1. Introduction
The pharmacological effects of inhibitors of poly-ADP-ribose polymerases (PARPs) in anticancer therapies are attributed to impairing DNA damage removal, since PARP1 plays a crucial role in the recruitment of repair machinery, mainly in an ADP-ribosylation-dependent manner [
1,
2]. Lesion recognition followed by the recruitment of poly-ADP-ribose polymerase 1 (PARP1) to sites of DNA damage and ADP-ribosylation of its automodification domain are prerequisites for the binding of XRCC1, POLB, LIG3, or ALC1, which are involved in base excision repair (BER), single-strand break repair (SSBR), and nucleotide excision repair (NER) [
3,
4]. However, PARP1 also facilitates alternative and conventional non-homologous end-joining (NHEJ) as well as homologous recombination (HR), therefore helping to protect genome integrity and preventing destabilization of the genome resulting from double-strand breaks [
5,
6,
7]. According to the “access–repair–restore” model, nucleic acid repair is preceded by chromatin reorganization, since DNA lesions are curtained by DNA-associated proteins [
8]. Thus, local chromatin rearrangements are required to allow the assembly of the multiprotein machinery that removes lesions. Recent findings identified a link between PARP1 activity, nucleosome density, and the efficiency of some repair pathways, including HR [
9]. In the study referred to, auto-ADP-ribosylated PARP1 serves as an indispensable anchor that provides a platform at the damage site for the functional interaction between the nucleosome-evicting brahma-related gene 1 (BRG1; the SWI/SNF chromatin-remodeling enzyme) and the NAD-dependent deacetylase sirtuin-1 (SIRT1), which activates BRG1 by erasing lysine acetylation, thus promoting DNA end-joining. BRG1 interacts with the poly-ADP-ribose polymer through its ATPase domain rather than the N- or C-terminal tails, and is recruited to genomic regions enriched in PARP1. A previous paper reported the interaction of BRG1 with PARP1 and other histone-remodeling enzymes at the genomic level, where nucleosome-evicting ATPase cooperated with PARP1 and histone deacetylases (HDACs: HDAC2 and HDAC9) at the promoters of
α-MHC and
β-MHC, thereby preventing cardiac differentiation and maintaining the proliferation potential of the precursors [
10]. However, the molecular mechanism that drives PARP1/BRG1-dependent up- or down-regulation of gene transcription has not yet been identified. The suggestion that PARP1 enables the binding of EP300 to the promoters of cell cycle-dependent genes in proliferating cells in an ADP-ribosylation-independent manner focused our attention on the possible role of PAR-synthesizing enzymes in the transcriptional regulation of genes controlled by BRG–EP300–HDAC1 complexes [
11]. Our recent discoveries regarding the above-mentioned chromatin-remodeling units showed that these enzymes control the transcription of proliferation and DNA repair genes in two considerably different breast cancer cell lines, MCF7 and MDA-MB-231, which differ in terms of their expressed hormone and HER2 receptors [
12]. The nucleosomes of E2F/CpG-driven promoters in the two studied gene groups were acetylated by histone acetyltransferase p300 (EP300), causing them to be marked for BRG1-mediated eviction, and enabling paused RNA polymerase II to become active, leading to active gene transcription. This mechanism operates only in proliferating cells, which are also characterized by a high abundance of PARP1. This is because the
PARP1 promoter is controlled by BRG1–EP300–HDAC1 complexes and is repressed with respect to the growth arrest seen in the great majority of normal primary cells [
11].
Based on these premises, we aimed to discover whether PARP1 co-regulates BRG1–EP300-dependent transcription, and if PARP1 can be considered an active component of such multiprotein complexes in the studied breast cancer cell lines. We also aimed to uncover the molecular mechanism that links PARP1 with BRG1-dependent transcription and verify possible PARP1 selectivity toward functionally related genes.
