Flavonoids in Cancer and Apoptosis
Abstract
1. Introduction
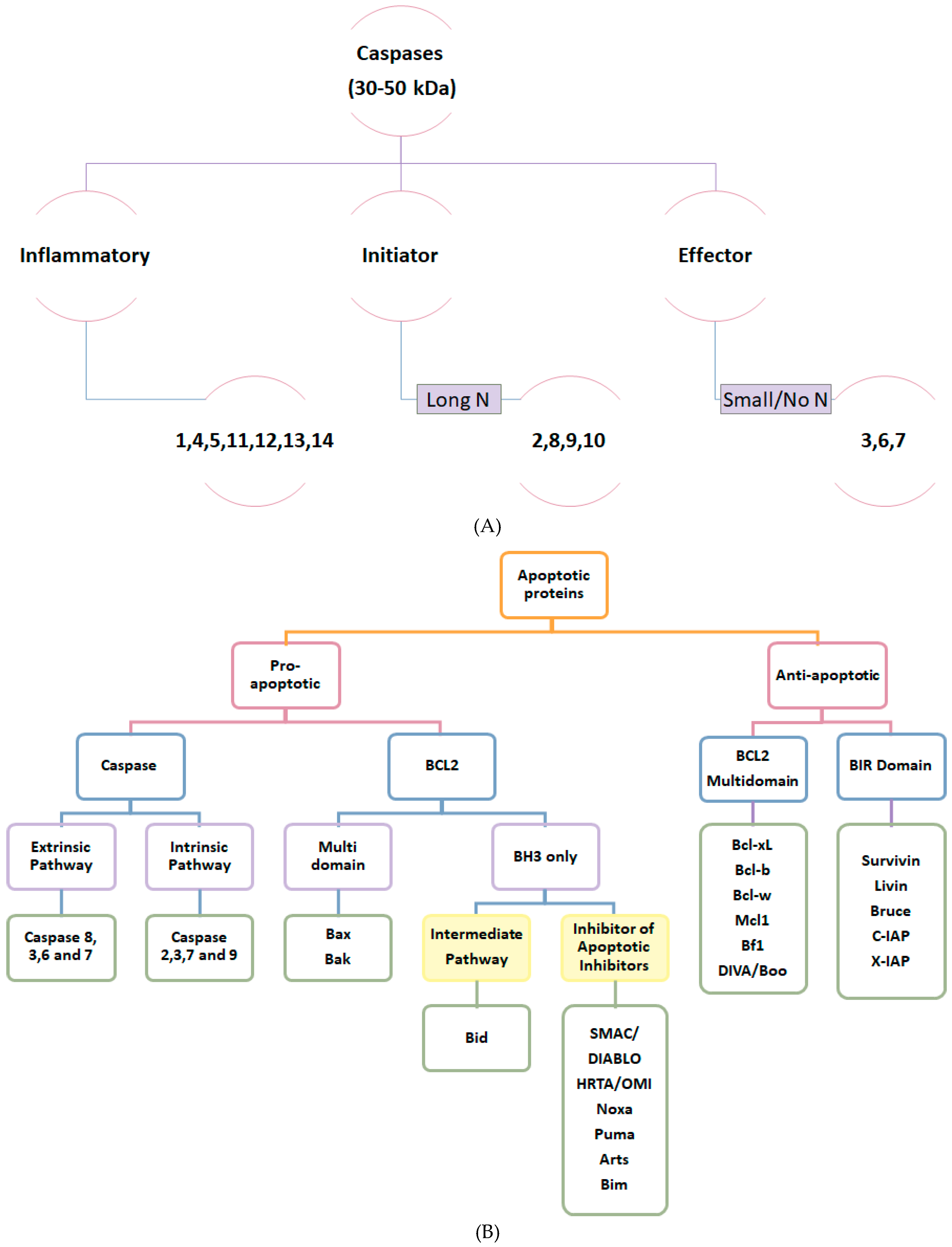
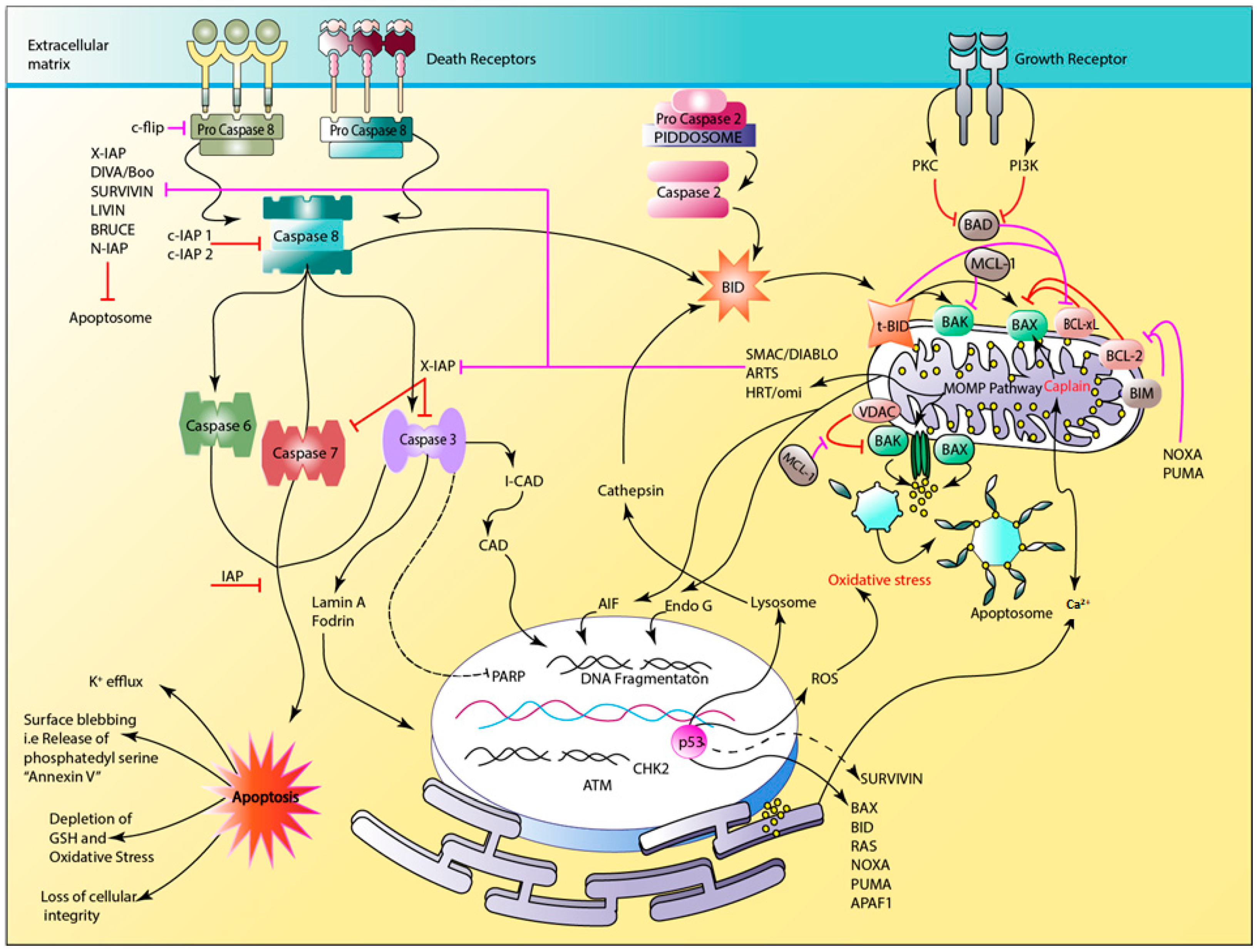
2. Apoptosis
2.1. Apoptotic Proteins
2.2. The Apoptotic Pathway
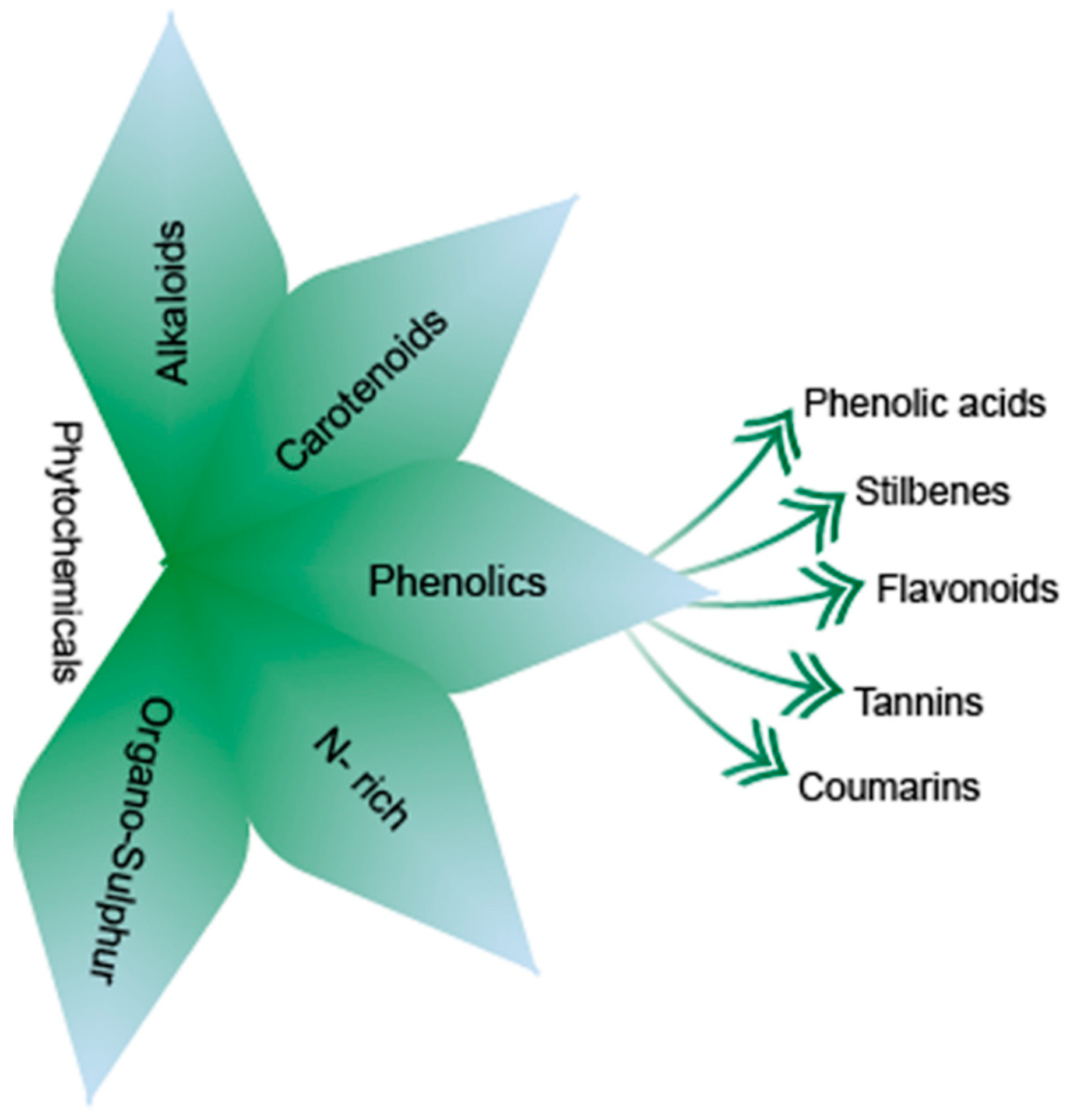
3. Phytochemicals
3.1. Classification of Phytochemicals
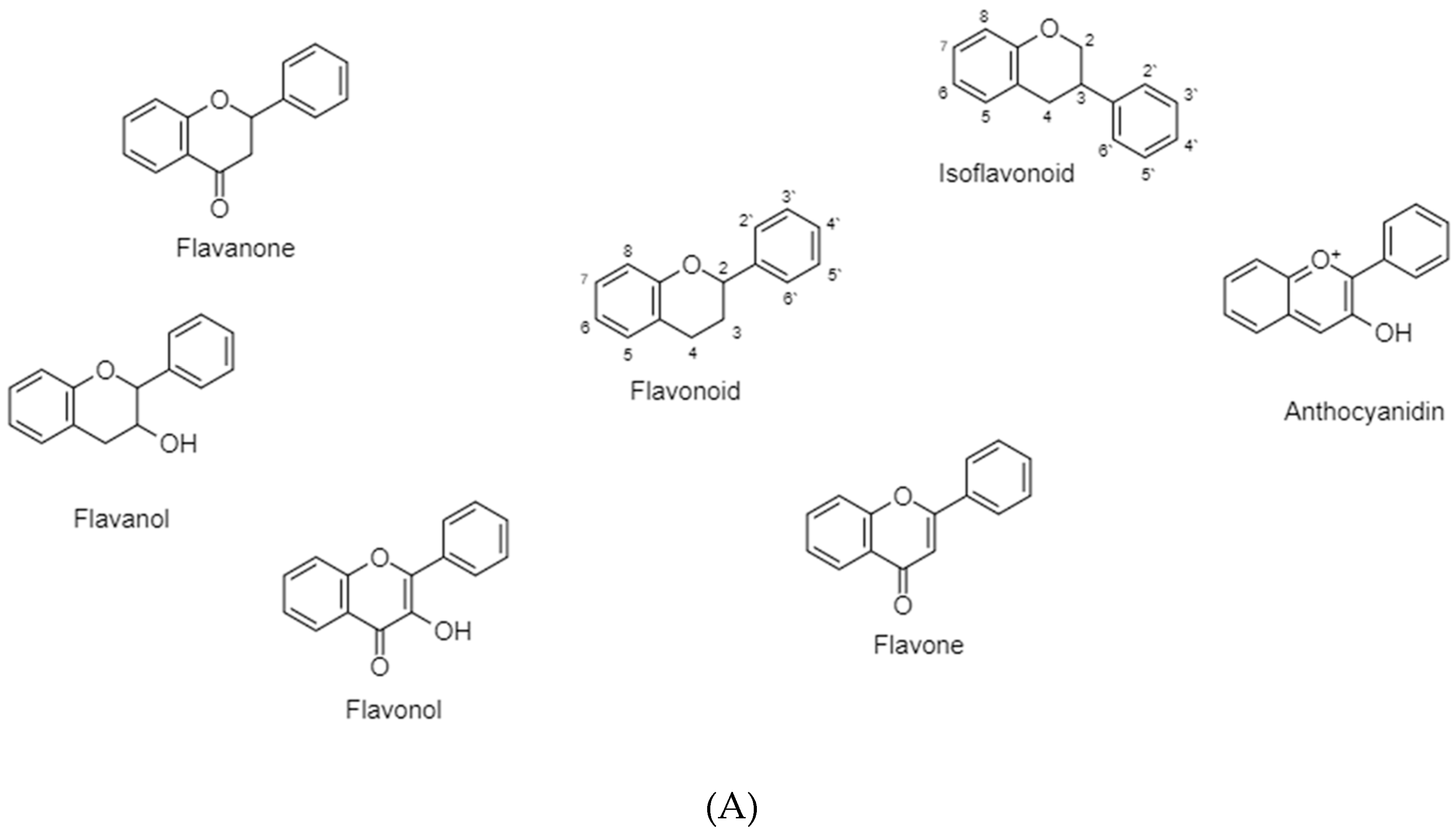
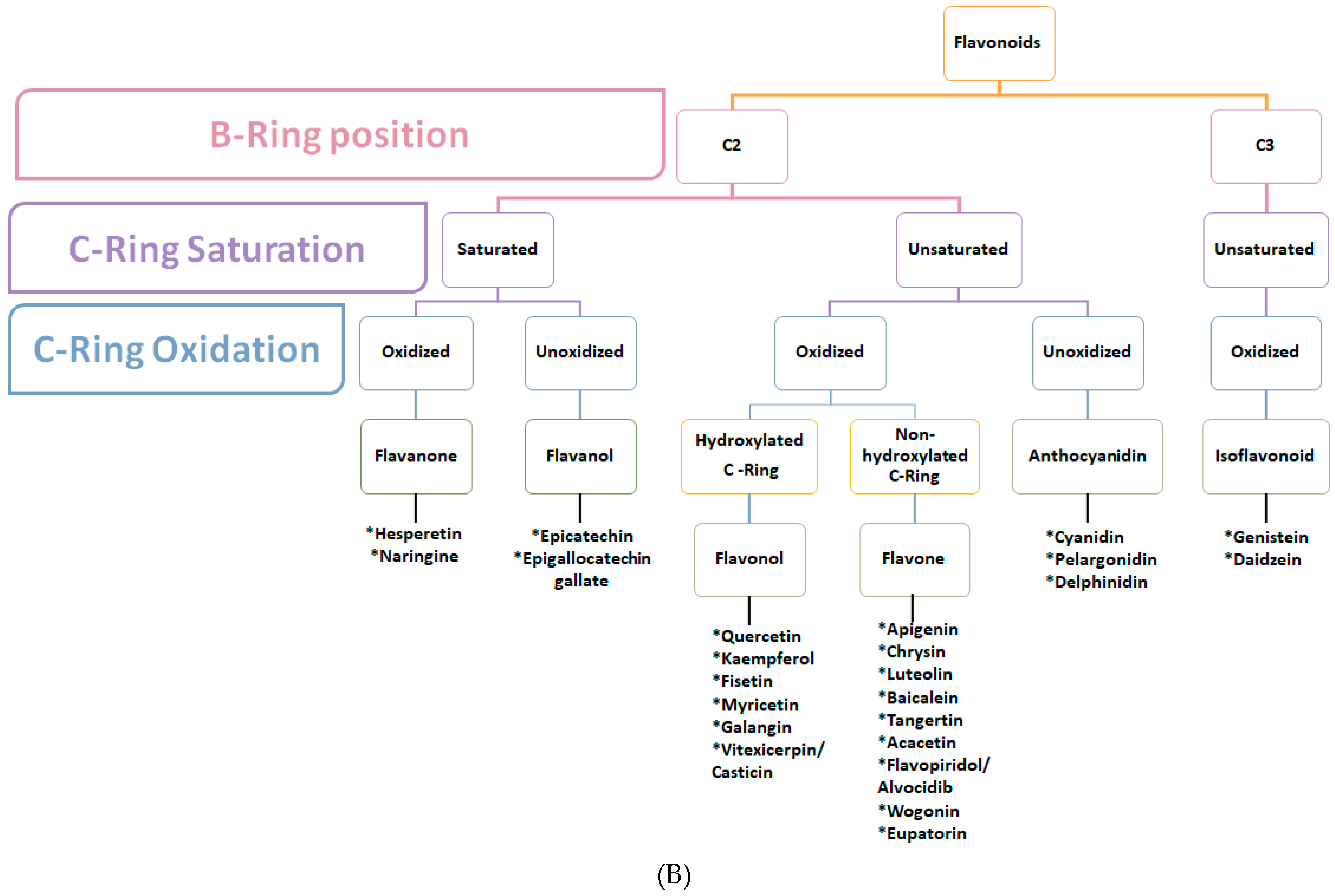
3.2. Flavonoids
3.2.1. Flavonoids (B Ring Attached to C2)-Saturated C-Ring
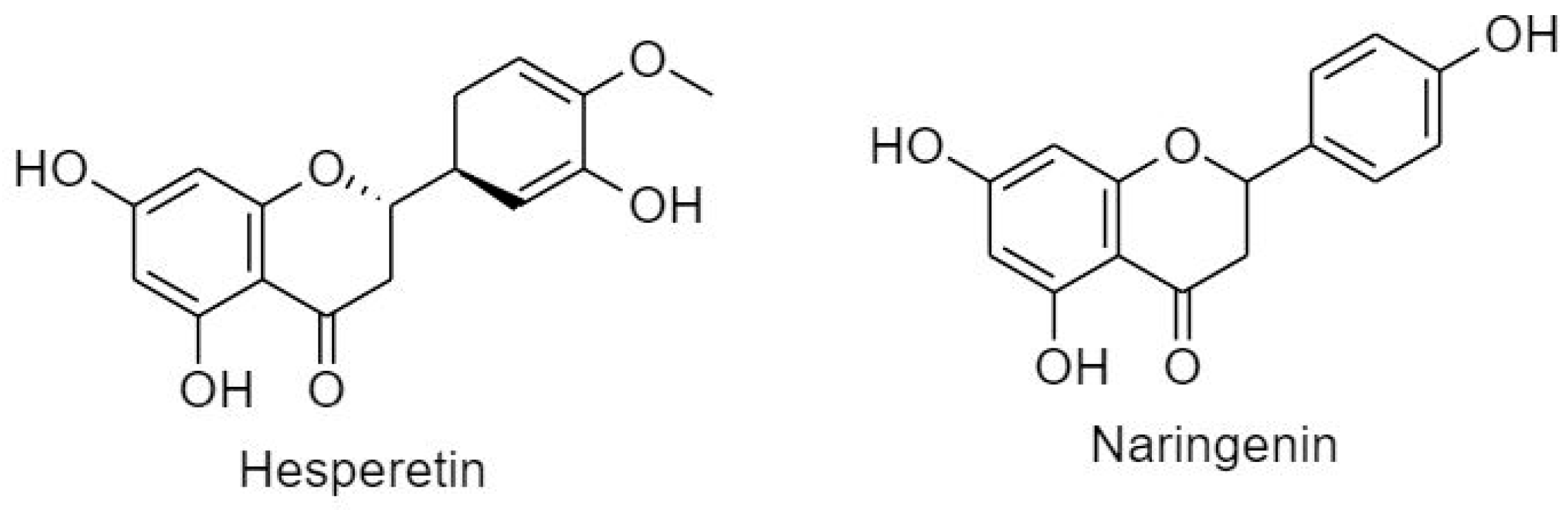
3.2.1.1. Flavanone
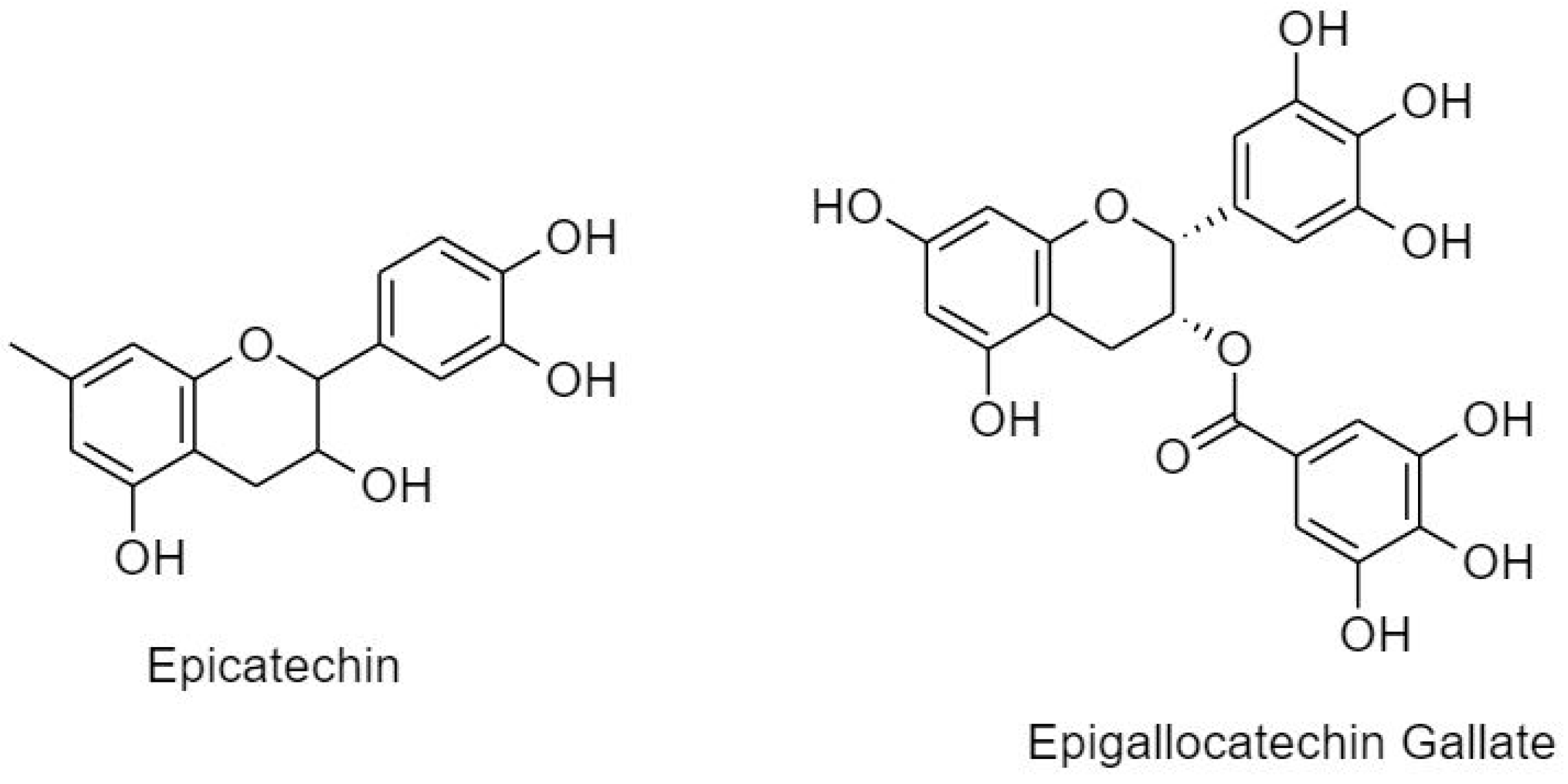
3.2.1.2. Flavanols
3.2.2. Flavonoids (B Ring Attached to C2)-Unsaturated C-Ring
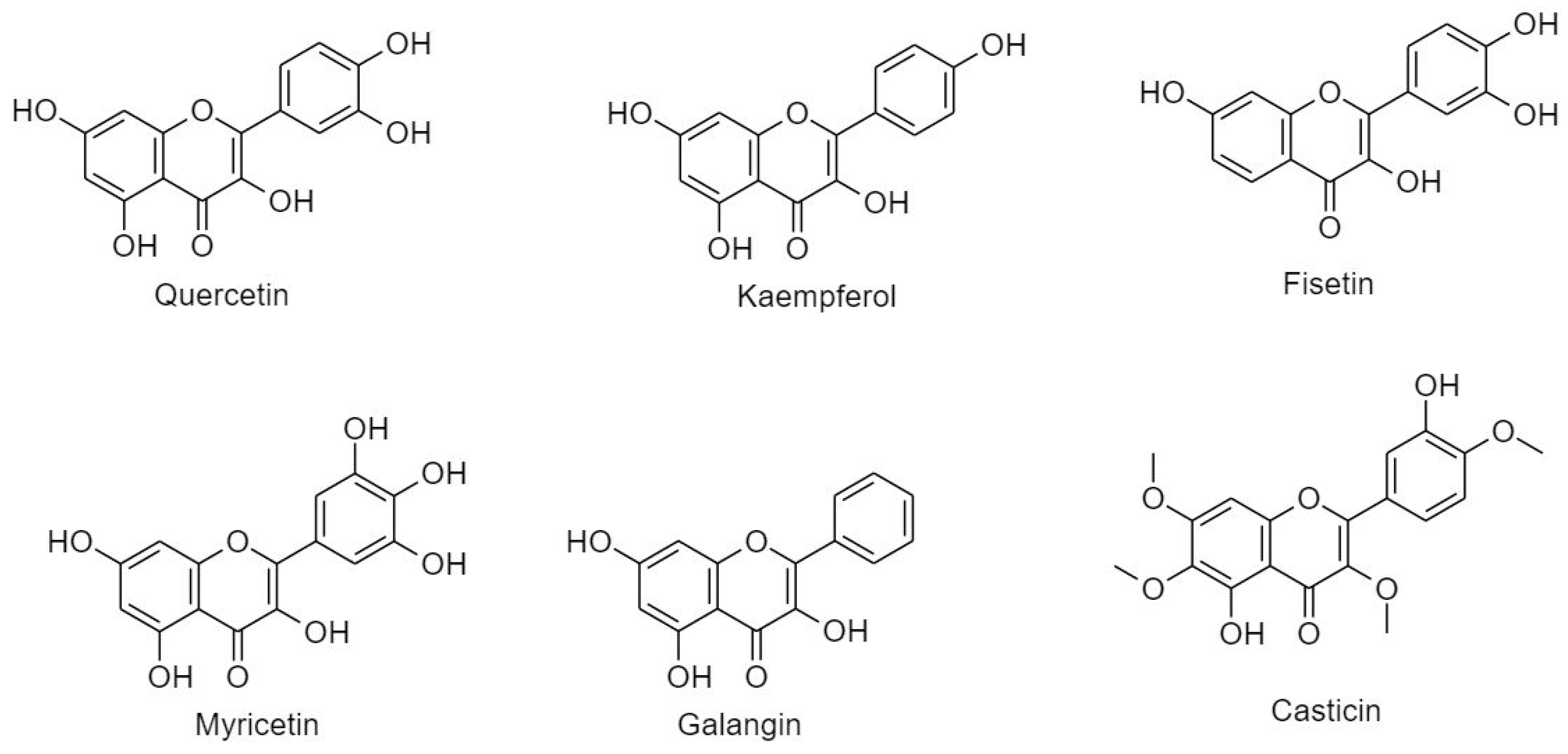
3.2.2.1. Flavonol
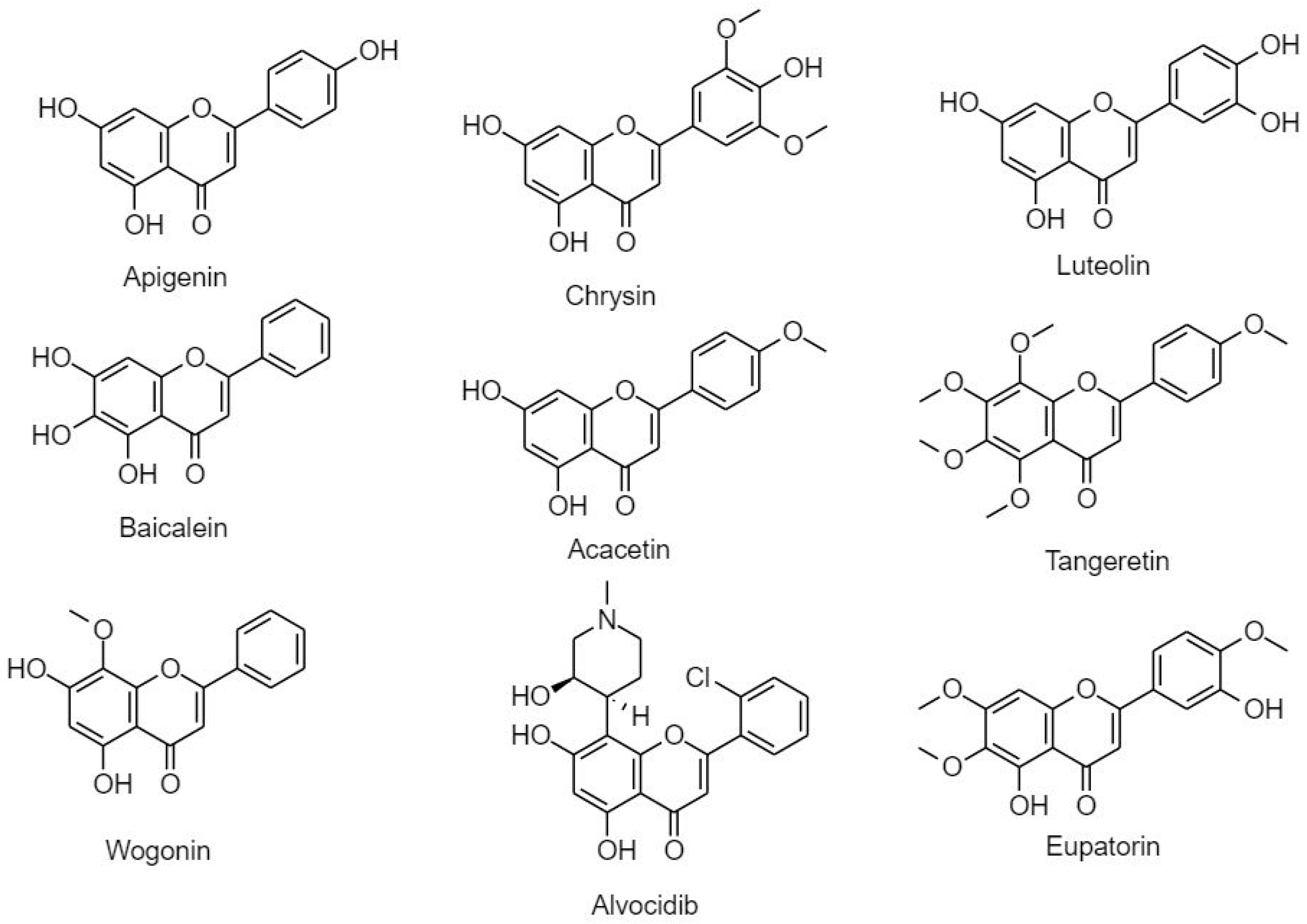
3.2.2.2. Flavones
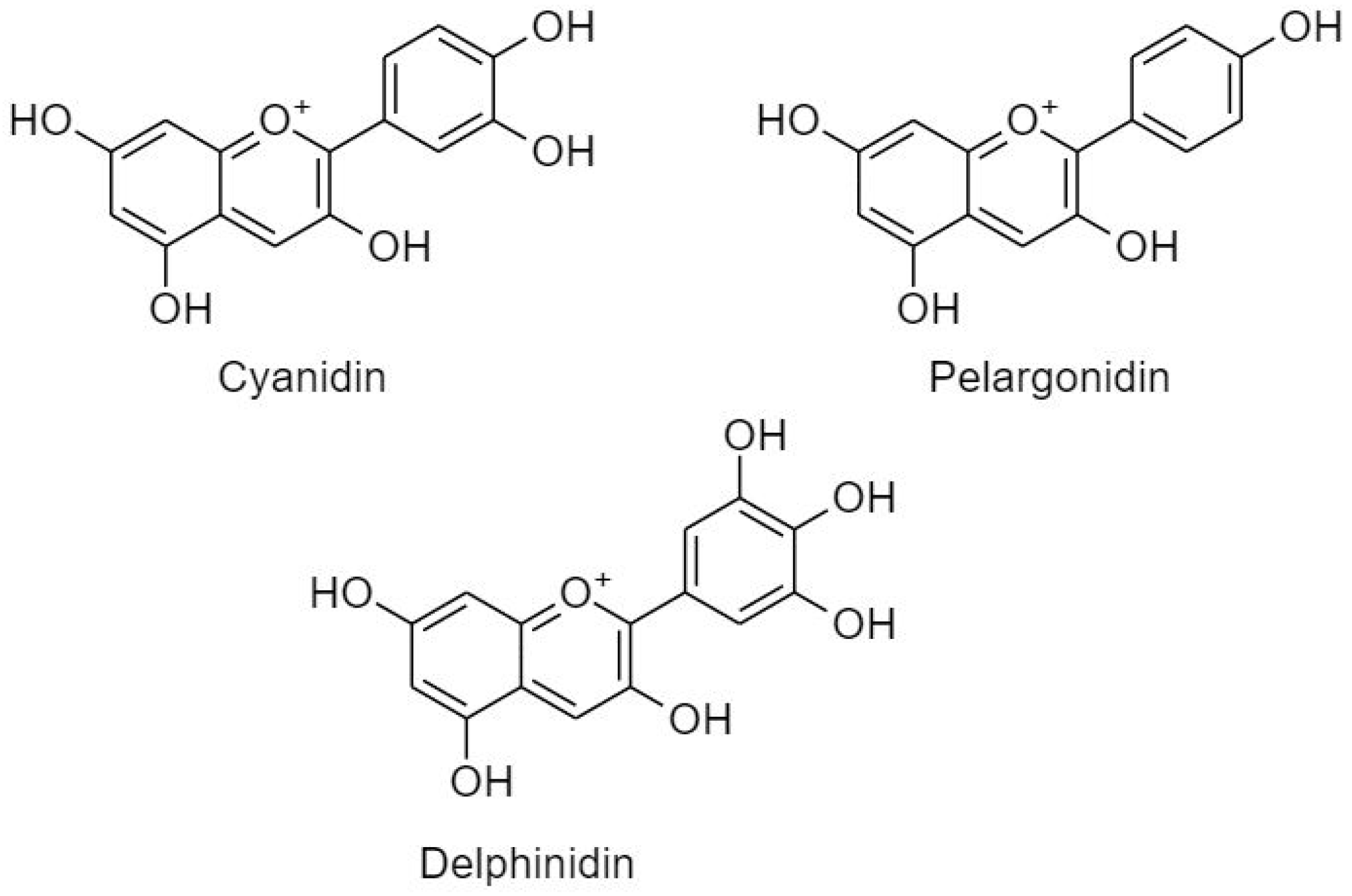
3.2.2.3. Anthocyanidins
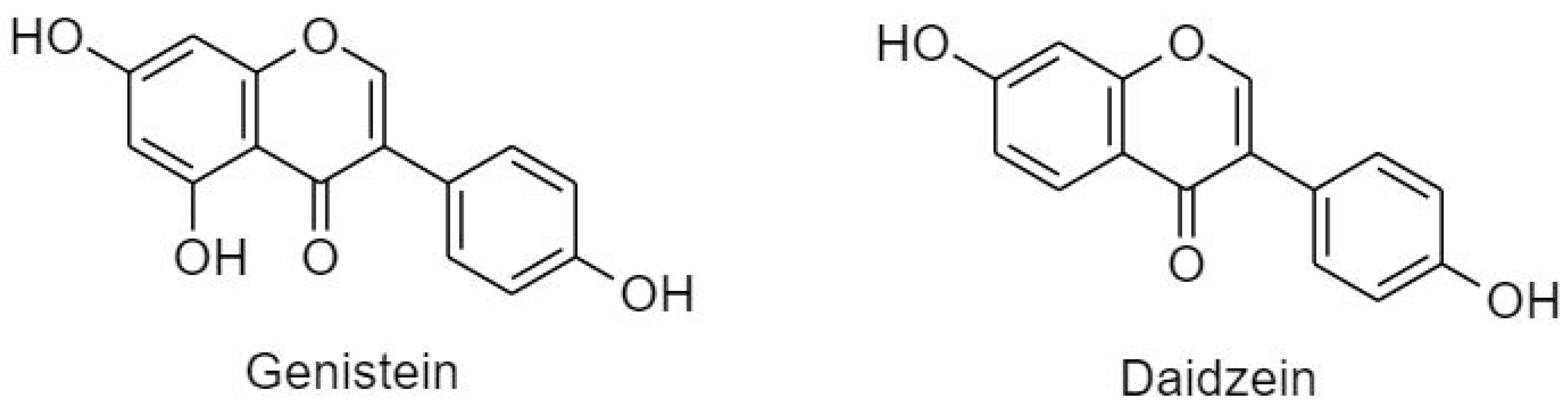
3.2.3. Isoflavonoids (B Ring Attached to C3)
4. Final Remarks, Future Perspectives and Conclusions
4.1. Bioavailability
4.2. Combination Therapy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ΔΨm | Mitochondrial Membrane Potential |
| 5-FU | 5-Fluorouracil |
| AIF | Apoptosis Inducing Factor |
| Akt | Protein Kinase B |
| AMPK | Adenosine Monophosphate-Activated Protein Kinase |
| APAF-1 | Apoptosis Protease Activating Factor 1 |
| Bad | Bcl-2 Associated Death Promoter |
| Bak | Bcl-2 Antagonist and Killer |
| Bax | Bcl-2 Associated X Protein |
| Bcl-2 | B Cell Lymphoma 2 Proteins |
| Bcl-xL | B Cell Lymphoma-Extra Large |
| BH3 | Bcl-2 Homology 3 |
| Bim | Bcl-2-Like Protein 11 |
| BIR | Baculoviral IAP Repeat Domains |
| CAT | Catalase |
| c-Flip | Caspase FLICE-Like Inhibitory Protein |
| CHOP | C/EBP Homologous Protein |
| c-IAP | Cellular Inhibitor of Apoptosis Protein |
| Cox-2 | Cyclooxygenase 2 |
| DISC | Death-Inducing Signaling Complex |
| DR | Death Receptor |
| EGCG | Epigallocatechin Gallate |
| EndoG | Endonuclease G |
| ER | Endoplasmic Reticulum |
| ESC | Esophageal Squamous Carcinoma |
| FADD | Fas-Associated Protein with Death |
| FasL | Fas Ligand |
| GPx | Glutathione Peroxidase |
| GRP | Glucose Related Protein |
| GSH | Glutathione |
| HCC | Hepatocellular Carcinoma |
| HGF | Hepatocyte Growth Factor |
| Her2 | Human Epidermal Growth Factor |
| HSP | Heat Shock Protein |
| MAPK | Mitogen-Activated Protein Kinase |
| Mcl-1 | Myeloid Cell Leukemia 1 |
| MDR | Multi Drug Resistance |
| MMP | Matrix Metalloproteinase |
| MOMP | Mitochondrial Outer Membrane Permeabilization |
| mTOR | Mammalian Target of Rapamycin |
| NAC | N Acetyl Cysteine |
| NF-κB | Nuclear Factor Kappa Beta Pathway |
| N-IAP | Neuronal Apoptosis Inhibitory Protein |
| NSLC | Non-Small Lung Carcinoma |
| Omi/HtrA2 | Temperature Requirement Protein A2 |
| PARP | Poly (ADP-Ribose) Polymerase |
| PI3K | Phosphatidylinositol 3-Kinase |
| PUMA | P53-Upregulated Modulator of Apoptosis |
| RCC | Renal Cell Carcinoma |
