Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Immunohistochemical (IHC) Detection of Irisin Expression in Tissue Microarrays (TMA) with NSCLC
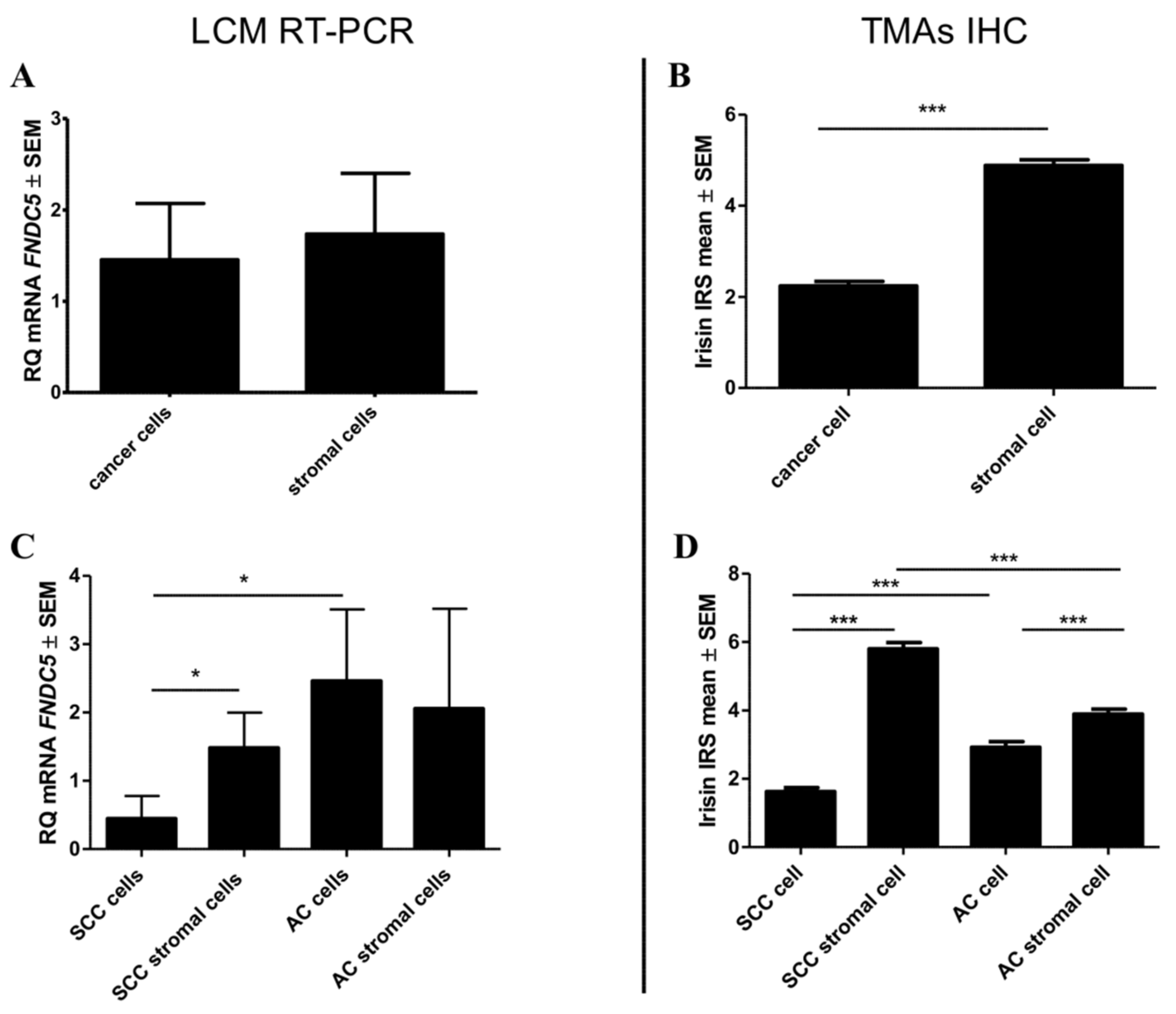
2.2. mRNA FNDC5 Expression Level in NSCLC
2.3. Comparison of the Expressions of Irisin with Vimentin, Alpha-smooth Muscle actin (αSMA) and Podoplanin (PDPN) Expression
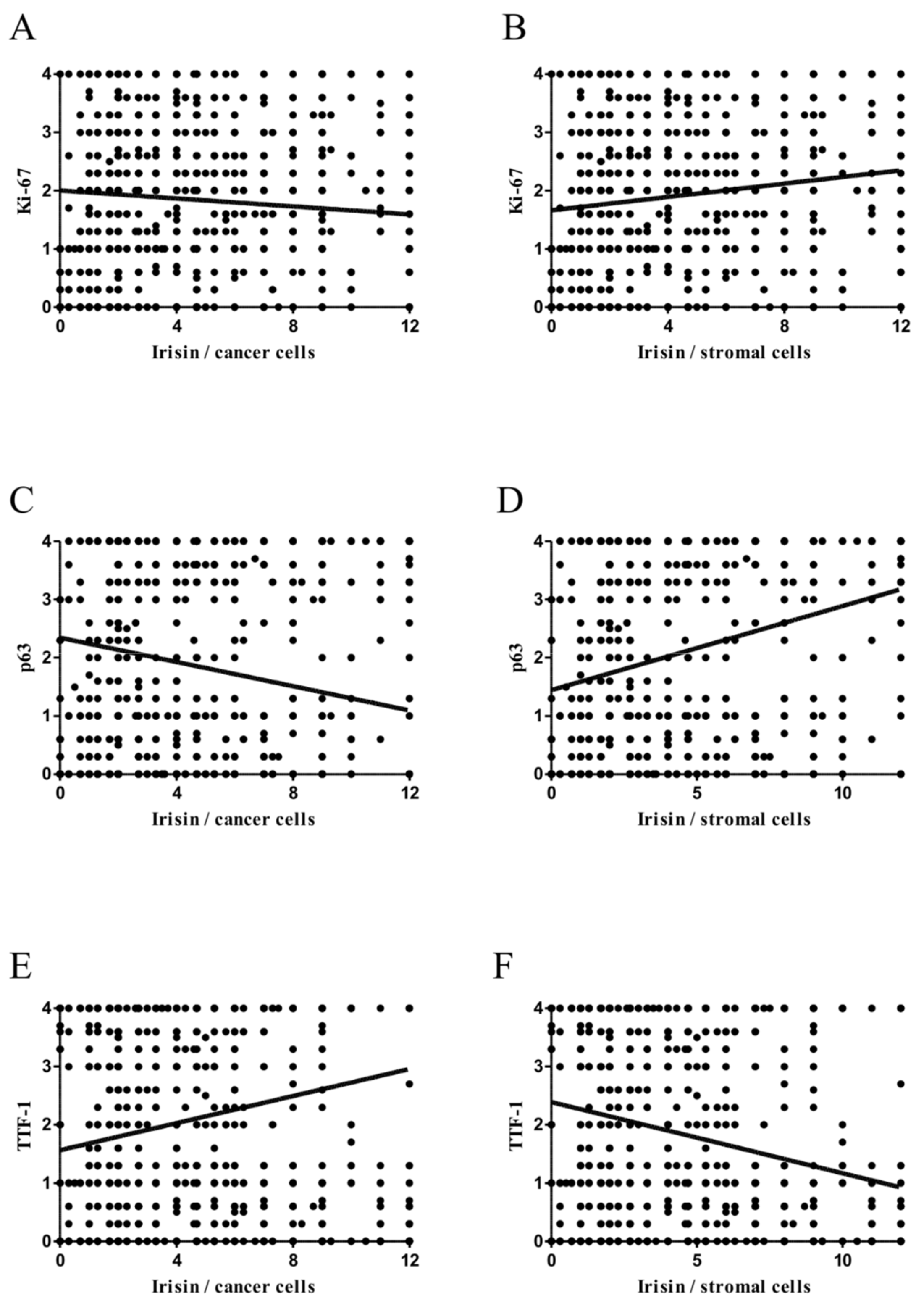
2.4. Associations between Irisin and Cancer Cell Proliferation
2.5. Comparison of Irisin with TTF-1 and p63 Expression Levels
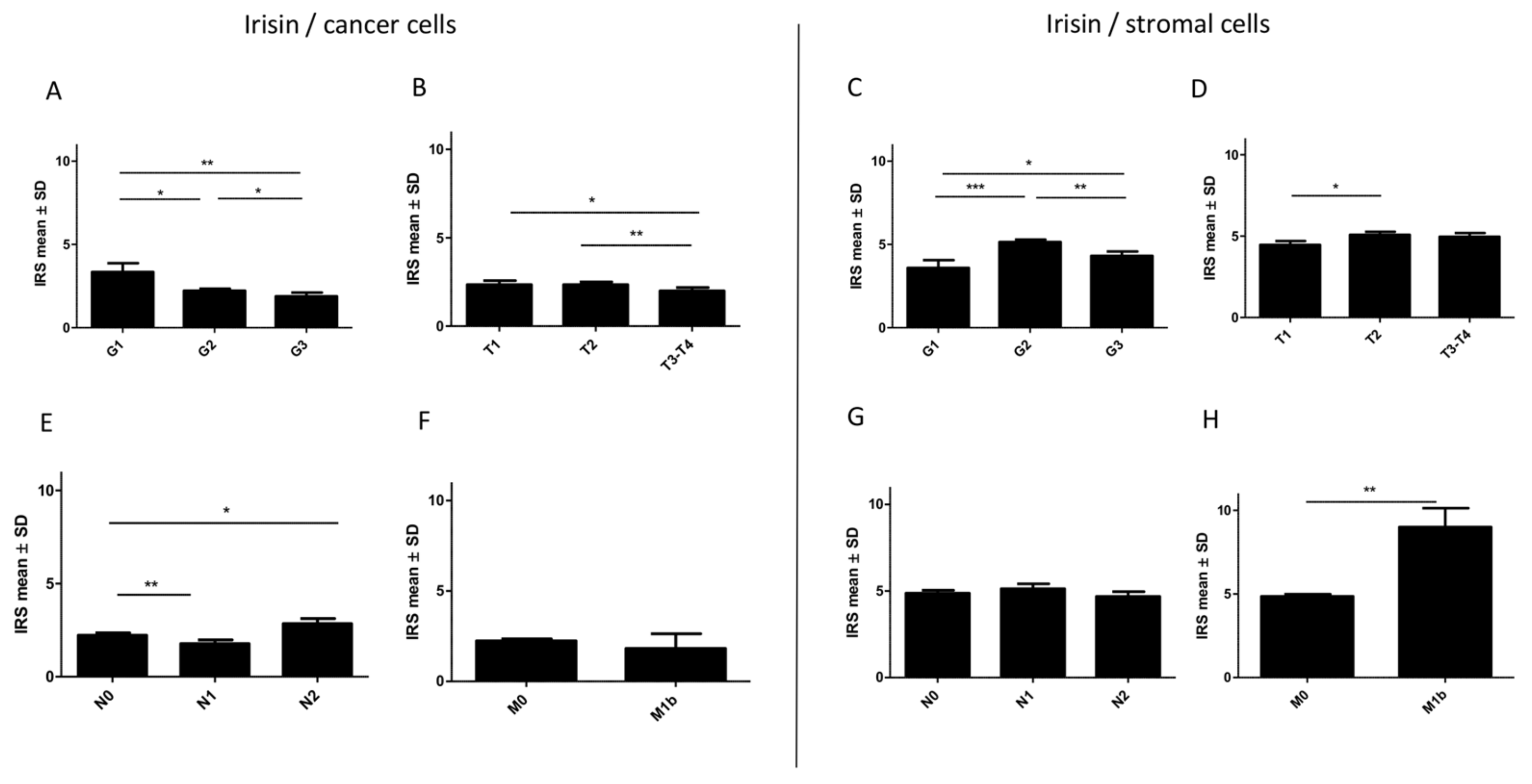
2.6. Associations between Irisin Expression in Cancer Cells and Clinicopathological Parameters
2.7. Associations between Irisin Expression in Stromal Cells and Clinicopathological Parameters
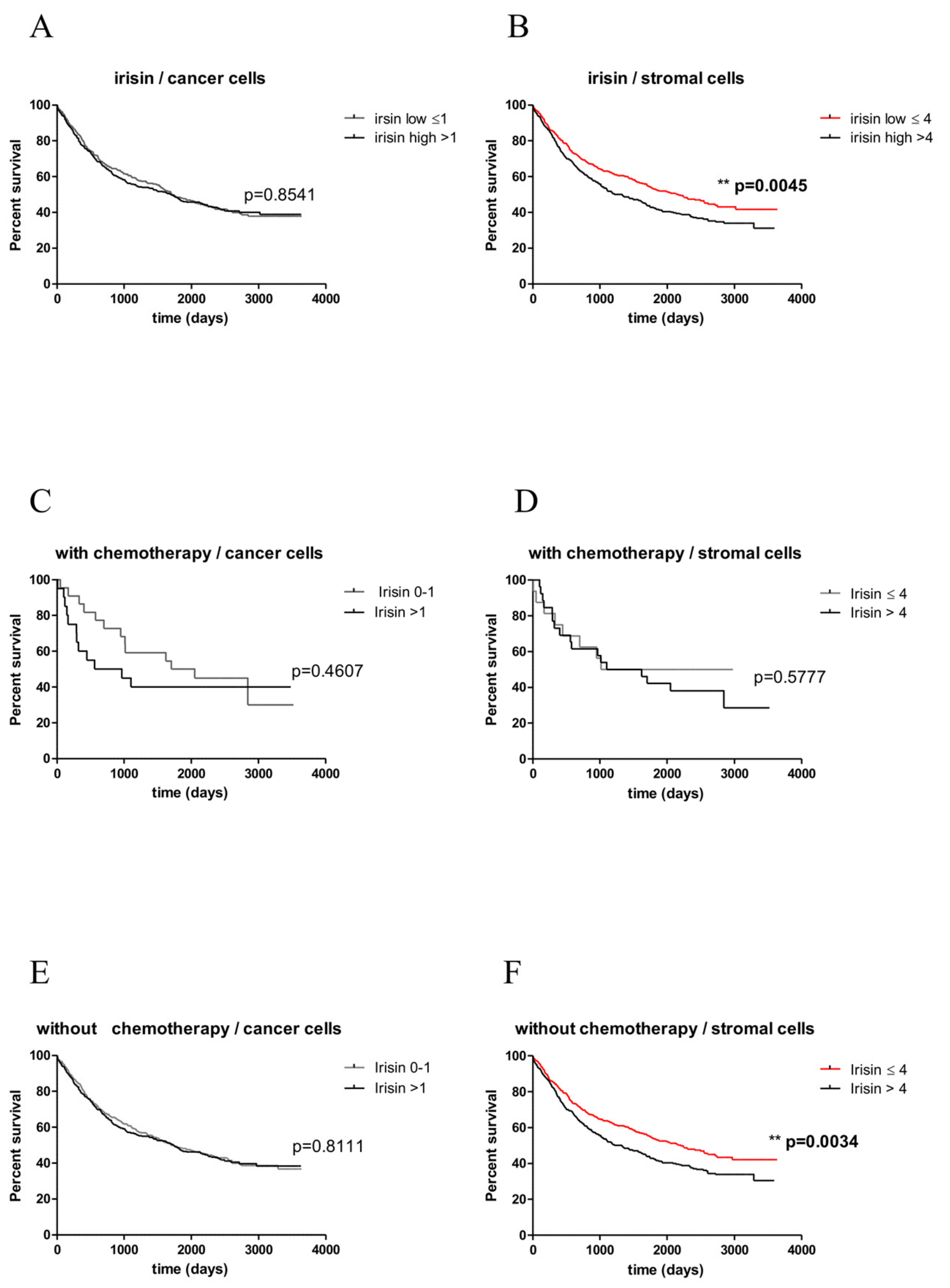
2.8. Associations between Irisin Expression and the Overall Survival (OS)
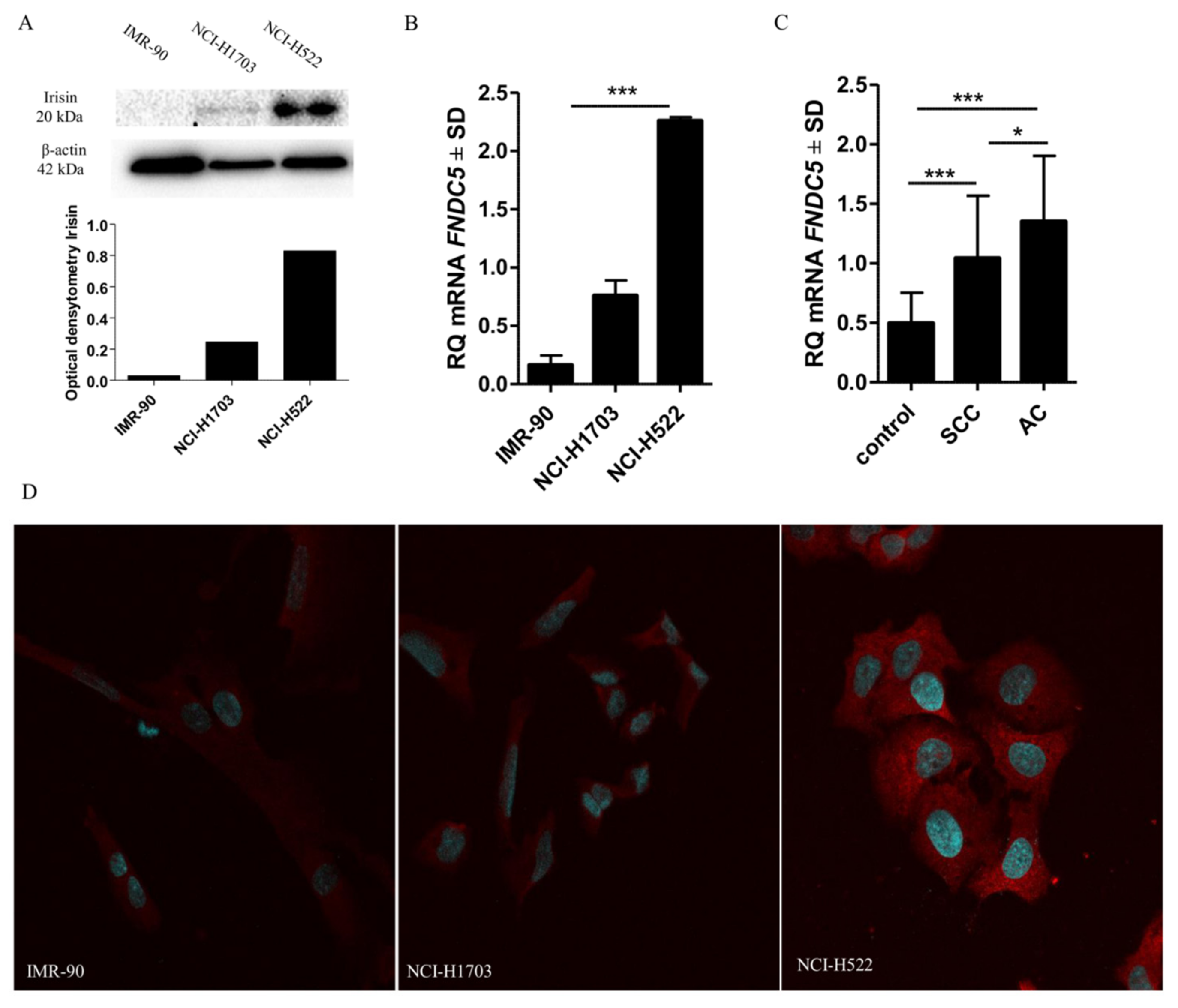
2.9. Comparison of Irisin Expression Levels in Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Cell Line Culture
4.3. Tissue Microarray (TMA) Immunohistochemical (IHC) Reactions
4.4. Laser Capture Microdissection (LCM)
4.5. Real-Time PCR (RT-PCR)
4.6. Western-Blot Analysis
4.7. Confocal Microscopy
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glatzel-Plucinska, N.; Piotrowska, A.; Grzegrzolka, J.; Olbromski, M.; Rzechonek, A.; Dzięgiel, P.; Podhorska-Okolow, M. SATB1 Level Correlates with Ki-67 Expression and Is a Positive Prognostic Factor in Non-small Cell Lung Carcinoma. Anticancer Res. 2018, 38, 723–736. [Google Scholar] [PubMed]