3. Discussion
Malignant transformation directs cellular changes and adaptive responses toward new requirements that cancer cells face during growth and metastasis. As long as the activation of oncogenes, loss of cell cycle checkpoint control, and impaired DNA repair capacity favor carcinogenesis, genome integrity and cell cycle re-entrance will eventually become threatened due to increased energy demand, mild but persistent oxidative stress, and the modulation of signaling cascades necessary for tumor growth and the invasion of other tissues [
17,
18]. The altered expression of many gene products in response to cell transformation is affected by reprogramming the epigenome, resulting in changes in the transcription and reconstruction of the cellular proteome to meet emerging needs [
19]. According to our findings, the activation of
PARP1 transcription as a consequence of the transition of a cell from quiescence to proliferation may help the cancer cell gain the necessary adaptive physiology by acting at the genomic level in two ways: directly, by contributing to DNA repair machineries, and indirectly, by affecting the transcription of BRG1–EP300 targets, among others, which enable the cancer cell to rapidly divide and resist DNA damaging agents [
12,
20,
21,
22]. Although the first aspects of this mechanism have been relatively deeply explored and the regulatory roles of PARP1 regarding the removal of DNA lesions and the transition between consecutive cell cycle phases in various modes—for example, the modulation of SP1 activity, H1 displacement at proliferation-relevant genes, and nuclear retention of PKM2—have been documented, the detailed molecular mechanism in regard to functionally linked genes has not been identified until now [
23,
24,
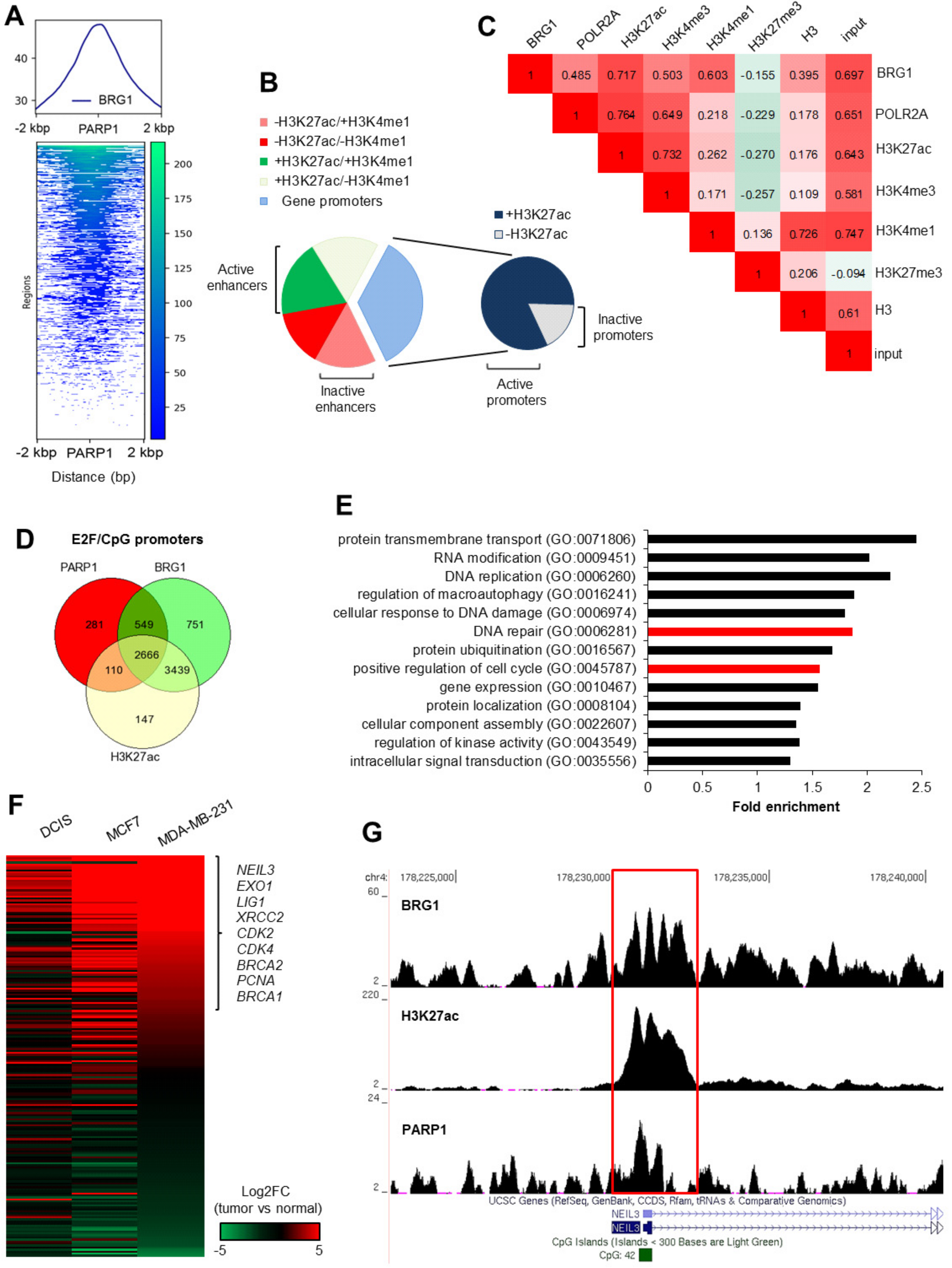
25]. In this study, PARP1 was shown to be an active component of the transcription machinery that drives BRG1-EP300-dependent gene expression by the poly-ADP-ribosylation of EP300 in breast cancer cells. PARP1 was highly enriched at gene promoters characterized by the occurrence of not only BRG1 and EP300, but also E2F binding motif(s) and CpG islands. Since these features apply to many functionally linked genes, the role of PARP1 in defining the breast cancer phenotype at the transcription level is likely to go far beyond cell cycle progression and the removal of DNA damage. As shown in
Figure 2E, triple-positive PARP1/BRG1/H3K27ac promoters represent the genes responsible for signal transduction and autophagy. Once poly-ADP-ribosylation is proved to be a co-activator of these genes, PARP inhibitors may be important to consider for pharmacological interventions that target and suppress mediators of pro-survival cascades at the genomic level. The role of poly-ADP-ribosylation in the fine-tuning of numerous intracellular processes simultaneously allows the maximization of the effectiveness of PARP1 inhibitors in rendering cancer cells vulnerable to anticancer drugs, which challenge PARP1-dependent or concurrent intracellular routes. Bearing in mind that the BRG1/EP300 complex was shown to operate at gene promoters in proliferating breast cancer cells and human macrophages due to an association with E2F transcription factors [
12,
26], the same PARP1-dependent mechanism of transcription control likely applies to other tumors, since the cell cycle status conditions both PARP1 expression and the activity of BRG1–EP300 complexes. Similarly, the energy status of proliferating cells demands a high ATP concentration; thus, the NAD
+/NADH redox ratio determines, for example, that PARP1 activity is five times higher in cancer cells than in normal, non-transformed cells [
27]. Therefore, it might be possible to adapt PARP1 inhibitors for the modulation of intracellular processes in a wider range of cancers.