| ROS | Reactive Oxygen Species |
| Smac/DIABLO | Second Mitochondrial Derived Activator of Caspase/Direct Inhibitor of Apoptosis-Binding with A Low Isoelectric Point |
| SOD | Super Oxide Dismutase |
| t-Bid | Truncated-Bid |
| TNF | Tumor Necrosis Factor |
| TRADD | TNF-Related Apoptosis-Inducing Ligand |
| TRIAL | TNFRSF1A-Associated Via Death Domain |
| uPA | Urokinase Plasminogen |
| VDAC | Voltage Dependent Anion Channel |
| VEGF | Vascular Endothelial Growth Factor |
| x-IAP | X-Linked Inhibitor of Apoptosis |
References
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [PubMed]
- Prakash, O.; Kumar, A.; Kumar, P. Anticancer potential of plants and natural products: A review. Am. J. Pharmacol. Sci. 2013, 1, 104–115. [Google Scholar] [CrossRef]
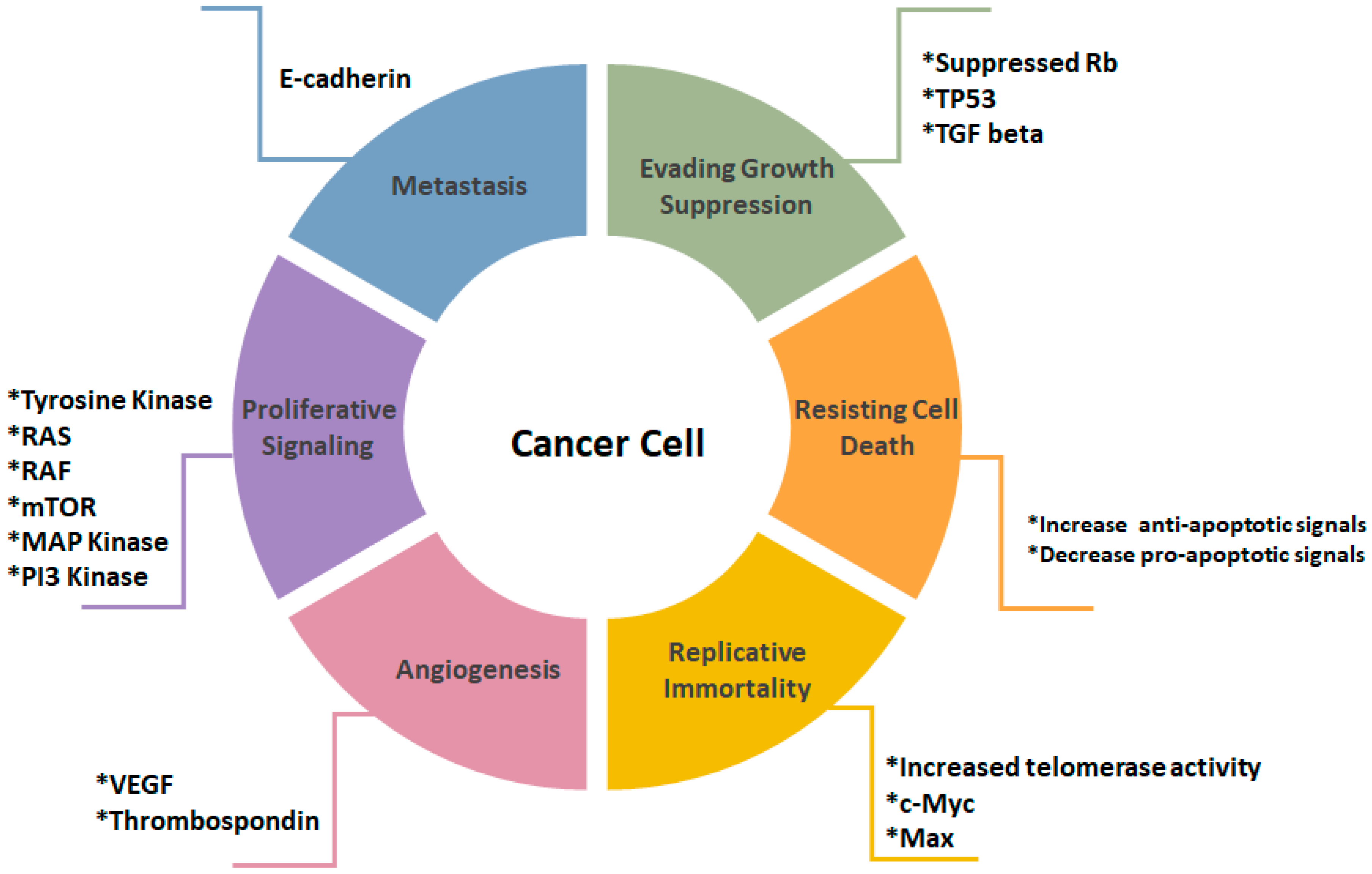
- Hanahan, D.; Weinberg, R.A. Review hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A.; Francisco, S. The hallmarks of cancer review university of california at san francisco. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta Bioenerget. 2011, 1807, 735–745. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Tseng, B. Why target apoptosis in cancer treatment? Mol. Cancer Ther. 2003, 2, 573–580. [Google Scholar]
- Opferman, J.T.; Kothari, A. Anti-apoptotic bcl-2 family members in development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Coloff, J.L.; Ferguson, E.C.; Jacobs, S.R.; Cui, K.; Rathmell, J.C. Glucose metabolism attenuates p53 and puma-dependent cell death upon growth factor deprivation. J. Biol. Chem. 2008, 283, 36344–36353. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Maria, A.; Büsselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (iaps). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Chwieralski, C.E.; Welte, T.; Bühling, F. Cathepsin-regulated apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Manzl, C.; Krumschnabel, G.; Bock, F.; Sohm, B.; Labi, V.; Baumgartner, F.; Logette, E.; Tschopp, J.; Villunger, A. Caspase-2 activation in the absence of piddosome formation. J. Cell Biol. 2009, 185, 291–303. [Google Scholar] [CrossRef]
- Boehning, D.; Patterson, R.L.; Sedaghat, L.; Glebova, N.O.; Kurosaki, T.; Snyder, S.H.; Insp, R. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003, 5, 1051. [Google Scholar] [CrossRef]
- Momeni, H.R.; Ph, D. Role of calpain in apoptosis. Cell J. 2011, 13, 65–72. [Google Scholar]
- Stewart, T.A.; Yapa, K.T.D.S.; Monteith, G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta 2015, 1848, 2502–2511. [Google Scholar] [CrossRef]
- Panche, A.D.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Varghese, E.; Samuel, S.M.; Abotaleb, M.; Cheema, S.; Mamtani, R.; Busselberg, D. The “yin and yang” of natural compounds in anticancer therapy of triple-negative breast cancers. Cancers 2018, 10, 346. [Google Scholar] [CrossRef]
