Prognostic Factors for Advanced Pancreatic Cancer Treated with Gemcitabine Plus S-1: Retrospective Analysis and Development of a Prognostic Model
Abstract
1. Introduction
2. Results
2.1. Patient Characteristic and GS Treatment Duration
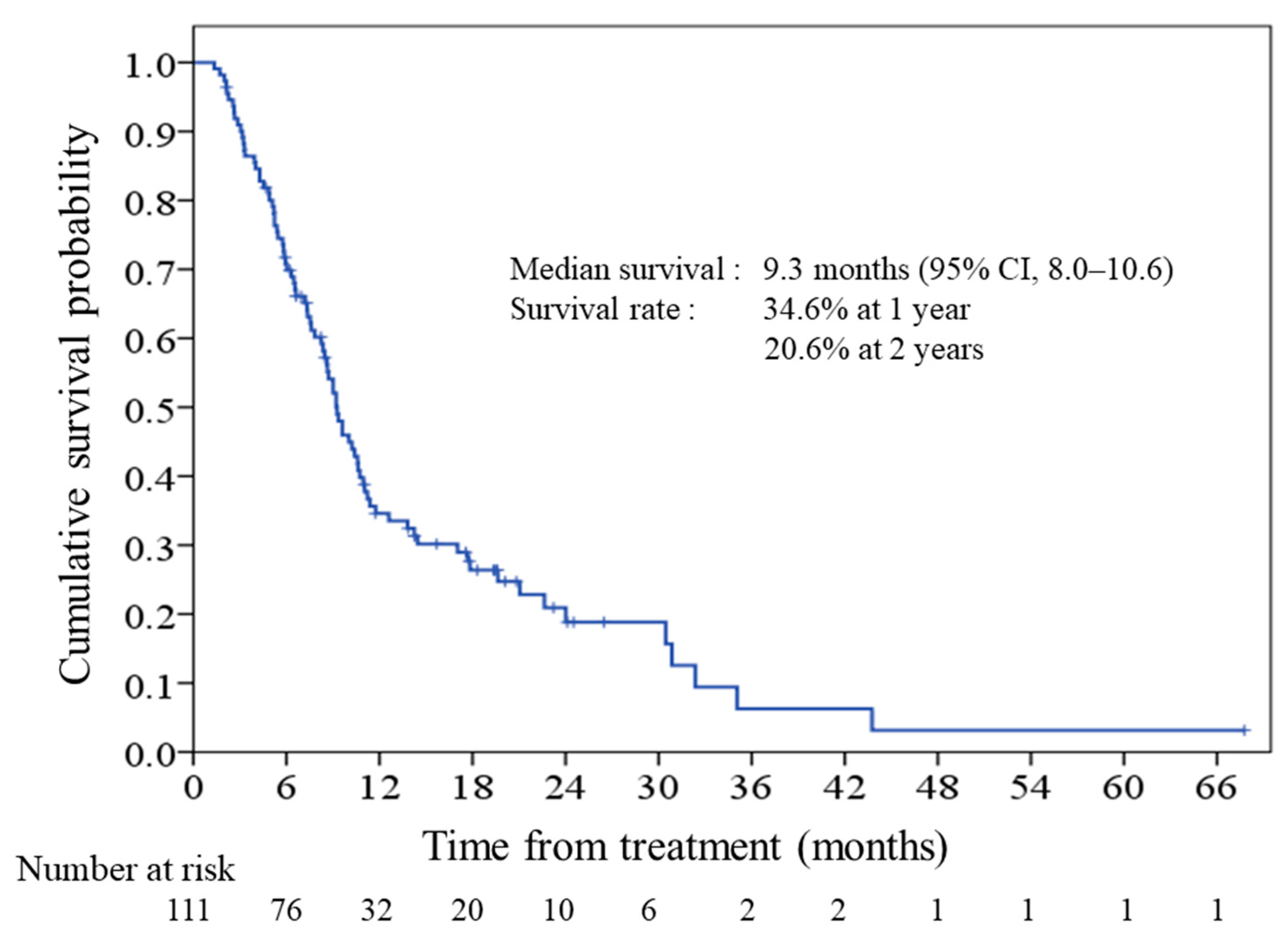