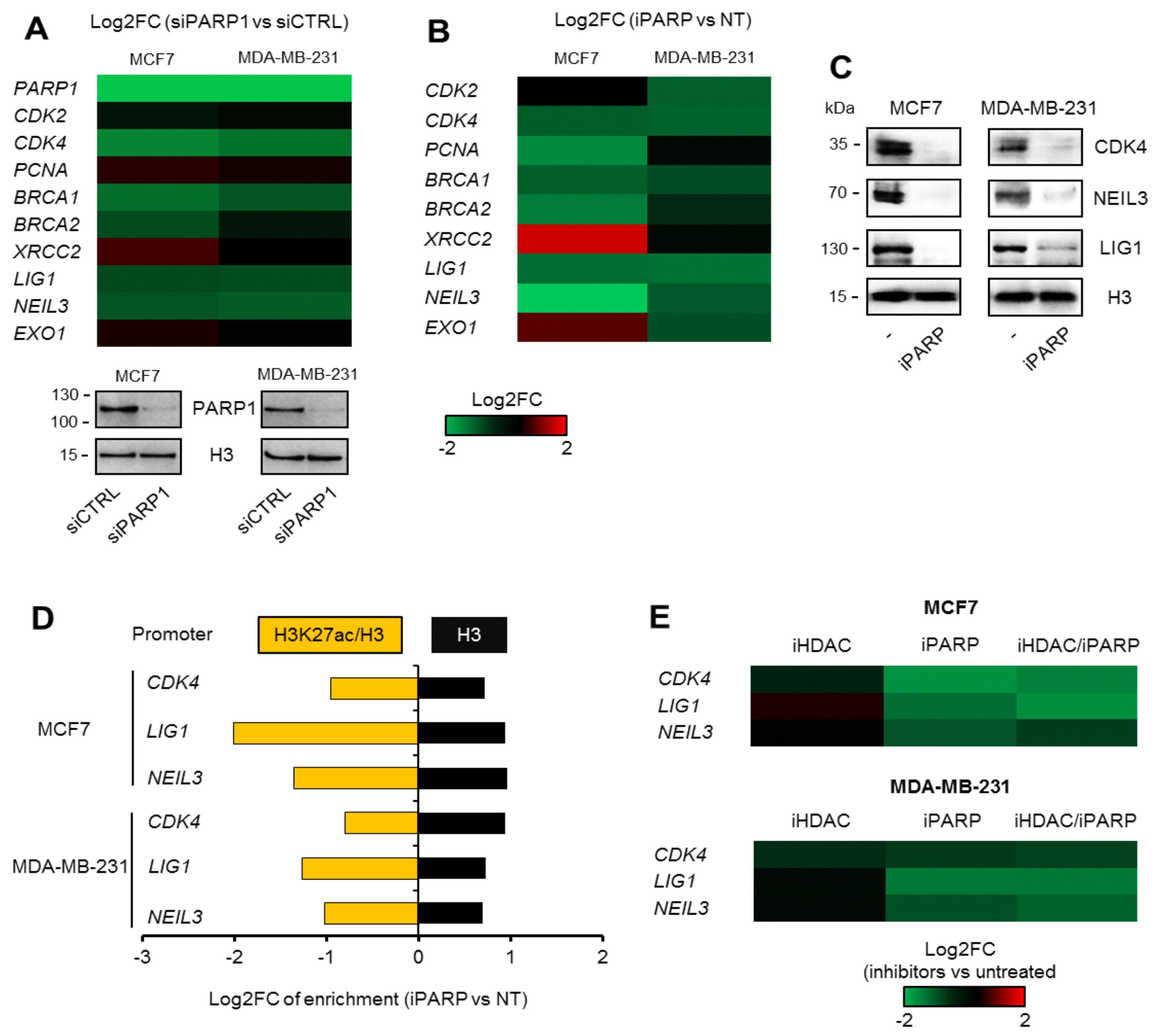
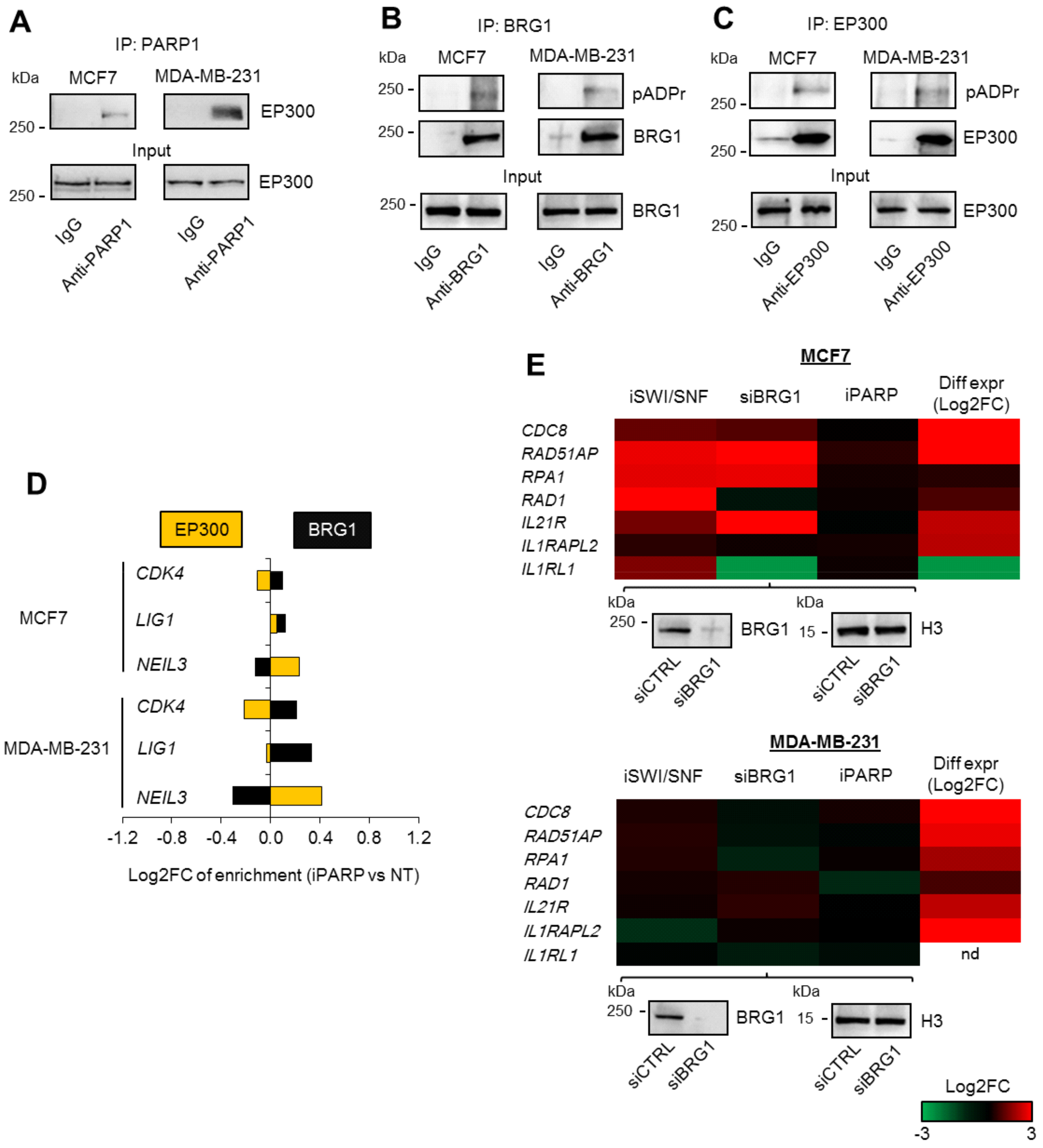
This study revealed a new mechanism that defines cancer cell responses to DNA lesions in a poly-ADP-ribosylation-dependent manner. Although the direct contribution of PARP1 to pathway repair at sites of damage is well acknowledged [
28,
29], we showed for the first time an impact on the repair mechanisms that is distant, gene promoter-related, and independent of lesion location. In this context, PARP inhibitors could be used to suppress the transcription of genes characterized by promoters enriched in PARP1, BRG1, and EP300, which represent executors that are crucial for BER, SSBR, NER, MMR, HR, and alt-NHEJ, since products of only the considered genes, i.e.,
BRCA1/2,
LIG1, and
NEIL3, contribute to more than one repair pathway [
30,
31]. Another benefit of using PARP inhibitors as DNA repair modulators acting at the level of the epigenome comes from the observation that poly-ADP-ribosylation is a co-activator of cell cycle-dependent genes that are simultaneously controlled by BRG1 and EP300, and are mostly over-expressed in the studied breast cancer cells, thus providing some selectivity toward this group. PARP1 was also found at gene promoters occupied by BRG1 alone, but cell treatment with olaparib did not reveal a considerable impact on the expression of these genes. Notably, the PARP1/BRG1 promoter response to an SWI/SNF inhibitor that is capable of impeding the activity of both ATPases (
Figure 4D) suggested that (a) EP300 has a co-activating role with BRG1 at the BRG1/EP300 promoters, and/or (b) the role of particular ATPases may be determined by EP300, and the lack of acetyltransferase may switch BRG1 to BRM activity. Although the scope of iSWI/SNFs is broader than that of iPARPs, the unexpected reaction of the chromatin to the loss of BRG1 and BRM activity may result in a coinciding repression and up-regulation of genes assigned to one regulatory circuit. Discrete inhibitors of the two ATPases mentioned, as well as further study on the mutual interdependence between these two enzymes, are needed for future clinical applications. Similarly, inhibitors of EP300 modulate the transcription of a significant number of genes at different levels; at the genome level by preventing acetylation of transcription co-factors and nucleosomes, and at the signaling cascade level by modifying signal transducers [
32,
33]. This all results in even less specificity toward the desired gene pool, and underlines the importance of the possible applications of PARP1 inhibitors. Some PARP-1 and PARP-2 inhibitors, such as olaparib, niraparib, rucaparib, veliparib, and talazoparib, which are small-molecule NAD
+ mimetics, are currently being studied in later-stage clinical trials or are already approved for breast and ovarian cancer treatment with deleterious germline
BRCA1 and
BRCA2 mutations, which predispose women to develop triple-negative and hormone-receptor-positive, human epidermal growth factor receptor 2-negative breast cancers, respectively [