- Cruz, C.S.D.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.A.; Fernández-Real, J.M.; Mantzoros, C. Irisin in humans: Recent advances and questions for future research. Metabolism 2014, 63, 178–180. [Google Scholar] [CrossRef]
- Komolka, K.; Albrecht, E.; Schering, L.; Brenmoehl, J.; Hoeflich, A.; Maak, S. Locus Characterization and Gene Expression of Bovine FNDC5: Is the Myokine Irisin Relevant in Cattle? PLoS ONE 2014, 9, e88060. [Google Scholar] [CrossRef]
- Thomas, R.J.; Kenfield, S.A.; Jimenez, A. Exercise-induced biochemical changes and their potential influence on cancer: A scientific review. Br. J. Sports Med. 2016, 51, 640–644. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Kuloglu, T.; Ozercan, M.; Albayrak, S.; Aydin, S.; Bakal, U.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Sarac, M.; Kazez, A.; et al. Irisin immunohistochemistry in gastrointestinal system cancers. Biotech. Histochem. 2016, 91, 242–250. [Google Scholar]
- Tekin, S.; Erden, Y.; Sandal, S.; Yilmaz, B. Is Irisin an Anticarcinogenic Peptide? Med. Sci. Int. Med. J. 2014, 4, 1. [Google Scholar] [CrossRef]
- Celik, O.; Yal, M.H. Irisin Immunostaining Characteristics of Breast and Ovarian Cancer Cells. Cell Mol. Biol. 2016, 62, 40–44. [Google Scholar]
- Hofmann, T.; Elbelt, U.; Stengel, A. Irisin as a muscle-derived hormone stimulating thermogenesis—A critical update. Peptides 2014, 54, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Cabiati, M.; Del Turco, S.; Navarra, T.; De Simone, P.; Filipponi, F.; Del Ry, S.; Gastaldelli, A.; Basta, G. Increased FNDC5/Irisin expression in human hepatocellular carcinoma. Peptides 2017, 88, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, T.; Celik, O.; Aydin, S.; Ozercan, I.H.; Acet, M.; Aydin, Y.; Artas, G.; Turk, A.; Yardim, M.; Ozan, G.; et al. Irisin immunostaining characteristics of breast and ovarian cancer cells. Cell. Mol. Biol. 2016, 62, 40–44. [Google Scholar] [PubMed]