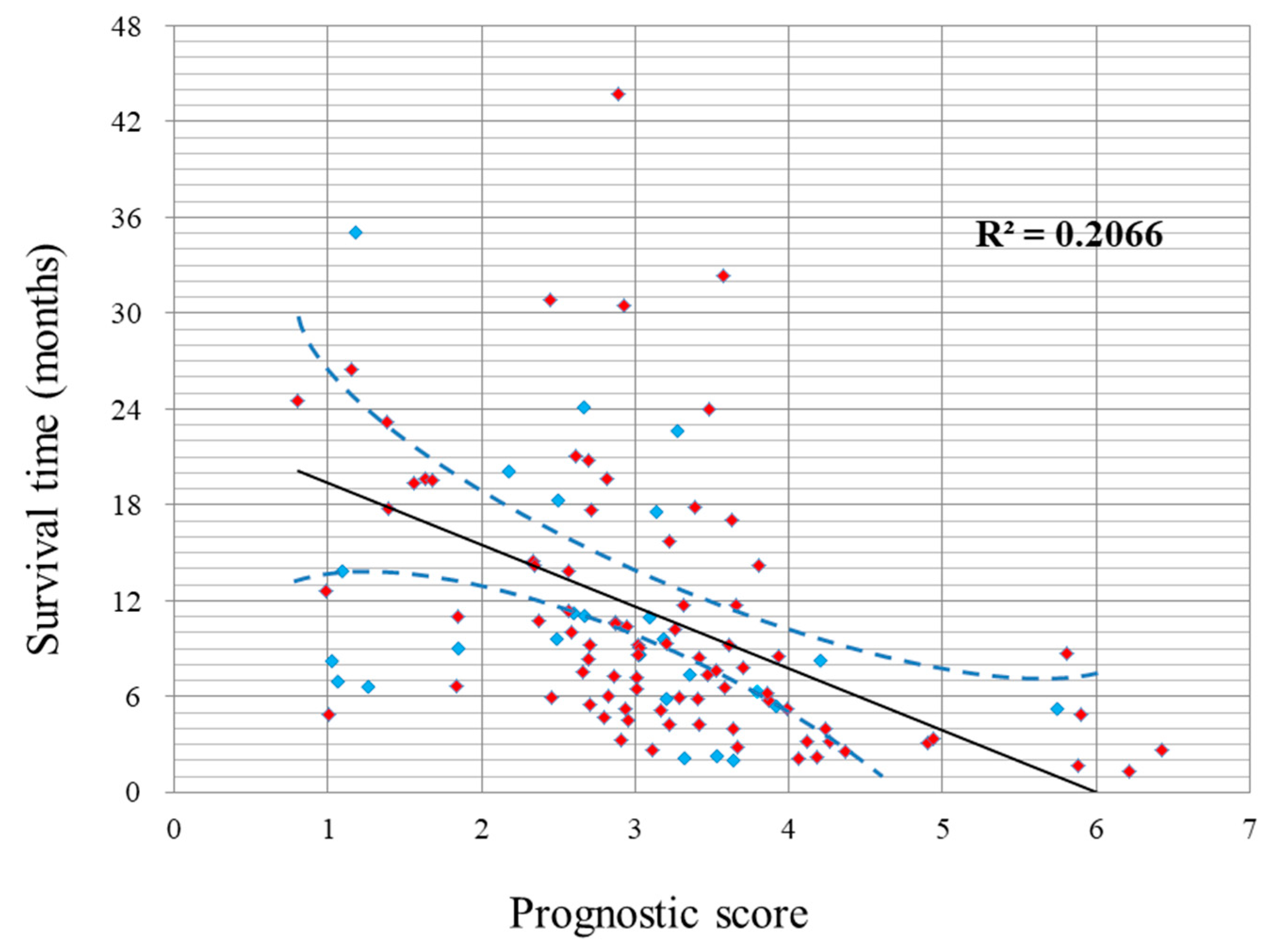
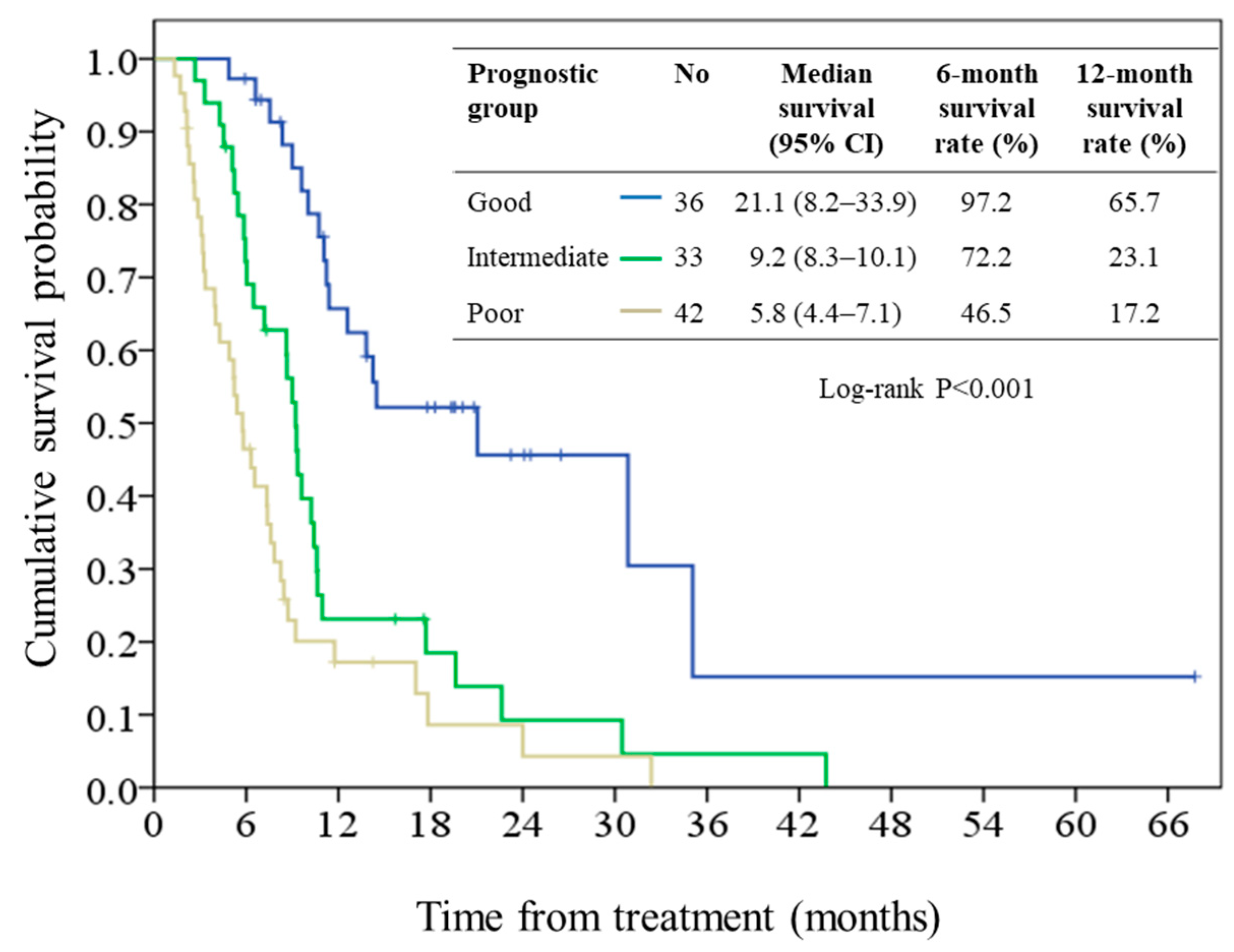
2.2. Survival and Prognostic Score
2.3. Performance of the Prognostic Model
3. Discussion
4. Materials and Methods
4.1. Patients and Treatment
4.2. Data Collection and Follow-Up
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Health Registry Annual Report 2013, Republic of China. Bureau of Health Promotion. Department of Health, Executive Yuan, 2015. Available online: http://www.hpa.gov.tw/BHPNet/Web/Stat/Statistics.aspx (accessed on 20 November 2018).