34,
35]. Since PARP inhibitor monotherapy strategies are effective in cancers with homologous recombination repair defects and are relatively well-tolerated by patients, they can be considered for the treatment of a wider range of cancers, both in combined therapies, due to the well-established fact that these drugs sensitize cells to DNA-damaging chemotherapy and radiation therapy, or as an alternative to taxanes and a supplement to anthracyclines [
36,
37,
38]. However, numerous phase I clinical trials utilizing a combination of cytotoxic chemotherapy with PARP inhibitors failed to confirm any beneficial effects of such combinations. Therefore, the use of these drugs in adjuvant or neoadjuvant settings may need substantial revision, while also taking into consideration the myelosuppressive effects of PARP inhibitors and the careful selection of anticancer agents in combination with DNA repair inhibitor(s). Nevertheless, the long list of promoters of functionally related genes that are enriched in PARP1 presented in this manuscript suggest the likely involvement of this enzyme regarding the modulation of other intracellular processes at the transcription level. These findings open the gate for new ideas and concepts regarding anticancer approaches, which require verification first in cell and animal models.
The described contribution of PARP1 to the regulation of BRG1–EP300 activity emphasizes the role of PARP1 in chromatin remodeling. Although a number of papers have documented this enzyme as a direct or indirect regulator of chromatin structure in a context-dependent fashion, none have provided an overall mechanism for functionally linked gene sets in particular. Transcription in cells is shaped by the poly-ADP-ribosylation of nucleosomes, histone writers and erasers (KDM5B), transcription factors (e.g., C/EBPβ), or POL2 regulating co-factors (NELF), as well as physical, activity-independent interactions with gene promoters that define chromatin composition (LSD1, EP300) [
13,
14,
15,
16,
39,
40]. However, DNA motifs or chromatin signatures, which determine PARP1 distribution in the genome, remain unidentified. According to Gibbson, PARP1 and ADP-ribosylation correlated with histone markers (H3K4me3 and H3K27ac) featuring actively transcribed genes and with POL2 pausing machinery in embryonic fibroblasts of mice [
40]. These findings agree with our own, in which PARP1 was enriched at highly acetylated CpG islands, allowing immediate POL2 pausing or release in response to received signals [
11,
12]. The association of PARP1 with GC-rich regions impedes the identification of the PARP1-specific motif in promoter sequences. Since only 19% of PARP1 peaks in the genome of MDA-MB-231 cells occurred at BRG1 and H3K27ac negative promoters, and less than 3% outside of BRG1 peaks, these two features of promoters, together with E2F-binding motifs and CpG islands, seem to direct the enzyme to its destination regarding chromatin, whereby the poly-ADP-ribosylation of BRG1 and EP300 enables gene expression.