- Liu, R.H. Nutrition, and cancer potential synergy of phytochemicals in cancer prevention: Mechanism of action 1. Int. Res. Conf. Food Nutr. Cancer Potential 2004, 134, 3479–3485. [Google Scholar]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Kelly, G.S. Quercetin. Altern. Med. Rev. 2011, 16, 172–194. [Google Scholar] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Vikash; Song, J.; Wang, J.; Yi, J.; Dong, W. Hesperetin induces the apoptosis of gastric cancer cells via activating mitochondrial pathway by increasing reactive oxygen species. Dig. Dis. Sci. 2015, 60, 2985–2995. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Wang, J.; Li, J.; Liao, F.; Dong, W. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumor Biol. 2016, 37, 3451–3459. [Google Scholar] [CrossRef]
- Palit, S.; Kar, S.; Sharma, G.; Das, P.K. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ros and activation of ask1/jnk pathway. J. Cell. Physiol. 2015, 230, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Sivagami, G.; Vinothkumar, R.; Preethy, C.P.; Riyasdeen, A.; Akbarsha, M.A.; Menon, V.P.; Nalini, N. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in ht-29 human colon adenocarcinoma cell line—A comparative study. Food Chem. Toxicol. 2012, 50, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Athinarayanan, J.; Subbarayan, V.P.; Lei, D.K.Y.; Alshatwi, A.A. Hesperetin induces an apoptosis-triggered extrinsic pathway and a p53- independent pathway in human lung cancer h522 cells. J. Asian Nat. Prod. Res. 2017, 6020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.S.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fund. Clin. Pharmacol. 2013, 27, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Sambantham, S.; Radha, M.; Paramasivam, A.; Anandan, B.; Malathi, R.; Chandra, S.R.; Jayaraman, G. Molecular mechanism underlying hesperetin-induced apoptosis by in silico analysis and in prostate cancer pc-3 cells. Asian Pac. J. Cancer Prev. 2013, 14, 4347–4352. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2014, 24, 551–560. [Google Scholar] [CrossRef]
- Bao, L.; Liu, F.; Guo, H.B.; Li, Y.; Tan, B.B.; Zhang, W.X.; Peng, Y.H. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer sgc7901 cell line by downregulation of akt pathway. Tumor Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, X.; Zhang, X.; Shang, D.; Zhou, Y.; Zhang, C. Enhanced anticancer effect of abt-737 in combination with naringenin on gastric cancer cells. Exp. Ther. Med. 2016, 11, 669–673. [Google Scholar] [CrossRef]
- Ahamad, M.S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M.; Arshad, M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ros generation and cell cycle arrest. PLoS ONE 2014, 9, e110003. [Google Scholar] [CrossRef]
