | 0.22–1.7 | 0.15 | 991/244 | 0.71 | 0.35–1.41 | 0.32 |
| DFS | ||||||||||||
| Variables | MST | HR | 95% CI | p | MST | HR | 95% CI | p | MST | HR | 95% CI | pValue |
| Age, ≤69/>69 (y) | 267/733 | 0.70 | 0.42–1.17 | 0.17 | 266/759 | 1.85 | 0.88–1.89 | 0.10 | 247/733 | 1.27 | 0.62–2.62 | 0.50 |
| Gender (female/male) | 539/357 | 0.63 | 0.35–1.16 | 0.14 | 444/522 | 0.92 | 0.41–2.05 | 0.83 | 753/156 | 0.42 | 0.16–1.01 | 0.07 |
| p-stage (I-II/III-IV) | 890/154 | 0.21 | 0.12–0.40 | <0.01 | 499/522 | 0.48 | 0.21–1.08 | 0.07 | 890/95 | 0.05 | 0.02–0.14 | <0.01 |
| ly (present/absent) | 336/890 | 1.73 | 1.03–2.88 | 0.03 | 444/1235 | 1.45 | 0.68–3.09 | 0.32 | 171/836 | 2.00 | 0.97–4.13 | 0.06 |
| v (present/absent) | 443/1235 | 1.63 | 0.95–2.81 | 0.07 | 499/1235 | 1.35 | 0.61–2.97 | 0.44 | 267/NR | 1.83 | 0.85–3.95 | 0.12 |
| pl (present/absent) | 267/765 | 2.04 | 1.22–3.39 | <0.01 | 411/765 | 1.86 | 0.88–3.93 | 0.09 | 187/1068 | 2.71 | 1.26–5.82 | 0.03 |
| VEGFR2 (high/low) | 286/765 | 2.4 | 1.37–4.19 | <0.01 | 411/654 | 1.71 | 0.81–3.61 | 0.16 | 147/912 | 3.98 | 1.75–9.05 | <0.01 |
| TUBB3 (high/low) | 357/765 | 1.65 | 0.98–2.78 | 0.05 | 443/765 | 1.37 | 0.65–2.87 | 0.39 | 247/836 | 1.97 | 0.95–4.08 | 0.06 |
| STMN1 (high/low) | 336/765 | 2.46 | 1.39–4.35 | <0.01 | 286/759 | 4.25 | 1.54–11.7 | <0.01 | 336/NR | 2.08 | 1.02–4.31 | 0.04 |
| Topo-II (high/low) | 443/522 | 1.03 | 0.59–1.78 | 0.92 | 443/539 | 1.38 | 0.61–3.13 | 0.60 | 340/336 | 0.90 | 0.42–1.91 | 0.78 |
| TS (high/low) | 357/753 | 1.57 | 0.94–2.61 | 0.08 | 362/759 | 2.14 | 0.99–4.64 | 0.05 | 336/753 | 1.22 | 0.58–2.56 | 0.59 |
| GRP78/BiP (high/low) | 444/443 | 1.09 | 0.61–1.93 | 0.30 | 1235/443 | 0.43 | 0.19–0.93 | 0.03 | 267/890 | 1.17 | 0.57–2.42 | 0.65 |
| OS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | All Patients | Patients with AC Component | Patients with Non-AC Component | |||||||||
| Univariate (p) | HR | 95% CI | p | Univariate (p) | HR | 95% CI | p | Univariate (p) | HR | 95% CI | p | |
| Age, ≤69/>69 (y) | 0.52 | 0.48 | 0.77 | |||||||||
| Gender (female/male) | 0.55 | 0.89 | 0.30 | |||||||||
| p-stage (I-II/III-IV) | <0.01 | 1.62 | 1.19–2.19 | <0.01 | 0.01 | 1.67 | 1.03–2.81 | 0.03 | <0.01 | 2.63 | 1.71–4.15 | <0.01 |
| Ly (present/absent) | 0.17 | 0.64 | 0.20 | |||||||||
| v (present/absent) | 0.21 | 0.72 | 0.26 | |||||||||
| Pl (present/absent) | 0.09 | 0.11 | 0.57 | |||||||||
| VEGFR2 (high/low) | <0.01 | 1.38 | 1.05–1.81 | 0.01 | 0.05 | 0.01 | 1.64 | 1.15–2.35 | <0.01 | |||
| TUBB3 (high/low) | <0.01 | 1.38 | 0.99–1.97 | 0.05 | 0.08 | 0.02 | 1.41 | 0.95–2.18 | 0.08 | |||
| STMN1 (high/low) | <0.01 | 0.96 | 0.69–1.34 | 0.85 | <0.01 | 0.92 | 0.51–1.66 | 0.78 | 0.12 | |||
| Topo-II (high/low) | 0.04 | 1.13 | 0.83–1.59 | 0.43 | 0.63 | 0.07 | ||||||