4. Materials and Methods
4.1. Materials
Two epithelial, breast cancer cell lines, derived from metastatic sites, MCF7 (estrogen and progesterone receptors-positive, HER2-negative) and MDA-MB-231 (triple negative) were purchased from ATCC and Sigma Aldrich (Poznan, Poland), respectively. DMEM high glucose with L-glutamine with sodium pyruvate for MCF7, fetal bovine serum, and antibiotics (penicillin and streptomycin) were from Biowest (CytoGen, Zgierz, Poland), L15 Medium for MDA-MB-231, iSWI/SNF (PFI-3), iHDAC (sodium butyrate), anti-rabbit IgG (A0545) and anti-mouse IgG (A4416) (whole molecule)–peroxidase antibody produced in goat, BLUeye prestained protein ladder (#94964), oligonucleotides for real-time PCR, SIGMAFAST™ Protease Inhibitor Tablets (PIC) were from Sigma Aldrich (Poznan, Poland). iPARP (olaparib, AZD-2281) was from Cayman Chemical (Biokom, Janki/Warsaw, Poland). Lipofectamine RNAiMAX, OptiMem, Dynabeads™ Protein G, glycogen, High-Capacity cDNA Reverse Transcription Kit, SuperSignal™ West Pico Chemiluminescent Substrate, TRI Reagent™, and RNase A were from Thermofisher Scientific (Thermofisher Scientific, Warsaw, Poland). KAPA HiFi™ HotStart ReadyMix (2×) from KapaBiosystems and Takyon™No ROX SYBR Core Kit blue dTTP from Eurogentec were purchased from Polgen (Lodz, Poland). EvaGreen® Dye, 20X in water was purchased from Biotium (Corporate Place Hayward, Fremont, CA, USA). WB antibodies: anti-DNA Ligase I (sc-271678), anti-CDK4 (sc-23896), anti-NEIL3 (sc-393703), anti-pADPr (sc-56198), siPARP1 (sc-29437), and gallotannin were purchased from Santa Cruz Biotechnology (AMX, Lodz, Poland). ChIP grade antibodies: normal rabbit IgG (#2729), anti-ARID1A (#12354), anti-SMARCC2 (#12760), anti-BRG1 (#49360), anti-H3K27ac (#4353), anti-histone H3 (#4620), and anti-PARP1 (#9532) were purchased from Cell Signaling Technology (LabJOT, Warsaw, Poland). Human Cytokine and Chemokine Receptor Primer Library (HCCR-I) were from RealTime Primers (Prospecta, Warsaw, Poland). For the mass spectrometric analysis, all materials were from Thermo Fisher Scientific (Loughborough, UK) unless otherwise indicated. Porcine Trypsin (Trypsin Gold Mass Spectrometry Grade) was from Promega (Southampton, UK), and general use Protease Inhibitor Cocktail (P2174) was from Sigma-Aldrich (Poole, UK).
4.2. Cell Culture and Treatment with Inhibitors
MCF7 were cultured in DMEM supplemented with 10% FBS, penicillin/streptomycin (50 U/mL and 50 µg/mL, respectively) in 5% CO2, whereas MDA-MB-231 was cultured in F15 medium supplemented with 15% FBS and penicillin/streptomycin (50 U/mL and 50 µg/mL, respectively) without CO2 equilibration. After freezing and thawing, cells were cultured in DMEM as described for MCF7 cells. iSWI/SNF (10 µM; PFI-3), iPARP1 (olaparib, 1 µM), and iHDAC1 (sodium butyrate, 250 µM) were added to cells 48 h prior to analysis.
4.3. Quantification of Gene Expression
mRNA quantification was conducted as described in Pietrzak et al. [
11] using Takyon™ No Rox SYBR
® MasterMix dTTP blue (Eurogentec—from local distributor—Polgen, Lodz, Poland) and CFX96 C1000 Touch (BioRad, Warsaw, Poland) for real-time PCR. The median average of ACTB, GAPDH, and B2M were used for normalization. Data in figures are shown as Log2FC with respect to untreated control or to siCTRL (indicated in figures or figure legends).
For protein detection, cell lysates were processed as previously described and visualized using SuperSignal™ West Pico Chemiluminescent Substrate. Pictures were acquired with ChemiDoc-IT2 (UVP, Meranco, Poznan, Poland).
