Enumerating Circulating Tumor Cells with a Self-Assembled Cell Array (SACA) Chip: A Feasibility Study in Patients with Colorectal Cancer
Abstract
1. Introduction
2. Results
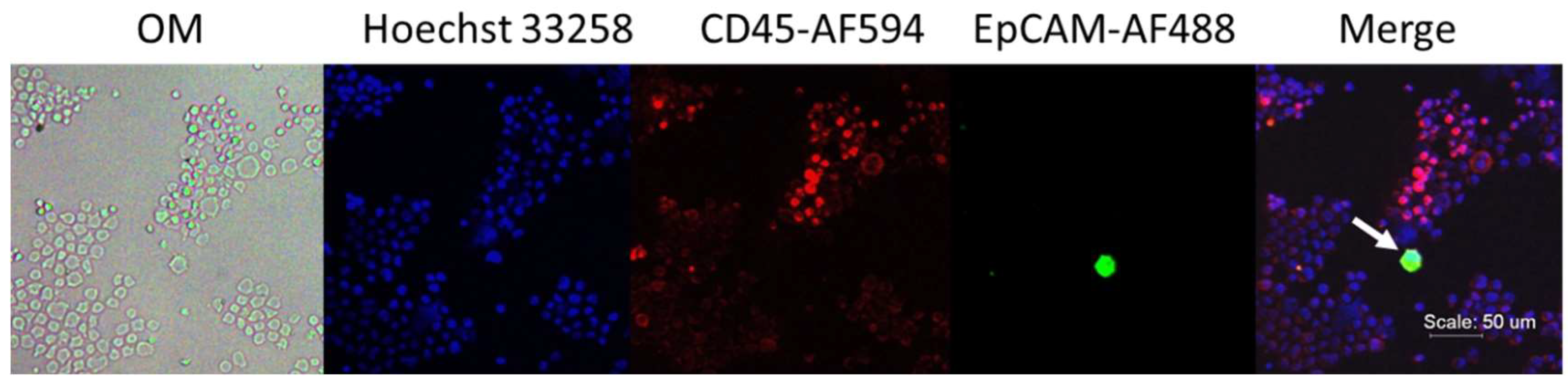
2.1. Fluorescent Micrograph-Based CTC Analysis
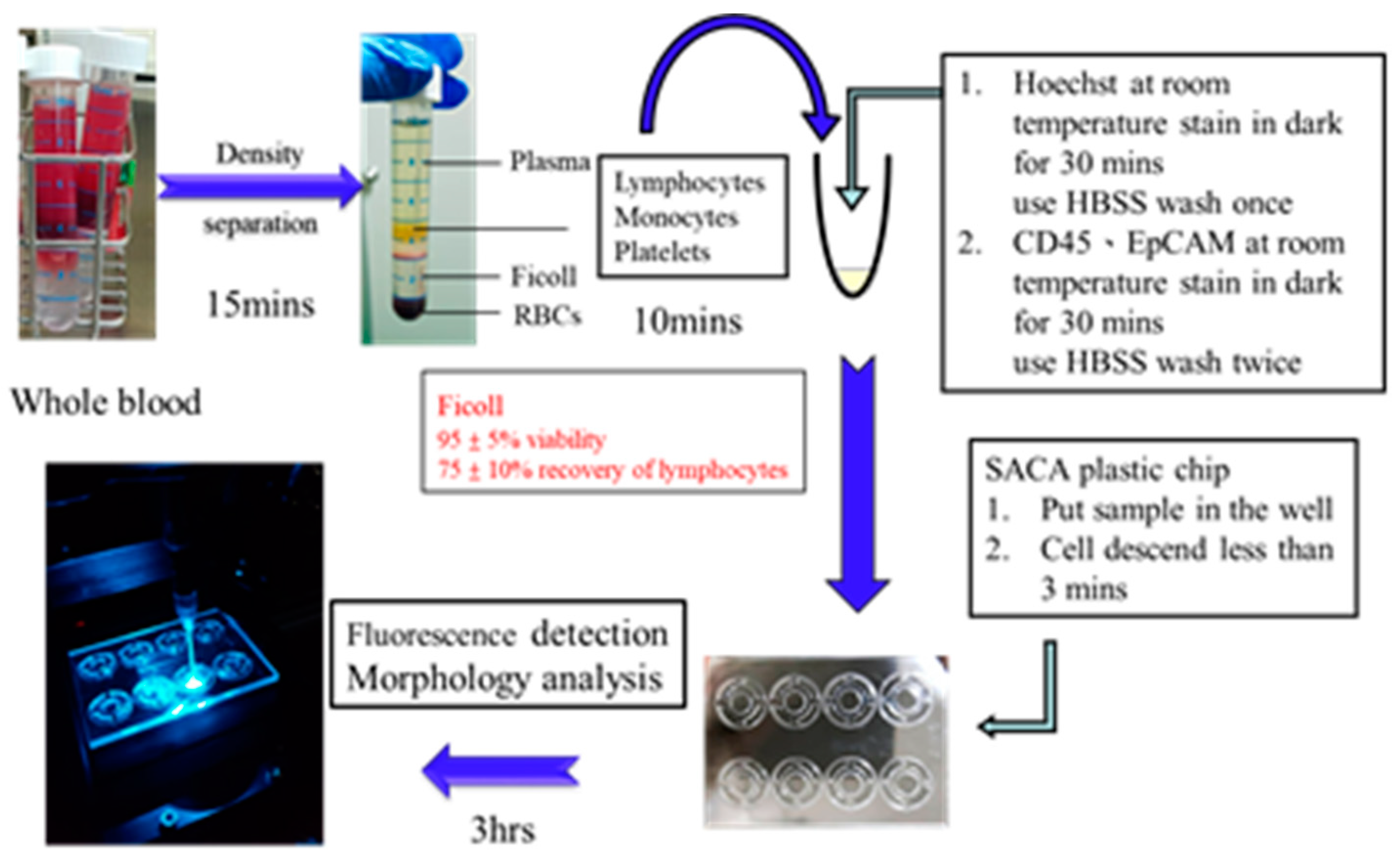
Process Development in Spike-In Samples
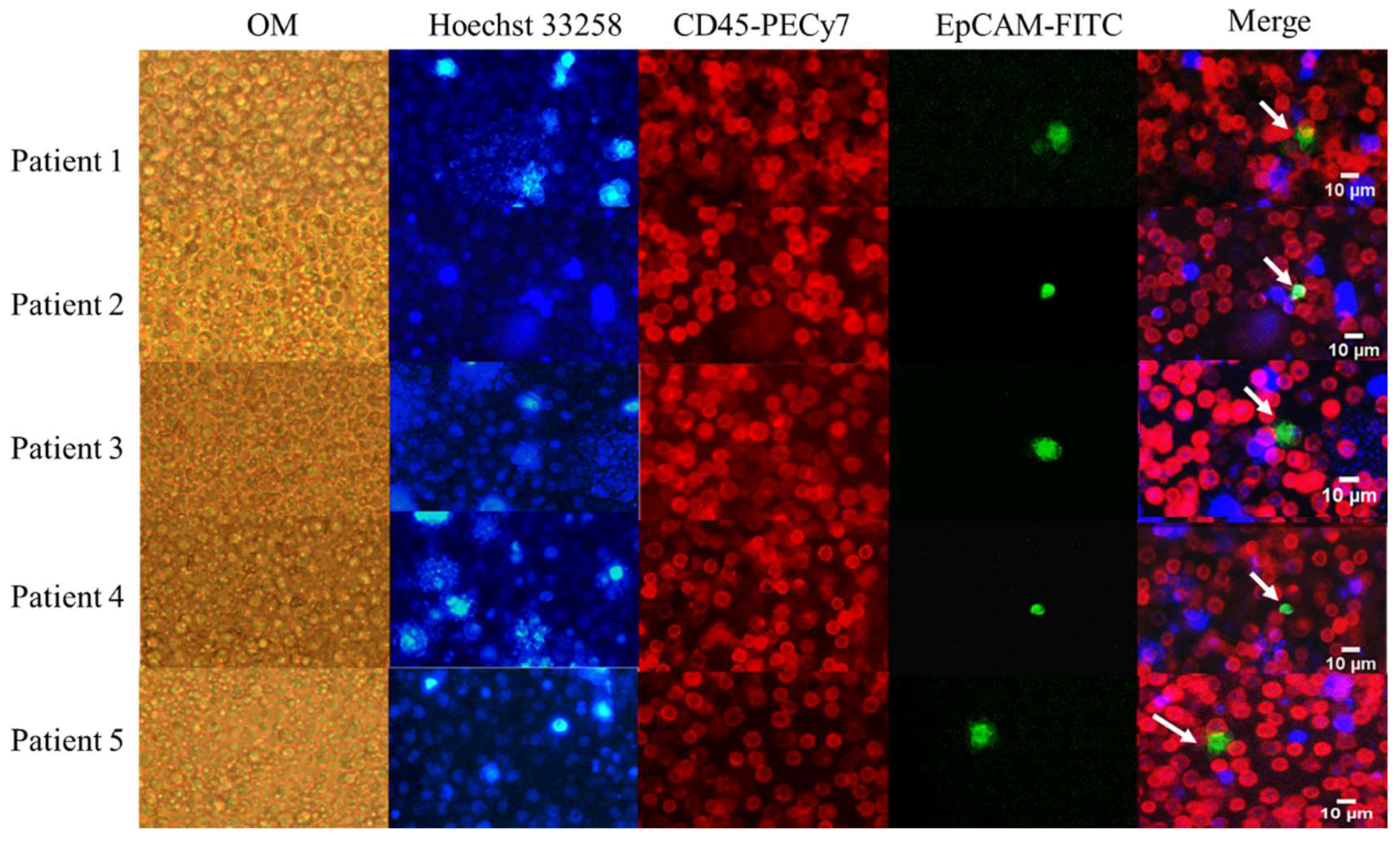
2.2. Validation in Clinical Samples
2.2.1. Demographics
2.2.2. CTC and Clinical Relevance
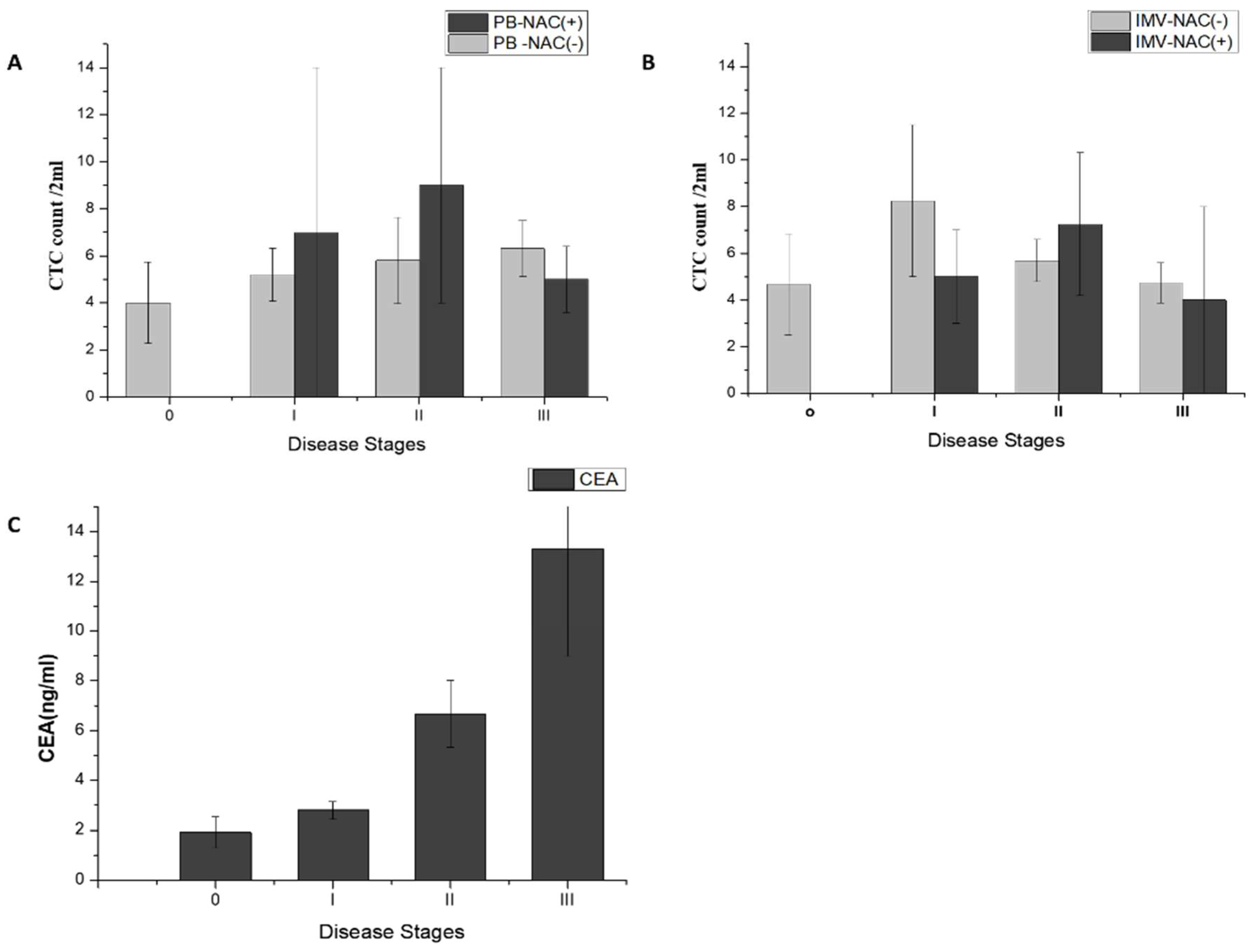
2.2.3. CTCs in PB and IMV of Patients with CRC
2.3. Clinical Implications of CTC in Colorectal Cancer
2.3.1. CTC Count Correlates with Stages of Non-Metastatic CRC
2.3.2. CTC Count and CEA Synergize to Predict Recurrence in Patients with Non-Metastatic CRC
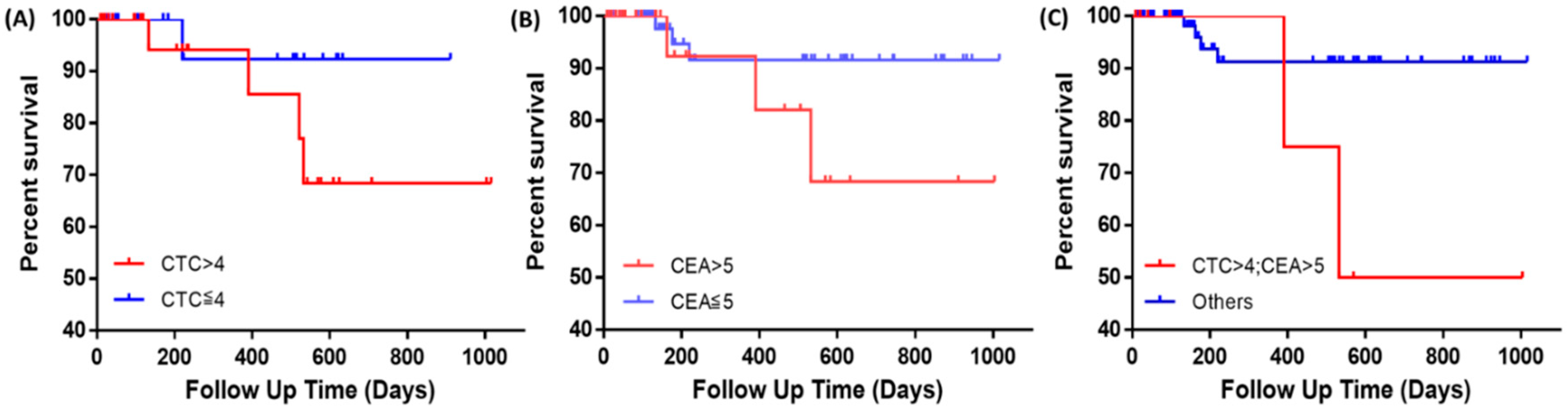
2.3.3. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Clinical Sample Acquisition
4.2. Sample Preparation Process for Clinical Research
4.2.1. Blood Collection and CTC Isolation
4.2.2. Immunofluorescence Staining
4.2.3. Imaging
4.2.4. The Preparation Time of Each Step of the Clinical Test Sample
4.3. Cell Line Culture
4.4. Cell Line Spike-In Controls
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Fabisiewicz, A.; Grzybowska, E. CTC clusters in cancer progression and metastasis. Med. Oncol. 2017, 34, 12. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 2006, 12, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539. [Google Scholar] [CrossRef] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Paterlini-Brechot, P.; Benali, N.L. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007, 253, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.J.; Rajasekaran, A.K. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006, 66, 8319–8326. [Google Scholar] [CrossRef]
- Cohen, S.; Punt, C.; Iannotti, N.; Saidman, B.; Sabbath, K.; Gabrail, N.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann. Oncol. 2009, 20, 1223–1229. [Google Scholar] [CrossRef]
- Van Dalum, G.; Stam, G.-J.; Scholten, L.F.; Mastboom, W.J.; Vermes, I.; Tibbe, A.G.; De Groot, M.R.; Terstappen, L.W. Importance of circulating tumor cells in newly diagnosed colorectal cancer. Int. J. Oncol. 2015, 46, 1361–1368. [Google Scholar] [CrossRef]
- Iinuma, H.; Watanabe, T.; Mimori, K.; Adachi, M.; Hayashi, N.; Tamura, J.; Matsuda, K.; Fukushima, R.; Okinaga, K.; Sasako, M. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J. Clin. Oncol. 2011, 29, 1547–1555. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chang, S.; Li, G.; Sun, Y. Application of liquid biopsy in precision medicine: Opportunities and challenges. Front. Med. 2017, 11, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Smith, R.A.; Ward, E. Worldwide variations in colorectal cancer. Cancer J. Clin. 2009, 59, 366–378. [Google Scholar] [CrossRef]