- Allen, P.J.; Kuk, D.; Castillo, C.F.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann. Surg. 2017, 265, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Balaban, E.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Mukherjee, S.; Crane, C.H.; Javle, M.M.; Eads, J.R.; Allen, P.; Ko, A.H.; et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2654–2668. [Google Scholar] [CrossRef]
- Seufferlein, T.; Bachet, J.B.; Van Cutsem, E.; Rougier, P.; Group, E.G.W. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. S7), vii33–vii40. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Pancreatic adenocarcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 20 November 2018).
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Smith, C.T.; Cunningham, D.; Starling, N.; Neoptolemos, J.P.; Ghaneh, P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J. Clin. Oncol. 2007, 25, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Lobo, D.N. Pancreatic cancer. Surgery (Oxford) 2010, 28, 198–204. [Google Scholar] [CrossRef]
- Koizumi, W.; Kurihara, M.; Nakano, S.; Hasegawa, K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 2000, 58, 191–197. [Google Scholar] [CrossRef]
- Sakata, Y.; Ohtsu, A.; Horikoshi, N.; Sugimachi, K.; Mitachi, Y.; Taguchi, T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur. J. Cancer 1998, 34, 1715–1720. [Google Scholar] [CrossRef]
- Ueno, H.; Ioka, T.; Ikeda, M.; Ohkawa, S.; Yanagimoto, H.; Boku, N.; Fukutomi, A.; Sugimori, K.; Baba, H.; Yamao, K.; et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J. Clin. Oncol. 2013, 31, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Miyakawa, H.; Fujii, H.; Nakamori, S.; Satoh, T.; Hamamoto, Y.; Ito, T.; Maguchi, H.; Matsumoto, S.; Ueno, H.; et al. Updated results from GEST study: A randomized, three-arm phase III study for advanced pancreatic cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Kogo, M.; Ishii, M.; Yoneyama, K.; Kitamura, K.; Shimada, K.; Shimizu, S.; Yoshida, H.; Kiuchi, Y. Practical prognostic index for survival in patients with unresectable pancreatic cancer treated with gemcitabine or S-1. Hepatogastroenterology 2015, 62, 478–484. [Google Scholar] [PubMed]
- Maréchal, R.; Demols, A.; Gay, F.; De Maertelaere, V.; Arvanitaki, M.; Hendlisz, A.; Van Laethem, J.L. Prognostic factors and prognostic index for chemonaïve and gemcitabine-refractory patients with advanced pancreatic cancer. Oncology 2007, 73, 41–51. [Google Scholar] [PubMed]
- Kuroda, T.; Kumagi, T.; Yokota, T.; Seike, H.; Nishiyama, M.; Imai, Y.; Inada, N.; Shibata, N.; Imamine, S.; Okada, S.; et al. Improvement of long-term outcomes in pancreatic cancer and its associated factors within the gemcitabine era: A collaborative retrospective multicenter clinical review of 1,082 patients. BMC Gastroenterol. 2013, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Inal, A.; Kos, F.T.; Algin, E.; Yildiz, R.; Berk, V.; Tugba Unek, I.; Colak, D.; Colak, D.; Kucukoner, M.; Tamer Elkiran, E.; et al. Prognostic factors in patients with advanced pancreatic cancer treated with gemcitabine alone or gemcitabine plus cisplatin: Retrospective analysis of a multicenter study. J. BUON 2012, 17, 102–105. [Google Scholar] [PubMed]