The following primer sets were used for the quantification of gene expression: CDK2, 5′-CAGGATGTGACCAAGCCAGT-3′ (forward) and 5′-TGAGTCCAAATAGCCCAAGG-3′ (reverse); CDK4, 5′-CTGGTGTTTGAGCATGTAGACC-3′ (forward) and 5′-AAACTGGCGCATCAGATCCTT-3′ (reverse), XRCC2, 5′-TCGCCTGGTTCTTTTTGCA-3′ (forward) and 5′-TCTGATGAGCTCGAGGCTTTC-3′ (reverse), BRCA2, 5′-CTTGCCCCTTTCGTCTATTTG-3′ (forward) and 5′-TACGGCCCTGAAGTACAGTCT-3′ (reverse), LIG1, 5′-CAGAGGGCGAGTTTGTCTTC-3′ (forward) and 5′-AGCCAGTTGTGCGATCTCTT-3′ (reverse), EXO1, 5’-AAACCTGAATGTGGCCGTGT-3′ (forward) and 5′CCTCATTCCCAAACAGGGACT-3′ (reverse), NEIL3, 5′-GGTCTCCACCCAGCTGTTAAAG-3′ (forward) and 5′-CACGTATCATTTTCATGAGGTGATG-3’ (reverse), PCNA, 5’-TCTGAGGGCTTCGACACCTA-3’ (forward) and 5′- TTCTCCTGGTTTGGTGCTTCA-3′ (reverse); BRG1, 5′-AAGAAGACTGAGCCCCGACATTC-3′ (forward) and 5′-CCGTTACTGCTAAGGCCTATGC-3′ (reverse), BRCA1, 5′-TGCCCACAGATCAACTGGAA-3′ (forward) and 5′- CACAGGTGCCTCACACATCT-3′ (reverse); ACTB, 5′- TGGCACCCAGCACAATGAA-3′ (forward) and 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse); PARP1, 5′- AAGCCCTAAAGGCTCAGAACG-3′ and 5′-ACCATGCCATCAGCTACTCGGT-3′. GAPDH and B2M were from the Human Toll-like Receptor Signaling Primer Library (HTLR-I, RealTime Primers – from local distributor - Prospecta, Warsaw, Poland)).
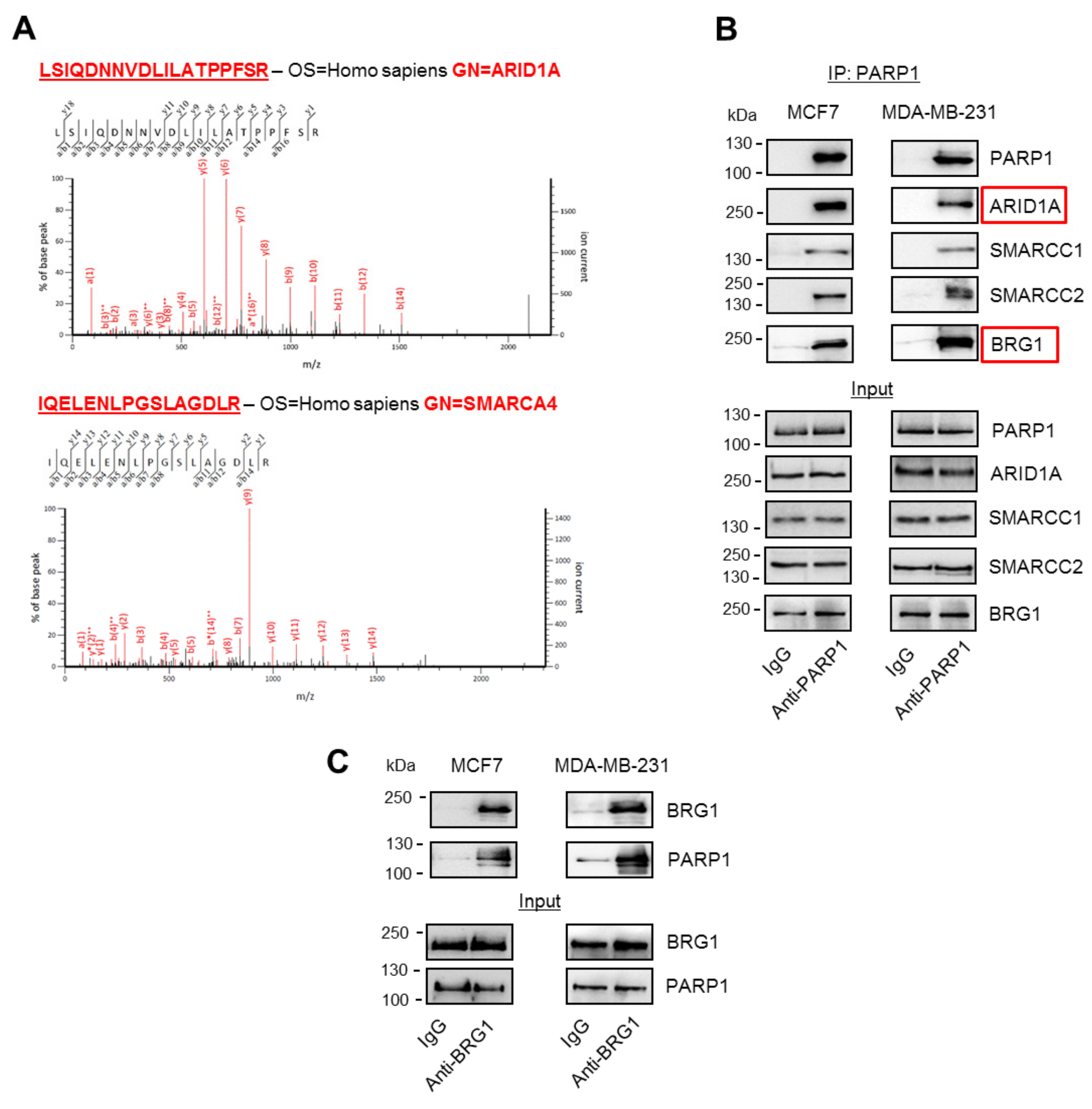
4.4. PARP1 Co-Immunoprecipitation for Mass Spectrometry
2 × 107 MCF7 cells were trypsinized, washed 3× with cold PBS, and lysed on ice in hypotonic buffer (50 mM HEPES-KOH, 10 mM NaCl, 1 mM EDTA, 10% glycerol, and 0.5% NP-40, 0.25% TritonX-100). Nuclei were washed twice in PBS supplemented with protease inhibitor cocktail (10× stock added at 10% of volume, Sigma P2714 General Purpose) and fixed in 1% formaldehyde in PBS on stirrer for 10 min at room temperature. Formaldehyde was quenched by the addition of 125 mM oglycine, and after 20 min of incubation, nuclei were washed twice in 10 mM Tri-HCl (pH = 8.0), 100 mM NaCl, and 1 mM EDTA, and finally resuspended in 10 mM Tri-HCl (pH = 8.0), 150 mM NaCl, 1 mM EDTA, 0.05 Na-deoxycholate, and 0.25% N-laurosarcosine. Nuclei were sonicated until the solution was transparent, TritonX-100 was added to 1% and then centrifuged (10,000× g, 4 °C, 10 min) to remove insoluble material. The supernatant was split into two samples, to which were added either control IgG–Dynabead or anti-PARP1–Dynabead conjugates (prepared by the incubation of 5 µg of antibody and 10 µL of magnetic beads in 10 mM Tri-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.05 Na-deoxycholate, and 0.25% N-laurosarcosine for at least 15 min). After overnight incubation on a roller shaker at 4 °C, the supernatant was removed, and the beads were washed 5× with 50 mM HEPES-KOH (pH = 7.5), 500 mM LiCl, 1 mM EDTA, 1% NP-40, and 0.7% Na-deoxycholate; twice with 50 mM NaCl in TE buffer (10 mM Tris pH = 8.0, 1 mM EDTA); and twice with 50 mM ammonium bicarbonate using a magnetic stand. Then, the beads were incubated with trypsin (Promega Gold, 1 µg/µL) in 50 mM ammonium bicarbonate for 6 h at 36 °C, after which the supernatant was transferred to new tubes and dried in a SpeedVac vacuum concentrator (Eppendorf, Stevenage, UK).