- Shah, S.A.; Haddad, R.; Al-Sukhni, W.; Kim, R.D.; Greig, P.D.; Grant, D.R.; Taylor, B.R.; Langer, B.; Gallinger, S.; Wei, A.C. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J. Am. Coll. Surg. 2006, 202, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Boursi, B.; Arber, N. Current and future clinical strategies in colon cancer prevention and the emerging role of chemoprevention. Curr. Pharm. Des. 2007, 13, 2274–2282. [Google Scholar] [CrossRef]
- Brenner, H.; Chang-Claude, J.; Jansen, L.; Knebel, P.; Stock, C.; Hoffmeister, M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014, 146, 709–717. [Google Scholar] [CrossRef]
- Cohen, S.J.; Alpaugh, R.K.; Gross, S.; O’hara, S.M.; Smirnov, D.A.; Terstappen, L.W.; Allard, W.J.; Bilbee, M.; Cheng, J.D.; Hoffman, J.P. Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 2006, 6, 125–132. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Maestro, M.; Puente, J.; Veganzones, S.; Alfonso, R.; Rafael, S.; Garcia-Saenz, J.; Vidaurreta, M.; Martin, M.; Arroyo, M. Circulating tumor cells in colorectal cancer: Correlation with clinical and pathological variables. Ann. Oncol. 2008, 19, 935–938. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, H.K.; Lu, B.; Williams, A.; Datar, R.; Cote, R.J.; Tai, Y.-C. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed. Microdevices 2011, 13, 203–213. [Google Scholar] [CrossRef]
- Harouaka, R.A.; Zhou, M.-D.; Yeh, Y.-T.; Khan, W.J.; Das, A.; Liu, X.; Christ, C.C.; Dicker, D.T.; Baney, T.S.; Kaifi, J.T. Flexible micro spring array device for high-throughput enrichment of viable circulating tumor cells. Clin. chem. 2013, 2013, 206805. [Google Scholar] [CrossRef]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.