- Aydin, S. Is irisin a decisive protein in cancer cachexia and death of cancer cells? Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3727–3729. [Google Scholar]
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum irisin levels are lower in patients with breast cancer: Association with disease diagnosis and tumor characteristics. BMC Cancer 2015, 15, 898. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef]
- Suchanski, J.; Tejchman, A.; Zacharski, M.; Piotrowska, A.; Grzegrzolka, J.; Chodaczek, G.; Nowińska, K.; Ryś, J.; Dzięgiel, P.; Kieda, C.; et al. Podoplanin increases the migration of human fibroblasts and affects the endothelial cell network formation: A possible role for cancer-associated fibroblasts in breast cancer progression. PLoS ONE 2017, 12, e0184970. [Google Scholar] [CrossRef]
- Kawase, A.; Ishii, G.; Nagai, K.; Ito, T.; Nagano, T.; Murata, Y.; Hishida, T.; Nishimura, M.; Yoshida, J.; Suzuki, K.; et al. Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int. J. Cancer 2008, 123, 1053–1059. [Google Scholar] [CrossRef]
- Hoshino, A.; Ishii, G.; Ito, T.; Aoyagi, K.; Ohtaki, Y.; Nagai, K.; Sasaki, H.; Ochiai, A. Podoplanin-Positive Fibroblasts Enhance Lung Adenocarcinoma Tumor Formation: Podoplanin in Fibroblast Functions for Tumor Progression. Cancer Res. 2011, 71, 4769–4779. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Tang, N.; Qiu, J.; Zhang, D.; Huang, F.; Cheng, Y.; Ding, K.; Li, W.; Zhang, P.; Tan, X. Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 493, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-S.; Mantzoros, C.S. Regulation of cell proliferation and malignant potential by irisin in endometrial, colon, thyroid and esophageal cancer cell lines. Metabolism 2014, 63, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Bermúdez, A.; Laza-Briviesca, R.; Vicente-Blanco, R.J.; García-Grande, A.; Coronado, M.J.; Laine-Menéndez, S.; Alfaro, C.; Sanchez, J.C.; Franco, F.; Calvo, V.; et al. Cancer-associated fibroblasts modify lung cancer metabolism involving ROS and TGF-β signaling. Free Radic. Biol. Med. 2019, 130, 163–173. [Google Scholar] [CrossRef]
- Deblois, G.; St-Pierre, J.; Giguère, V. The PGC-1/ERR signaling axis in cancer. Oncogene 2013, 32, 3483–3490. [Google Scholar] [CrossRef]
- Dang, C.V. Links between metabolism and cancer. Genes Dev. 2012, 26, 877–890. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.-I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef]
- Sarnowska, E.; Balcerak, A.; Olszyna-Serementa, M.; Kotlarek, D.; Sarnowski, T.J.; Siedlecki, J.A. AMP-activated protein kinase (AMPK) as therapeutic target. Postępy Hig. Med. Dosw. 2013, 67, 750–760. [Google Scholar] [CrossRef]
- Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Lin, Z.; Ertel, A.; Flomenberg, N.; Witkiewicz, A.K.; Birbe, R.C.; Howell, A.; Pavlides, S.; Gandara, R.; et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 2011, 10, 1772–1783. [Google Scholar] [CrossRef]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; De Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal Metabolic Reprogramming through Lactate