4.5. Mass Spectrometry Analysis of PARP1 Co-Immunoprecipitates
Tryptic digest samples were resuspended in 25 µL of 2% acetonitrile in water and 0.5% formic acid. Peptides were separated and analyzed using a U3000 nanoflow LC system (Thermo) connected to a 5600 Triple ToF mass spectrometer (Sciex, Warrington, UK). Then, 10 µL of sample was loaded onto a 0.5 × 5 mm PepMap C18 trap, washed with buffer A (2% acetonitrile 98% water containing 0.5% formic acid) for 4 min, and then separated by a 90-minute gradient from 2% to 40% buffer B (98% acetonitrile, 2% water containing 0.5% formic acid) on a 0.075 × 150 mm PepMap C18 column (Thermo Fisher Scientific, Loughborough, UK)). MSMS data were collected for precursors of 2+ to 5+ charge state in the range m/z 350–1250 Th using a top 10 data dependent acquisition method, collecting MS data for 200 ms and MSMS data for 100 ms, with dynamic exclusion for 15 s and a standard rolling collision energy settings. MSMS data was collected in the range of m/z 50–2000 Th. All other settings were optimized for peptides using a standard mixture. Samples were run as biological replicates. MSMS data was analyzed using Progenesis QIP (Waters, Manchester, UK) for label-free quantitative analysis and Mascot (Matrix Science, London) for protein identification. Data was loaded as .wiff files into Progenesis QIP, automatically aligned, and peak picked using the default settings; then, the alignment was manually improved where necessary. Default settings were used for peak picking, and Hi-N was used for quantification. Data was exported to Mascot using the default settings. For the Mascot analysis, the SwissProt Mammalian database (2018_09) was searched, allowing 50 ppm error for MS and 100 ppm for MSMS data, two missed cleaves, methionine oxidation as a variable modification, and an overall FDR of <1%. Data was re-imported into Progenesis QIP for further quantitative analysis. Protein identifications were deemed significant if more than two peptides were identified with an overall confidence score greater than 50, but more stringent criteria were applied for proteins to be further investigated. Quantification data was considered significant where the ANOVA p-value was less than 0.05, the fold change was greater than 2, and the highest mean was in the PARP immunoprecipitation.
4.6. Co-Immunoprecipitation and Western Blot
MCF7 and MDA-MB-2331 cells were lysed in 20 mM Tris-HCl (pH = 7.5), 75 mM KCl, 5 mM MgCl2, 0.2 mM EDTA, 10% glycerol, 0.1% Tween20, and PIC (IP buffer); sonicated with the ultrasonic homogenizer Bandelin Sonopuls (HD 2070; 10 impulses, 60%); and centrifuged (10,000× g, 4 °C, 10 min). Supernatant was incubated with anti-PARP1, anti-BRG1, anty-EP300, and corresponding IgG at 4 °C for 2 h. For another 1 h, lysates were added with Dynabeads (5 µL); then, they were washed 5× with the IP buffer and once in 50 mM NaCl in TE buffer (10 mM Tris pH = 8.0, 1 mM EDTA). Beads were suspended in gel loading buffer supplemented with 5% β-mercaptoethanol, and heated at 70 °C for 10 min. Beads were collected on a magnetic stand and supernatant was separated by PAGE. BRG1, ARID1A, SMARCC1, SMARCC2, PARP1, EP300, H3, and poly-ADP-ribose were detected on nitrocellulose membranes after overnight staining with corresponding antibodies. For the detection of poly-ADP-ribosylation, cells were lysed and processed in the presence of a PARG inhibitor: 0.5 mM gallotannin.
Each immunoprecipitation followed by Western blot was repeated in three biological replicates. Each time, the striking difference in protein level being detected was observed between IgG (weak or lack of signal) versus anti-PARP1 (or anti-BRG1; strong and clear bands). Representative images were taken for figures.