- Yi, J.H.; Lee, J.; Park, S.H.; Lee, K.T.; Lee, J.K.; Lee, K.H.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Lim, D.H.; et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology 2011, 80, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2140–2141. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, C.; Zhou, Y.; Chen, R.; Fan, X.; Bi, Z.; Li, Z.; Liu, Y. Gemcitabine compared with gemcitabine and s-1 combination therapy in advanced pancreatic cancer: A systematic review and meta-analysis. Medicine 2015, 94, e1345. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Okada, S.; Nose, H.; Yoshimori, M.; Aoki, K.; Okusaka, T. Prognostic factors in patients with advanced pancreatic cancer treated with systemic chemotherapy. Pancreas 1996, 12, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Cubiella, J.; Castells, A.; Fondevila, C.; Sans, M.; Sabater, L.; Navarro, S.; Fernández-Cruz, L. Prognostic factors in nonresectable pancreatic adenocarcinoma: A rationale to design therapeutic trials. Am. J. Gastroenterol. 1999, 94, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Von Meyenfeldt, M. Cancer-associated malnutrition: An introduction. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. S2), S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Moshage, H.J.; Janssen, J.A.; Franssen, J.H.; Hafkenscheid, J.C.; Yap, S.H. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J. Clin. Investig. 1987, 79, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Cancer related circulating and tumor-associated neutrophils—Subtypes, sources and function. FEBS J. 2018. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Q.; Fan, J.; Cheng, S.; Ding, W.; Hua, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin. Chim. Acta 2018, 479, 181–189. [Google Scholar] [CrossRef]
- Cheng, H.; Long, F.; Jaiswar, M.; Yang, L.; Wang, C.; Zhou, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis. Sci. Rep. 2015, 5, 11026. [Google Scholar] [CrossRef] [PubMed]
- Ben, Q.; An, W.; Wang, L.; Wang, W.; Yu, L.; Yuan, Y. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 2015, 44, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, I.; Peacock, O.; Lloyd, G.; Larvin, M.; Hall, R.I. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am. J. Surg. 2010, 200, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Guo, M.; Liu, Z.; Xiao, Z.; Jin, K.; Long, J.; Liu, L.; Liu, C.; Xu, J.; Ni, Q.; et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann. Surg. Oncol. 2015, 22, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Ohara, K.; Kiberu, A.; Van Hagen, T.; Davidson, A.; Khattak, M.A. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern. Med. J. 2014, 44, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Sasaki, T.; Sasahira, N.; Tsujino, T.; Kogure, H.; Yagioka, H.; Yashima, Y.; Togawa, O.; Arizumi, T.; et al. Comorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine-based chemotherapy. Crit. Rev. Oncol. Hematol. 2011, 78, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