Shuttle Coordinately Influences Tumor-Stroma Interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, J.; Guo, H.; Xu, H.; Zhang, G. PGC-1 alpha interacts with microRNA-217 to functionally regulate breast cancer cell proliferation. Biomed. Pharmacother. 2017, 85, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K. Gong, F. Serum and adipose tissue mRNA levels of ATF3 and FNDC5/irisin in colorectal cancer patients with or without obesity. Front. Physiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Altay, D.U.; Keha, E.E.; Karaguzel, E.; Mentese, A.; Yaman, S.O.; Alver, A. The Diagnostic Value of FNDC5/Irisin in Renal Cell Cancer. Int. Braz. J. Urol 2018, 44, 44,734–739. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Dziegiel, P.; Salwa-Zurawska, W.; Zurawski, J.; Wojnar, A.; Zabel, M. Prognostic significance of augmented metallothionein (MT) expression correlated with Ki-67 antigen expression in selected soft tissue sarcomas. Histol. Histopathol. 2005, 20, 83–89. [Google Scholar] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
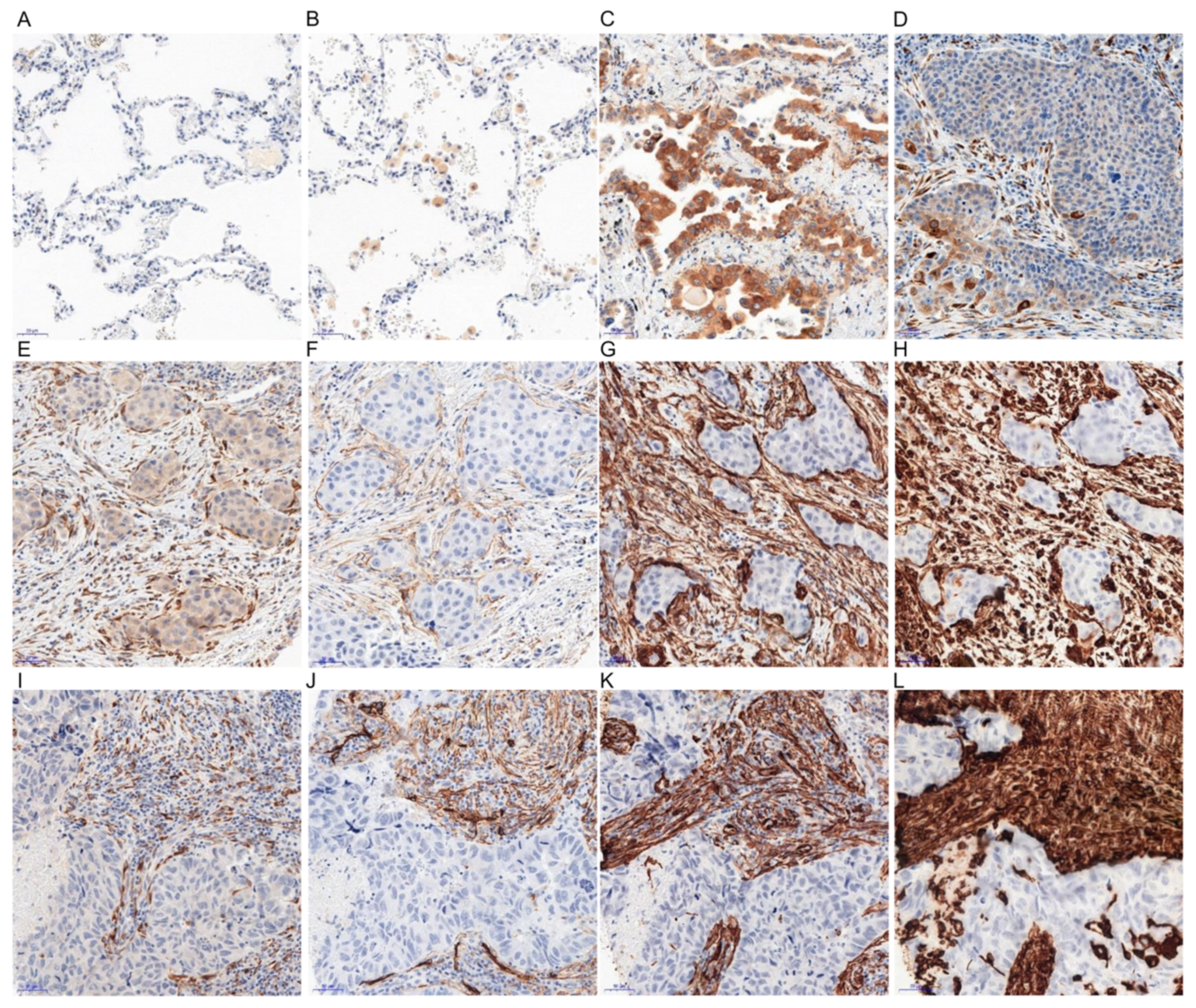
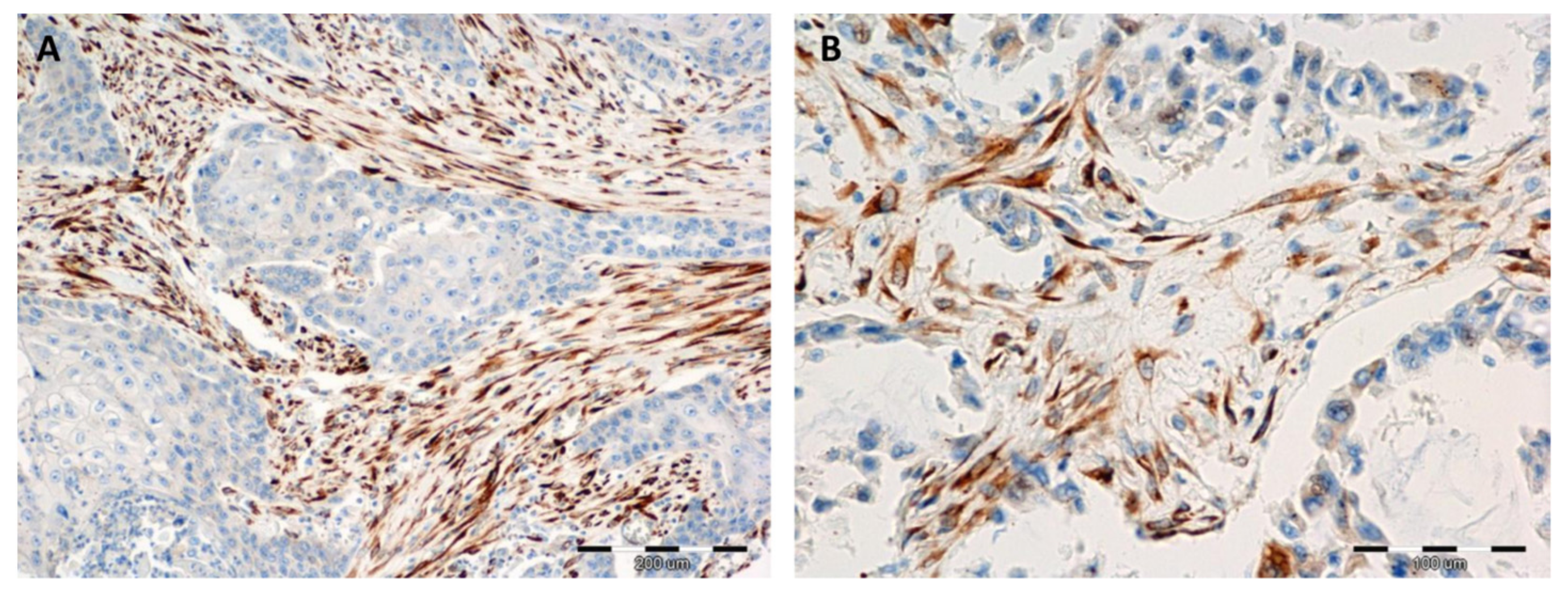
| Clinicopathological Parameter | n 729 (%) | Irisin Expression in NSCLC Cancer Cells | Irisin Expression in NSCLC Stromal Cells | ||||
|---|---|---|---|---|---|---|---|
| Low 1–4 | High 5–12 | Chi2Test p Value | Low 1–4 | High 5–12 | Chi2Test p Value | ||
| Age ≤60 | 290 (39.8) | 248 (85.5) | 42 (14.5) | 0.3911 | 155 (53.4) | 135 (46.6) | 0.7797 |
| > 60 | 439 (60.2) | 365 (83.1) | 74 (16.9) | 230 (52.4) | 209 (47.6) | ||
| Sex Male | 540 (74) | 458 (84.8) | 82 (15.2) | 0.3644 | 275 (50.8) | 265 (49.2) | 0.0846 |