4.7. Chromatin Immunoprecipitation
The immunoprecipitation of chromatin-bound proteins and histones was carried out according to the protocol previously described [
11]. For the quantification of H3K27 acetylation, cells were lysed and processed in the presence of iHDAC (0.5 mM). Fragments spanning PARP1/BRG1/H3K27ac sites in selected gene promoters were amplified using KAPA HiFi™ HotStart ReadyMix supplemented with EvaGreen
® Dye and 4% DMSO. Primers for
CDK4,
LIG1, NEIL3, and
XRCC1 promoters were as follows:
CDK4 prom, 5′-ATAACCAGCTCGCGAAACGA-3′ and 5′-AGAGCAATGTCAAGCGGTCA-3′,
LIG1 prom, 5′-AACACACTCAGATCCGCCAG-3′ and 5′-GCTTCCACCGATTCCTCCTC-3′,
NEIL3 prom, 5′-GTAGGGAGCGACCTCAACAG-3′ and 5′-AGTACAGCCTGGTCCTTCCA-3′,
XRCC1 prom, 5′- TGGCCAGAAGGATGAGGTAG-3′ (forward) and 5′-AGGAAACGCTCGTTGCTAAG-3′ (reverse).
4.8. Transient Gene Silencing
For PARP1 and BRG1 silencing, MCF7 and MCD-MB-23 were seeded at the density of 100,000 cells per well, transfected on the following day with RNAiMAX-siRNA complexes (3 µL of transfection reagent and 20 nmol siRNA incubated in OptiMem for 20 min). The silencing was confirmed by real-time PCR and Western blot 48 h after cell transfection.
4.9. ChIP-Seq Analysis in Galaxy Version 19.05.dev [41]
The following, publically available, generated by other groups and deposited in the
PubMed Central Database data from MDA-MB-231 cells were taken for ChIP-Seq analysis: PARP1—GSM1517306 (SRR1593959), BRG1—GSM1856026 (SRR2171350), GSM1856027 (SRR2171351), and GSM1856028 (SRR2171352), H3K27ac—GSM1855991 (SRR2171311) and GSM1855992 (SRR2171312); H3K4me3—GSM1700392 (SRR2044734), H3K4me1—GSM2036932 (SRR3096750 and SRR3096751), H3K27me3—GSM949581 (SRR513994), H3K9ac—GSM1619765 (SRR1820123 and SRR1820124), H3—GSM2531568 (SRR5332805), POLR2A—GSM2309434 (SRR4240635), and Input—GSM1964894 (SRR2976843). FASTQ quality formats were unified to Sanger formatted with a FASTQ Groomer [
26]. Reads were aligned to Human Genome version 19 using a Map with Bowtie for Illumina, and unmapped reads were filtered out. ChIP-seq peaks were called in MACS with a
p-value cutoff for peak detection set at 10
−3. BRG1 co-occurrence at the PARP1 peaks was monitored by computeMatrix/plotProfile (PARP1 peaks in bed were used as regions to plot, while mapped BRG1 reads were used for scoring) [
42]. The co-distribution of BRG1, POLR2A, and histone modifications at the PARP1-enriched regions was studied by MulitBamSummary/plotCorrelation (regions of the genome were limited to PARP1 peaks in bed, mapped reads for scoring) [
42]. Regions simultaneously enriched in BRG1, PARP1, H3K4me1, and H3K27ac were identified by returning intersects of the peaks in bed using bedtools Intersect intervals [
43]. Regions localized outside of gene promoters and double positive for H3K4me1 and H3K27ac were assigned as active enhancers high in H3K4me1 and low in H3K27ac as inactive enhancers, while gene promoters were recognized by returning intersects of BRG1/PARP1 peaks and genomic regions ±2000 bp centered on TSS (overlapping intervals of both datasets). Promoters defined by high H3K27ac and associated with the presence of gene transcripts (RNA-Seq results for MDA-MB-231) downstream of corresponding TSS were assumed as active, while the lack of promoter acetylation marked inactive gene promoters. Genomic intervals for E2F (overlaps of E2F1 and E2F4) and CpG islands were taken from the UCSC main tables wgEncodeRegTfbsClusteredV3 and cpgIslandExt, respectively. Intersects of TSS ±2 kbp and CpG or E2F intervals were compared using bedtools Intersect intervals. The characteristics of gene promoters (occurrence of particular proteins and CpGs, histone modifications, E2F binding sites) were studied using Venn diagrams, which were created in
http://www.interactivenn.net/ from gene lists. The annotation of PARP1/BRG1/H3K27ac promoters to biological processes was carried out in AmiGO2 (test type—binominal, correction—FDR) [
44]. PARP1, BRG1, and H3K27ac peaks were visualized in the UCSC Genome Browser.
The following, publically available datasets for various breast cells, which have been generated by other groups, were downloaded from the PubMed Central Database data and used for ChIP-Seq analysis: data from normal breast, ductal carcinoma in situ (DCIS; pre-invasive malignancy of the breast), MCF7 and MDA-MB-231 cells were taken for RNA-Seq analysis: normal breast—GSM1695870 (SRR2040339), GSM1695872 (SRR2040341), GSM1695873 (SRR2040342), GSM1695874 (SRR2040343), GSM1695877 (SRR2040346), and GSM1695878 (SRR2040347); DCIS—GSM1695891 (SRR2040360), GSM1695898 (SRR2040367), GSM1695899 (SRR2040368), GSM1695882 (SRR2040351), GSM1695890 (SRR2040359), and GSM1695894 (SRR2040363); MCF7—GSM2422725 (SRR5094305), GSM2422726 (SRR5094306), GSM2422727 (SRR5094307), GSM2422728 (SRR5094308), GSM2422729 (SRR5094309), and GSM2422730 (SRR5094310); MDA-MB-231—GSM2422731 (SRR5094311), GSM2422732 (SRR5094312), GSM2422733 (SRR5094313), GSM2422734 (SRR5094314), GSM2422735 (SRR5094315), and GSM2422736 (SRR5094316). All samples were processed as described in Sobczak et al. [
12]. Differential gene expression in cancer versus normal breast cells was calculated with Cuffdiff and shown as a heatmap for two selected GOs (positive regulation of cell cycle and DNA repair) [
45].