-i.; Morgan, B.; Trautwein, J. Inertial focusing for tumor antigen–dependent and–independent sorting of rare circulating tumor cells. Sci. Transl. Med. 2013, 5, 179ra147. [Google Scholar] [CrossRef]
- Karabacak, N.M.; Spuhler, P.S.; Fachin, F.; Lim, E.J.; Pai, V.; Ozkumur, E.; Martel, J.M.; Kojic, N.; Smith, K.; Chen, P.-I. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 2014, 9, 694. [Google Scholar] [CrossRef]
- Chen, T.-J.; Wu, J.-K.; Chang, Y.-C.; Fu, C.-Y.; Wang, T.-P.; Lin, C.-Y.; Chang, H.-Y.; Chieng, C.-C.; Tzeng, C.-Y.; Tseng, F.-G. High-efficiency rare cell identification on a high-density self-assembled cell arrangement chip. Biomicrofluidics 2014, 8, 036501. [Google Scholar] [CrossRef]
- Martin, V.; Siewert, C.; Scharl, A.; Harms, T.; Heinze, R.; Ohl, S.; Radbruch, A.; Miltenyi, S.; Schmitz, J. Immunomagnetic enrichment of disseminated epithelial tumor cells from peripheral blood by MACS. Exp. Hematol. 1998, 26, 252–264. [Google Scholar]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235. [Google Scholar] [CrossRef]
- Cho, W.; Pradhan, R.; Chen, H.Y.; Weng, Y.-H.; Chu, H.Y.; Tseng, F.-G.; Lin, C.-P.; Jiang, J.-K. Rapid Staining of Circulating Tumor Cells in Three-Dimensional Microwell Dialysis (3D-μDialysis) Chip. Sci. Rep. 2017, 7, 11385. [Google Scholar] [CrossRef]

| (A)Peripheral Vein Blood (PB) | ||
| Groups | Patients (n) | Percentage (%) |
| Age (years) | ||
| <60 | 55 | 43% |
| ≥60 | 74 | 57% |