- Arul, D.; Subramanian, P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Kapoor, R.; Rizvi, F.; Kakkar, P. Naringenin prevents high glucose-induced mitochondria-mediated apoptosis involving aif, endo-g and caspases. Apoptosis 2013, 18, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Bulzomi, P.; Bolli, A.; Galluzzo, P.; Acconcia, F.; Ascenzi, P.; Marino, M. The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high bisphenol a background. IUBMB Life 2012, 64, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, N.; Sulfikkarali, N.; RajendraPrasad, N.; Karthikeyan, S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (hela) cancer cells. Biomed. Prev. Nutr. 2011, 1, 223–231. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.W.; Kim, M.S.; Bak, Y.; Park, Y.S.; Jung, K.Y.; Lim, Y.H.; Yoon, D.Y. The apoptotic effects of the flavonoid n101-2 in human cervical cancer cells. Toxicol. In Vitro 2012, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, Y.J.; Lee, J.H.; Nam, M.J. Naringenin causes ask1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food Chem. Toxicol. 2017, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H. Molecular mechanisms and therapeutic effects of (−) -epicatechin and other polyphenols in cancer, inflammation, diabetes, andneurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Amin, A.; Gali-muhtasib, H.; Ocker, M.; Schneider-stock, R. Overview of major classes of plant-derived anticancer drugs. Int. J. Biomed. Sci. 2009, 5, 1–11. [Google Scholar]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer t47d cells through down-regulation of pi3k/akt and telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Li, M.-J. Green tea compounds in breast cancer prevention and treatment. World J. Clin. Oncol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Liu, L.; Hou, L.; Gu, S.; Zuo, X.; Meng, D.; Luo, M.; Zhang, X.; Huang, S.; Zhao, X. Molecular mechanism of epigallocatechin-3-gallate in human esophageal squamous cell carcinoma in vitro and in vivo. Oncol. Rep. 2015, 33, 297–303. [Google Scholar] [CrossRef]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of akt, erk1/2 and alternative p38mapk signalling pathways in human colon cancer cell apoptosis induced by green tea egcg. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef]
- Kwak, T.W.; Park, S.B.; Kim, H.J.; Jeong, Y.I.L.; Kang, D.H. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. OncoTargets Ther. 2017, 10, 137–144. [Google Scholar] [CrossRef]
- Sonoda, J.I.; Ikeda, R.; Baba, Y.; Narumi, K.; Kawachi, A.; Tomishige, E.; Nishihara, K.; Takeda, Y.; Yamada, K.; Sato, K.; et al. Green tea catechin, epigallocatechin-3-gallate, attenuates the cell viability of human non-small-cell lung cancer a549 cells via reducing bcl-xl expression. Exp. Ther. Med. 2014, 8, 59–63. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef]
- Gupta, S.; Hastak, K.; Afaq, F.; Ahmad, N.; Mukhtar, H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa b and induction of apoptosis. Oncogene 2004, 23, 2507–2522. [Google Scholar] [CrossRef]