| TS (high/low) | <0.01 | 1.43 | 0.98–2.11 | 0.05 | 0.01 | 1.71 | 0.99–2.99 | 0.05 | 0.21 | |||
| GRP78/BiP (high/low) | 0.04 | 1.36 | 1.03–1.81 | 0.02 | 0.15 | 0.32 | ||||||
| DFS | ||||||||||||
| Variables | Univariate (p) | HR | 95% CI | p | Univariate (p) | HR | 95% CI | p | Univariate (p) | HR | 95% CI | p |
| Age, ≤69/>69 (y) | 0.17 | 0.10 | 0.50 | |||||||||
| Gender (female/male) | 0.14 | 0.83 | 0.07 | |||||||||
| p-stage (I-II/III-IV) | <0.01 | 1.67 | 1.26–2.23 | <0.01 | 0.07 | <0.01 | 3.07 | 1.96–5.29 | <0.01 | |||
| ly (present/absent) | 0.03 | 0.95 | 0.71–1.28 | 0.77 | 0.32 | 0.06 | ||||||
| v (present/absent) | 0.07 | 0.44 | 0.12 | |||||||||
| pl (present/absent) | <0.01 | 1.33 | 1.02–1.77 | 0.03 | 0.09 | 0.03 | ||||||
| VEGFR2 (high/low) | <0.01 | 1.36 | 1.05–1.76 | 0.01 | 0.16 | <0.01 | 1.83 | 1.26–2.66 | <0.01 | |||
| TUBB3 (high/low) | 0.05 | 0.39 | 0.06 | |||||||||
| STMN1 (high/low) | <0.01 | 1.33 | 1.01–1.74 | 0.03 | <0.01 | 1.94 | 1.26–2.94 | <0.01 | 0.04 | |||
| Topo-II (high/low) | 0.92 | 0.60 | 0.78 | |||||||||
| TS (high/low) | 0.08 | 0.05 | 0.59 | |||||||||
| GRP78/BiP (high/low) | 0.30 | 0.03 | 1.66 | 1.13–2.46 | 0.01 | 0.65 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, H.; Shimizu, K.; Kawashima, O.; Endoh, H.; Imaizumi, K.; Goto, Y.; Kamiyoshihara, M.; Sugano, M.; Yamamoto, R.; Tanaka, S.; et al. Clinical Significance of Various Drug-Sensitivity Markers in Patients with Surgically Resected Pulmonary Pleomorphic Carcinoma. Cancers 2019, 11, 1636. https://doi.org/10.3390/cancers11111636
Imai H, Shimizu K, Kawashima O, Endoh H, Imaizumi K, Goto Y, Kamiyoshihara M, Sugano M, Yamamoto R, Tanaka S, et al. Clinical Significance of Various Drug-Sensitivity Markers in Patients with Surgically Resected Pulmonary Pleomorphic Carcinoma. Cancers. 2019; 11(11):1636. https://doi.org/10.3390/cancers11111636
Chicago/Turabian StyleImai, Hisao, Kimihiro Shimizu, Osamu Kawashima, Hideki Endoh, Kazuyoshi Imaizumi, Yasuhiro Goto, Mitsuhiro Kamiyoshihara, Masayuki Sugano, Ryohei Yamamoto, Shigebumi Tanaka, and et al. 2019. "Clinical Significance of Various Drug-Sensitivity Markers in Patients with Surgically Resected Pulmonary Pleomorphic Carcinoma" Cancers 11, no. 11: 1636. https://doi.org/10.3390/cancers11111636
APA StyleImai, H., Shimizu, K., Kawashima, O., Endoh, H., Imaizumi, K., Goto, Y., Kamiyoshihara, M., Sugano, M., Yamamoto, R., Tanaka, S., Fujita, A., Kogure, Y., Seki, Y., Mogi, A., Oyama, T., Minato, K., Asao, T., & Kaira, K. (2019). Clinical Significance of Various Drug-Sensitivity Markers in Patients with Surgically Resected Pulmonary Pleomorphic Carcinoma. Cancers, 11(11), 1636. https://doi.org/10.3390/cancers11111636