| Female | 189 (26) | 155 (82) | 34 (18) | 110 (58.2) | 79 (41.8) | ||
| Smoking Status Yes | 631 (86.5) | 535 (84.8) | 96 (15.2) | 0.1909 | 325 (51.5) | 306 (48.5) | 0.0475 |
| No | 98 (13.5) | 78 (79.6) | 20 (20.4) | 61 (62.2) | 37 (37.8) | ||
| Histology Type AC | 348 (47.6) | 273 (78.5) | 75 (21.5) | <0.0001 | 221 (63.5) | 127 (36.5) | <0.0001 |
| SCC | 381 (52.4) | 340 (89.2) | 41 (10.8) | 164 (43) | 217 (57) | ||
| Tumour Size (T) T1 | 177 (24.3) | 148 (83.5) | 29 (16.4) | <0.0001 | 102 (57.5) | 75 (42.5) | 0.3027 |
| T2 | 329 (45.1) | 272 (82.8) | 57 (17.2) | 166 (50.4) | 163 (49.6) | ||
| T3–4 | 223 (30.6) | 120 (53.7) | 103 (46.3) | 117 (52.5) | 106 (47.5) | ||
| Lymph Nodes (N) N0 | 474 (65) | 400 (84.4) | 74 (15.6) | 0.0076 | 252 (53.2) | 222 (46.8) | 0.3235 |
| N1 | 134 (18.4) | 121 (90.3) | 13 (9.7) | 64 (47.8) | 70 (52.2) | ||
| N2 | 121 (16.6) | 92 (76) | 29 (24) | 69 (57.2) | 52 (42.8) | ||
| Metastasis (M) M0 | 723 (99.2) | 608 (84.1) | 115 (15.9) | 0.9595 | 723 | 0 | <0.0001 |
| M1b | 6 (0.8) | 5 (83.3) | 1 (16.7) | 0 | 6 | ||
| Stage I | 267 (36.6) | 113 (42.3) | 154 (57.7) | <0.0001 | 150 (56.2) | 117 (43.8) | 0.1049 |
| II | 245 (33.6) | 213 (86.9) | 32 (13.1) | 116 (47.3) | 129 (52.7) | ||
| III–IV | 217 (29.8) | 176 (81.1) | 41 (18.9) | 119 (54.8) | 98 (45.2) | ||
| Grade of malignancy (G) G1 | 47 (6.4) | 34 (72.5) | 13 (27.5) | 0.0607 | 28 (59.6) | 19 (40.4) | 0.0183 |
| G2 | 548 (75.2) | 456 (83.2) | 92 (16.8) | 270 (49.3) | 278 (50.7) | ||
| G3 | 134 (18.4) | 117 (87.3) | 12 (12.7) | 83 (61.9) | 51 (38.1) | ||
| NSCLC | P Value (U-Mann–Whitney Test) | Mean Value ± SD | |||
|---|---|---|---|---|---|