- Pan, M.H.; Lin, C.C.; Lin, J.K.; Chen, W.J. Tea polyphenol (-)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase cascade signaling. J. Agric. Food Chem. 2007, 55, 5030–5037. [Google Scholar] [CrossRef]
- Pianetti, S.; Guo, S.; Kavanagh, K.T.; Sonenshein, G.E. Green tea polyphenol epigallocatechin-3 gallate inhibits her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002, 62, 652–655. [Google Scholar]
- Masuda, M.; Suzui, M.; Lim, J.T.; Deguchi, A.; Soh, J.W.; Weinstein, I.B. Epigallocatechin-3-gallate decreases vegf production in head and neck and breast carcinoma cells by inhibiting egfr-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002, 2, 350–359. [Google Scholar] [CrossRef]
- Ranganathan, S.; Halagowder, D. Quercetin suppresses twist to induce apoptosis in mcf-7 breast cancer cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef]
- Damnjanovic, I.; Najman, S.; Stojanovic, S.; Stojanovic, D.; Veljkovic, A.; Kocic, H.; Langerholc, T.; Damnjanovic, Z.; Pesic, S. Quercetin induces apoptosis and necroptosis in mcf-7 breast cancer cells. Bratislavské Lekárske Listy 2017, 116, 227–232. [Google Scholar]
- Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via bcl-2 and bax regulation. Mol. Med. Rep. 2012, 1453–1456. [Google Scholar] [CrossRef]
- Chou, C.-C.; Yang, J.-S.; Lu, H.-F.; Ip, S.-W.; Lo, C.; Wu, C.-C.; Lin, J.-P.; Tang, N.-Y.; Chung, J.-G.; Chou, M.-J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer mcf-7 cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Choi, Y.K.; Woo, J.K.; Kim, M.; Kim, I.; Na, C.H.; Hur, H.; Jang, B.H.; et al. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in her2-overexpressing bt-474 breast cancer cells. Oncol. Rep. 2016, 36, 31–42. [Google Scholar] [CrossRef]
- Niu, G.; Yin, S.; Xie, S.; Li, Y.; Nie, D.; Ma, L.; Wang, X.; Wu, Y. Quercetin induces apoptosis by activating caspase-3 and regulating bcl-2 and cyclooxygenase-2 pathways in human hl-60 cells. Acta Biochim. Biophys. Sin. 2011, 43, 30–37. [Google Scholar] [CrossRef]
- Granato, M.; Rizzello, C.; Montani, M.S.G.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting pi3k/akt/mtor and stat3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells. Nutr. Cancer 2012, 64, 580–587. [Google Scholar] [CrossRef]
- Sun, S.; Gong, F.; Liu, P.; Miao, Q. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of vegf/pi3k/akt signaling pathway. Gene 2018, 664, 50–57. [Google Scholar] [CrossRef]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Li, L.Y.; Lee, Y.J. Quercetin-induced ubiquitination and down-regulation of her-2/neu. J. Cell. Biochem. 2008, 105, 585–595. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martin, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates nf-kappa b and ap-1/jnk pathways to induce cell death in human hepatoma cells. Nutr. Cancer 2010, 62, 390–401. [Google Scholar] [CrossRef]