| Characteristic | Value (%) |
|---|---|
| Median age, year (range) | 62 (32–82) |
| Male sex | 66 (59.5) |
| Median BMI, kg/m2 (range) | 22.5 (15.6–32.5) |
| ECOG PS | |
| 0 or 1 | 89 (80.2) |
| 2 | 19 (17.1) |
| 3 | 3 (2.7) |
| Charlson comorbidity index | |
| 0 | 31 (27.9) |
| 1 | 39 (35.1) |
| 2 | 21 (18.9) |
| 3 | 17 (15.3) |
| 4 | 2 (1.8) |
| 5 | 1 (0.9) |
| Smoking | |
| never | 56 (50.5) |
| ever or active | 55 (49.5) |
| Primary tumor site | |
| head | 32(28.8) |
| body | 24 (21.6) |
| tail | 32 (28.8) |
| overlapping | 23 (20.7) |
| Presence of jaundice | |
| No | 84 (75.7) |
| Yes | 27 (24.3) |
| Primary tumor size, cm (range) | 4.9 (1.8–13.1) |
| T-classification | |
| 1 | 2 (1.8) |
| 2 | 14 (12.6) |
| 3 | 30 (27.0) |
| 4 | 65 (58.6) |
| N-classification | |
| 0 | 19 (17.1) |
| 1 | 92 (82.9) |
| M-classification | |
| 0 | 22 (19.8) |
| 1 | 89 (80.2) |
| AJCC tumor stage | |
| III | 22 (19.8) |
| IV | 89 (80.2) |
| Site of metastases | |
| liver | 57 (51.4) |
| peritoneum | 31 (27.9) |
| distant lymph nodes | 17 (15.3) |
| lung | 13 (11.7) |
| others | 4 (3.6) |
| Variable | Category | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age | per year | 1.02 (0.99–1.04) | 0.14 | ||
| BMI | per kg/m2 | 0.97 (0.91–1.04) | 0.41 | ||
| Sex | male | 1 | 1 | ||
| female | 0.68 (0.44–1.06) | 0.090 | 0.71 (0.42–1.20) | 0.200 | |
| ECOG PS | 0 or 1 | 1 | 1 | ||
| 2 | 2.95 (1.70–5.12) | <0.001 | 1.43 (0.74–2.77) | 0.29 | |
| 3 | 4.58 (1.41–14.9) | 0.011 | 15.0 (3.11–72.6) | 0.001 | |
| CCI | per index | 1.15 (0.94–1.40) | 0.17 | ||
| Smoking | never | 1 | |||
| ever | 1.39 (0.90–2.15) | 0.16 | |||
| Tumor site | head | 1 | 1 | ||
| body | 0.75 (0.39–1.44) | 0.40 | 1.58 (0.73–3.42) | 0.250 | |
| tail | 1.77 (1.01–3.08) | 0.045 | 1.85 (0.96–3.59) | 0.090 | |
| overlapping | 1.14 (0.60–2.15) | 0.69 | 1.95 (0.93–4.06) | 0.080 | |
| Presence of jaundice | no | 1 | |||
| yes | 1.35 (0.80–2.25) | 0.26 | |||
| Primary tumor size | per cm | 1.08 (0.94–1.25) | 0.15 | ||
| T-classification | 1 | 1 | |||
| 2 | 1.74 (0.23–13.4) | 0.600 | |||
| 3 | 1.14 (0.15–8.45) | 0.90 | |||
| 4 | 0.60 (0.081–4.44) | 0.62 | |||
| N-classification | 0 | 1 | |||
| 1 | 1.57 (0.90–2.73) | 0.19 | |||
| M-classification | 0 | 1 | 1 | ||
| 1 | 4.57 (2.19–9.54) | <0.001 | 5.13 (2.05–12.8) | 0.001 | |
| Hemoglobin, g/dL | >12 | 1 | |||
| ≤12 | 1.39 (0.58–2.17) | 0.14 | |||
| Platelet count, 109/L | ≥150 | 1 | |||
| <150 | 0.99 (0.57–1.71) | 0.99 | |||
| Leukocyte count, 109/L | <11,000 | 1 | |||
| ≥11,000 | 1.36 (0.93–2.11) | 0.18 | |||
| AST, U/L | ≤34 | 1 | |||