| Cancer Cells | Stromal Cells | Cancer Cells | Stromal Cells | ||
| Lymph Nodes | Lymph Nodes | ||||
| N0 vs. N1 | 0.0607 | 0.1477 | N0 | 2.2 ± 0.13 | 4.8 ± 0.16 |
| N0 vs. N2 | 0.0124 | 0.4401 | N1 | 1.8 ± 0.20 | 5.1 ± 0.27 |
| N1 vs. N2 | 0.0011 | 0.1502 | N2 | 2.8 ± 0.28 | 4.6 ± 0.27 |
| Tumour Size | Tumour Size | ||||
| T1 vs. T2 | 0.4377 | 0.0228 | T1 | 2.3 ± 0.22 | 4.4 ± 0.24 |
| T1 vs. T3–4 | 0.0215 | 0.0719 | T2 | 2.3 ± 0.15 | 5.1 ± 0.19 |
| T2 vs. T3–4 | 0.0087 | 0.3162 | T3–4 | 1.9 ± 0.17 | 4.9 ± 0.23 |
| Stage | Stage | ||||
| I vs. II | 0.0275 | 0.0023 | I | 2.3 ± 0.17 | 4.4 ± 0.20 |
| I vs. III–IV | 0.5178 | 0.3322 | II | 2.0 ± 0.17 | 5.4 ± 0.21 |
| II vs. III–IV | 0.1637 | 0.0324 | III–IV | 2.3 ± 0.19 | 4.7 ± 0.21 |
| Metastases | Metastases | ||||
| M0 vs. M1b | 0.8822 | 0.0054 | M0 | 2.2 ± 0.10 | 4.8 ± 0.12 |
| M1b | 1.8 ± 0.82 | 9.0 ± 1.12 | |||
| Clinicopathological Parameters | NSCLC | ||
|---|---|---|---|
| Univariate Analysis HR (95% CI) p | Multivariate Analysis HR (95% CI) p | ||
| Age ≤60 vs. >60 | 1.25 (1.02–1.54) 0.0292 | 1.35 (1.10–1.66) 0.0039 | |
| Sex Male vs. Female | 1.39 (1.11–1.75) 0.0045 | 1.28 (1.02–1.61) 0.0343 | |
| Smoking History Yes vs. No | 1.31(0.97–1.76) 0.0702 | ||
| Pt T1–T2 vs. T3–T4 | 1.85 (1.52–2.25) <0.0001 | 1.49 (1.17–1.88) <0.0001 | |
| pN N0 vs. N+ | 1.78 (1.47–2.16) <0.0001 | 1.44 (1.12–1.85) 0.0029 | |
| Grade G1 vs. G2–G3 | 1.19 (0.80–1.77) 0.3869 | ||
| Stage I–II vs. III–IV | 2.09 (1.72–2.55) <0.0001 | 1.39 (1.04–1.85) 0.0233 | |
| Irisin Cancer Cells <25% vs. ≥25% | 1.05 (0.77–1.43) 0.7535 | ||
| Irisin Stromal Cells <25% vs. ≥25% | 1.33 (1.08–1.63) 0.0054 | 1.30 (1.06–1.60) 0.0111 | |