- Calder, M.; Burgos, E. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, K.C. Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 2013, 29, 229–234. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar]
- Li, W.; Du, B.; Wang, T.; Wang, S.; Zhang, J. Kaempferol induces apoptosis in human hct116 colon cancer cells via the ataxia-telangiectasia mutated-p53 pathway with the involvement of p53 upregulated modulator of apoptosis. Chem.-Biol. Interact. 2009, 177, 121–127. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Li, Z.; DePriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar]
- Moradzadeh, M.; Tabarraei, A.; Sadeghnia, H.R.; Ghorbani, A.; Mohamadkhani, A.; Erfanian, S.; Sahebkar, A. Kaempferol increases apoptosis in human acute promyelocytic leukemia cells and inhibits multidrug resistance genes. J. Cell. Biochem. 2018, 119, 2288–2297. [Google Scholar] [CrossRef]
- Luo, H.; Daddysman, M.K.; Rankin, G.O.; Jiang, B.H.; Chen, Y.C. Kaempferol enhances cisplatin’s effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cmyc. Cancer Cell Int. 2010, 10, 1–9. [Google Scholar] [CrossRef]
- Guo, H.; Ren, F.; Zhang, L.I.; Zhang, X.; Yang, R. Kaempferol induces apoptosis in hepg2 cells via activation of the endoplasmic reticulum stress pathway. Mol. Med. Rep. 2016, 13, 2791–2800. [Google Scholar] [CrossRef]
- Jeong, J.C.; Kim, M.S.; Kim, T.H.; Kim, Y.K. Kaempferol induces cell death through erk and akt-dependent down-regulation of xiap and survivin in human glioma cells. Neurochem. Res. 2009, 34, 991–1001. [Google Scholar] [CrossRef]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol increases apoptosis in human cervical cancer hela cells via pi3k/akt and telomerase pathways. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Syed, D.N. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer lncap cells. Carcinogenesis 2018, 29, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Helewski, K.J.; Mizgala, E.; Krol, W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of trail in prostate cancer cells. Int. J. Oncol. 2011, 39, 771–779. [Google Scholar] [PubMed]
- Sabarwal, A.; Agarwal, R.; Singh, R.P. Fisetin inhibits cellular proliferation and induces mitochondria-dependent apoptosis in human gastric cancer cells. Mol. Carcinogen. 2017, 514, 499–514. [Google Scholar] [CrossRef]
- Lin, M.-T.; Lin, C.-L.; Lin, T.-Y.; Cheng, C.-W.; Yang, S.-F.; Lin, C.-L.; Wu, C.-C.; Hsieh, Y.-H.; Tsai, J.-P. Synergistic effect of fisetin combined with sorafenib in human cervical cancer hela cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biol. 2015, 60, 1396–1405. [Google Scholar] [CrossRef]
- Ying, T.H.; Yang, S.F.; Tsai, S.J.; Hsieh, S.C.; Huang, Y.C.; Bau, D.T.; Hsieh, Y.H. Fisetin induces apoptosis in human cervical cancer hela cells through erk1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch Toxicol 2012, 86, 263–273. [Google Scholar]