| >34 | 1.02 (0.65–1.60) | 0.93 | |||
| Alkaline phosphatase, IU/L | ≤140 | 1 | |||
| >140 | 1.43 (0.91–2.24) | 0.13 | |||
| CEA, ng/mL | ≤5 | 1 | |||
| >5 | 1.16 (0.75–1.79) | 0.51 | |||
| CA19-9, u/mL | ≤37 | 1 | |||
| >37 | 0.87 (0.52–1.46) | 0.60 | |||
| Albumin, g/dL | per gm/dL | 0.52 (0.33–0.84) | 0.008 | 0.58 (0.33–0.98) | 0.043 |
| NLR | per ratio | 1.15 (1.07–1.23) | <0.001 | 1.20 (1.11–1.31) | <0.001 |
| Factor | β | SE | p | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|
| ECOG PS | |||||
| 0 or 1 | Reference | ||||
| 2 | 0.359 | 0.337 | 0.286 | 1.432 | 0.74–2.77 |
| 3 | 2.709 | 0.804 | 0.001 | 15.0 | 3.11–72.6 |
| Tumor stage | |||||
| III | reference | ||||
| IV | 1.636 | 0.469 | 0.001 | 5.13 | 2.05–12.9 |
| NLR, 100% | 0.186 | 0.044 | <0.001 | 1.204 | 1.11–1.31 |
| Albumin, g/dL | −0.547 | 0.282 | 0.043 | 0.579 | 0.33–0.98 |
| Constant | 3 | 0.911 | 0.001 | 0.041 | - |
| Prognostic Group | Prognostic Score | N (%) | Median Survival, Months (95% CI) | Hazard Ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| Good | < 2.7 | 36 (32.4) | 21.1 (8.2–33.9) | 1 (reference) | |
| Intermediate | 2.7 to 3.3 | 33 (29.7) | 9.2 (8.3–10.1) | 2.80 (1.55–5.06) | 0.001 |
| Poor | >3.3 | 42 (37.8) | 5.8 (4.4–7.1) | 4.81 (2.71–8.56) | <0.001 |
| Patient | ECOG PS | Stage | Neutrophil | Lymphocyte | NLR | Albumin (g/dL) | Sum of Scores | Prognostic Group |
|---|---|---|---|---|---|---|---|---|
| A | 0 | III | 60 | 20 | 3 | 4.0 | 1.37 | good |
| B | 0 | IV | 60 | 15 | 4 | 4.0 | 2.916 | intermediate |
| C | 3 | IV | 60 | 12 | 5 | 3.0 | 3.634 | poor |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-F.; Huang, P.-W.; Chen, J.-S.; Chen, Y.-Y.; Lu, C.-H.; Chang, P.-H.; Hung, Y.-S.; Chou, W.-C. Prognostic Factors for Advanced Pancreatic Cancer Treated with Gemcitabine Plus S-1: Retrospective Analysis and Development of a Prognostic Model. Cancers 2019, 11, 57. https://doi.org/10.3390/cancers11010057
Chang C-F, Huang P-W, Chen J-S, Chen Y-Y, Lu C-H, Chang P-H, Hung Y-S, Chou W-C. Prognostic Factors for Advanced Pancreatic Cancer Treated with Gemcitabine Plus S-1: Retrospective Analysis and Development of a Prognostic Model. Cancers. 2019; 11(1):57. https://doi.org/10.3390/cancers11010057
Chicago/Turabian StyleChang, Ching-Fu, Pei-Wei Huang, Jen-Shi Chen, Yen-Yang Chen, Chang-Hsien Lu, Pei-Hung Chang, Yu-Shin Hung, and Wen-Chi Chou. 2019. "Prognostic Factors for Advanced Pancreatic Cancer Treated with Gemcitabine Plus S-1: Retrospective Analysis and Development of a Prognostic Model" Cancers 11, no. 1: 57. https://doi.org/10.3390/cancers11010057
APA StyleChang, C.-F., Huang, P.-W., Chen, J.-S., Chen, Y.-Y., Lu, C.-H., Chang, P.-H., Hung, Y.-S., & Chou, W.-C. (2019). Prognostic Factors for Advanced Pancreatic Cancer Treated with Gemcitabine Plus S-1: Retrospective Analysis and Development of a Prognostic Model. Cancers, 11(1), 57. https://doi.org/10.3390/cancers11010057