| Ki-67 <25% vs. ≥25% | 0.94 (0.77–1.14) 0.5464 | ||
| TTF-1 <25% vs. ≥25% | 1.04 (0.86–1.27) 0.6235 | ||
| p63 <25% vs. ≥25% | 0.85 (0.70–1.03) 0.0998 | ||
| TTF-1 <25% vs. ≥25% | 0.98 (0.82–1.19) 0.1572 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowinska, K.; Jablonska, K.; Pawelczyk, K.; Piotrowska, A.; Partynska, A.; Gomulkiewicz, A.; Ciesielska, U.; Katnik, E.; Grzegrzolka, J.; Glatzel-Plucinska, N.; et al. Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-Small Cell Lung Cancer. Cancers 2019, 11, 1538. https://doi.org/10.3390/cancers11101538
Nowinska K, Jablonska K, Pawelczyk K, Piotrowska A, Partynska A, Gomulkiewicz A, Ciesielska U, Katnik E, Grzegrzolka J, Glatzel-Plucinska N, et al. Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-Small Cell Lung Cancer. Cancers. 2019; 11(10):1538. https://doi.org/10.3390/cancers11101538
Chicago/Turabian StyleNowinska, Katarzyna, Karolina Jablonska, Konrad Pawelczyk, Aleksandra Piotrowska, Aleksandra Partynska, Agnieszka Gomulkiewicz, Urszula Ciesielska, Ewa Katnik, Jedrzej Grzegrzolka, Natalia Glatzel-Plucinska, and et al. 2019. "Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-Small Cell Lung Cancer" Cancers 11, no. 10: 1538. https://doi.org/10.3390/cancers11101538
APA StyleNowinska, K., Jablonska, K., Pawelczyk, K., Piotrowska, A., Partynska, A., Gomulkiewicz, A., Ciesielska, U., Katnik, E., Grzegrzolka, J., Glatzel-Plucinska, N., Ratajczak-Wielgomas, K., Podhorska-Okolow, M., & Dziegiel, P. (2019). Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-Small Cell Lung Cancer. Cancers, 11(10), 1538. https://doi.org/10.3390/cancers11101538