- Kang, K.A.; Piao, M.J.; Ruwan, S.; Madduma, K. Fisetin induces apoptosis and endoplasmic reticulum stress in human non-small cell lung cancer through inhibition of the mapk signaling pathway. Tumor Biol. 2016, 37, 9615–9624. [Google Scholar] [CrossRef]
- Yi, C.; Zhang, Y.; Yu, Z.; Xiao, Y.; Wang, J.; Qiu, H.; Yu, W.; Tang, R.; Yuan, Y.; Guo, W.; et al. Melatonin enhances the anti-tumor effect of fisetin by inhibiting cox-2/inos and nf-kb/p300 signaling pathways. PLoS ONE 2014, 9, e99943. [Google Scholar] [CrossRef]
- Young, K.; Jeong, S.-J.; Kim, S.-H.; Hoon, J.; Kim, J.-H.; Koh, W.; Chen, C.-Y.; Kim, S.-H. Activation of reactive oxygen species/amp activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma u266 cells. Cancer Lett. 2012, 319, 197–202. [Google Scholar]
- Devi, K.P.; Rajavel, T.; Habtemariam, S.; Nabavi, S.F.; Nabavi, S.M. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015, 142, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Ha, T.K.; Yoon, J.H.; Lee, J.S. Myricetin induces cell death of human colon cancer cells. Anticancer Res. 2014, 34, 701–706. [Google Scholar] [PubMed]
- Kim, W.; Yang, H.J.; Youn, H.; Yun, Y.J.; Seong, K.M.; Youn, B. Myricetin inhibits akt survival signaling and induces bad-mediated apoptosis in a low dose ultraviolet (uv)-b-irradiated hacat human immortalized keratinocytes. J. Radiat. Res. 2010, 51, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, Q.; Wu, S.; Yi, D.; Yu, Y.; Liu, S.; Li, S.; Li, Z. Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double-strand breaks in human ovarian cancer cells. Mol. Med. Rep. 2016, 13, 2094–2100. [Google Scholar] [CrossRef]
- Huang, H.; Chen, A.Y.; Ye, X.; Li, B.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Myricetin inhibits proliferation of cisplatin-resistant cancer cells through a p53-dependent apoptotic pathway. Int. J. Oncol. 2015, 47, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Knickle, A.; Fernando, W.; Greenshields, A.L.; Rupasinghe, H.P.V.; Hoskin, D.W. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem. Toxicol. 2018, 118, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Ruchirawat, S.; Svasti, J.; Saiki, I. Chrysin overcomes trail resistance of cancer cells through mcl-1 downregulation by inhibiting stat3 phosphorylation. Int. J. Oncol. 2013, 43, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.A.; Jeon, Y.K.; Nam, M.J. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme l1 and glutathione s-transferase p. Food Chem. Toxicol. 2012, 50, 684–688. [Google Scholar] [CrossRef]
- Zhang, H.-T. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J. Gastroenterol. 2010, 16, 3377. [Google Scholar] [CrossRef]
- Zhang, H.T.; Wu, J.; Wen, M.; Su, L.J.; Luo, H. Galangin induces apoptosis in hepatocellular carcinoma cells through the caspase 8/t-bid mitochondrial pathway. J. Asian Nat. Prod. Res. 2012, 14, 626–633. [Google Scholar] [CrossRef]