| Sex | ||
| Male | 75 | 58% |
| Female | 54 | 42% |
| Tumor location of the disease | ||
| Colon | 89 | 69% |
| Others | 40 | 31% |
| T classification (TNM Stage) | ||
| Earlier than T2 | 123 | 72% |
| Later than T3 | 48 | 28% |
| (B)Inferior Mesenteric Vein Blood (IMV) | ||
| Groups | Patients (n) | Percentage (%) |
| Age (years) | ||
| <60 | 47 | 49% |
| ≥60 | 48 | 51% |
| Sex | ||
| Male | 56 | 59% |
| Female | 39 | 41% |
| Tumor location of the disease | ||
| Colon | 64 | 67% |
| Others | 31 | 33% |
| T classification (TNM Stage) | ||
| Earlier thanT2 | 60 | 63% |
| Later than T3 | 35 | 37% |
| (C)Lymph Node Metastasis | ||
| Groups | Patients (n) | Percentage (%) |
| Negative | 82 | 73% |
| Positive | 30 | 27% |
| Preoperative Serum CEA (ng/mL) | ||
| Patients (n) | Percentage (%) | |
| ≤5 | 81 | 65% |
| >5 | 44 | 35% |
| Preoperative Serum CA19-9 (U/mL) | ||
| Patients (n) | Percentage (%) | |
| <35 | 106 | 87% |
| ≥35 | 16 | 13% |
| (A)CTC Count (PB)—2 mL of Blood | ||||
| Disease stages | No. of cases | Mean ± SEM | Range (median) | Mode |
| Non-colorectal cancer cases, NAC (−) | ||||
| Benign | 7 | 3.28 ± 0.7 | 0–6 (3) | 3 |
| Non-colorectal cancer cases, NAC (+) | ||||
| Benign | 1 | 1 ± N/A | N/A | N/A |
| Colorectal cancer cases, NAC (−) | ||||
| Stage 0 | 3 | 4 ± 1.7 | 1–7 (4) | N/A |
| Stage I | 29 | 5.62 ± 1.12 | 1–26 (4) | 4 |
| Stage II | 34 | 5.8 ± 1.83 | 0–57 (4) | 4 |
| Stage III | 29 | 6.3 ± 1.18 | 0–30 (3.5) | 4 |
| Stage IV | 10 | 4.6 ± 1.04 | 1–12 (3) | 3 |
| Colorectal cancer cases, NAC (+) | ||||
| Stage 0 | 0 | N/A | N/A | N/A |
| Stage I | 2 | 7 ± 7.0 | 0–14 (7) | N/A |
| Stage II | 5 | 9 ± 5.4 | 1–30 (3) | N/A |
| Stage III | 2 | 5 ± 1.0 | 4–6 (5) | N/A |
| Stage IV | 7 | 2 ± 0.48 | 1–5 (2) | 2 |
| (B)CTC Count (IMV)—2 mL of Blood | ||||
| Disease stages | No. of cases | Mean ± SEM | Range (median) | Mode |
| Non-colorectal cancer cases, NAC (−) | ||||
| Benign | 4 | 6.5 ± 1.50 | 3–9 (5) | 3 |
| Non-colorectal cancer cases, NAC(+) | ||||
| Benign | 1 | 0 ± N/A | N/A | N/A |
| Colorectal cancer cases, NAC (−) | ||||
| Stage 0 | 3 | 4.6 ± 2.18 | 2–9 (3) | N/A |
| Stage I | 20 | 8.25 ± 3.26 | 0–69 (5) | 4 |
| Stage II | 27 | 5.6 ± 0.91 | 0–21 (6) | 7 |
| Stage III | 19 | 4.7 ± 0.86 | 0–14 (3) | 3 |
| Stage IV | 7 | 6.8 ± 1.14 | 4–13 (6) | 5 |
| Colorectal cancer cases, NAC (+) | ||||
| Stage 0 | 0 | N/A | N/A | N/A |
| Stage I | 2 | 7 ± 2.0 | 0–14 (7) | N/A |
| Stage II | 4 | 7.2 ± 3.06 | 1–13 (7) | N/A |
| Stage III | 2 | 4 ± 4.0 | 0–8 (4) | N/A |
| Stage IV | 7 | 4.7 ± 0.83 | 1–7 (5) | 7 |
| (A) | No. of Cases | Probability Ratio | |
| Disease stages 0–III | Recurrence (+) | Recurrence (−) | (odds ratio, OR) |
| CTC > 4 | 3 | 27 | 4.00 |
| CTC ≤ 4 | 1 | 36 | |
| CEA > 5 | 3 | 25 | 2.68 |
| CEA ≤ 5 | 3 | 67 | |
| CEA > 5; CTC > 4 | 2 | 6 | 7.16 |
| Others | 3 | 86 | |
| (B) | No. of Cases | Probability Ratio | |
| Disease stage IV | Recurrence (+) | Recurrence (−) | (odds ratio, OR) |
| CTC > 4 | 1 | 1 | N/A |
| CTC ≤ 4 | 0 | 7 | |
| CEA > 5 | 3 | 12 | N/A |
| CEA ≤ 5 | 0 | 3 | |
| CEA > 5; CTC > 4 | 1 | 1 | 4.66 |
| Others | 3 | 14 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.-Y.; Lu, L.-S.; Cho, W.; Wu, S.-Y.; Chang, Y.-C.; Lin, C.-P.; Yang, C.-Y.; Lin, C.-H.; Jiang, J.-K.; Tseng, F.-G. Enumerating Circulating Tumor Cells with a Self-Assembled Cell Array (SACA) Chip: A Feasibility Study in Patients with Colorectal Cancer. Cancers 2019, 11, 56. https://doi.org/10.3390/cancers11010056
Chu H-Y, Lu L-S, Cho W, Wu S-Y, Chang Y-C, Lin C-P, Yang C-Y, Lin C-H, Jiang J-K, Tseng F-G. Enumerating Circulating Tumor Cells with a Self-Assembled Cell Array (SACA) Chip: A Feasibility Study in Patients with Colorectal Cancer. Cancers. 2019; 11(1):56. https://doi.org/10.3390/cancers11010056
Chicago/Turabian StyleChu, Hsueh-Yao, Long-Sheng Lu, Wanying Cho, Shin-Yao Wu, Yu-Cheng Chang, Chien-Ping Lin, Chih-Yung Yang, Chi-Hung Lin, Jeng-Kai Jiang, and Fan-Gang Tseng. 2019. "Enumerating Circulating Tumor Cells with a Self-Assembled Cell Array (SACA) Chip: A Feasibility Study in Patients with Colorectal Cancer" Cancers 11, no. 1: 56. https://doi.org/10.3390/cancers11010056
APA StyleChu, H.-Y., Lu, L.-S., Cho, W., Wu, S.-Y., Chang, Y.-C., Lin, C.-P., Yang, C.-Y., Lin, C.-H., Jiang, J.-K., & Tseng, F.-G. (2019). Enumerating Circulating Tumor Cells with a Self-Assembled Cell Array (SACA) Chip: A Feasibility Study in Patients with Colorectal Cancer. Cancers, 11(1), 56. https://doi.org/10.3390/cancers11010056