- Zhang, W.; Lan, Y.; Huang, Q.; Hua, Z. Galangin induces b16f10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 mapk. Cytotechnology 2013, 65, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Zhao, B.J.; Liu, J.; Liu, B.; Sun, J.X.; Li, J.; Li, X.M. Molecular mechanisms of casticin action: An update on its antitumor functions. Asian Pac. J. Cancer Prev. 2014, 15, 9049–9058. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Y.; Mao, Q.Q.; Li, X.; Chen, M.W.; Su, J.; Tian, L.; Mao, N.Q.; Long, L.Z.; Quan, M.F.; et al. Casticin induces caspase-mediated apoptosis via activation of mitochondrial pathway and upregulation of dr5 in human lung cancer cells. Asian Pac. J. Trop. Med. 2013, 6, 372–378. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, L.; Long, L.; Quan, M.; Liu, F.; Cao, J. Casticin potentiates trail-induced apoptosis of gastric cancer cells through endoplasmic reticulum stress. PLoS ONE 2013, 8, e58855. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Khan, M.; Zheng, B.; Yang, J.; Zhong, L.; Ma, T. Casticin induces apoptosis and mitotic arrest in pancreatic carcinoma panc-1 cells. Afr. J. Pharm. Pharmacol. 2012, 6, 412–418. [Google Scholar]
- Meng, F.-M.; Yang, J.-B.; Yang, C.-H.; Jiang, Y.; Zhou, Y.-F.; Yu, B.; Yang, H. Vitexicarpin induces apoptosis in human prostate carcinoma pc-3 cells through g2/m phase arrest. Asian Pac. J. Cancer Prev. 2012, 13, 6369–6374. [Google Scholar] [CrossRef] [PubMed]
- Chonghao, W. Vitexicarpin induces apoptosis-independent mitotic arrest in u87 glioblastoma cells. Afr. J. Pharm. Pharmacol. 2012, 6, 1874–1882. [Google Scholar] [CrossRef]
- Liu, E.; Kuang, Y.; He, W.; Xing, X.; Gu, J. Casticin induces human glioma cell death through apoptosis and mitotic arrest. Cell. Physiol. Biochem. 2013, 31, 805–814. [Google Scholar] [CrossRef]
- Song, X.-L.; Zhang, Y.-J.; Wang, X.-F.; Zhang, W.-J.; Wang, Z.; Zhang, F.; Zhang, Y.-J.; Lu, J.-H.; Mei, J.-W.; Hu, Y.-P.; et al. Casticin induces apoptosis and g0/g1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Mason, A. Plant flavone apigenin inhibits hdac and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinogen. 2012, 51, 952–962. [Google Scholar]
- Shukla, S.; Fu, P.; Gupta, S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and ku70-bax interaction in prostate cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef]
- Chan, L.P.; Chou, T.H.; Ding, H.Y.; Chen, P.R.; Chiang, F.Y.; Kuo, P.L.; Liang, C.H. Apigenin induces apoptosis via tumor necrosis factor receptor- and bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhu, Y.; Li, J.-F.; Wang, X.; Liang, Z.; Li, S.-Q.; Xu, X. Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget 2017, 8, 19834–19842. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.D.; Shiao, C.K.; Lee, Y.C.; Shih, Y.W. Apigenin, a dietary flavonoid, inhibits proliferation of human bladder cancer T-24 cells via blocking cell cycle progression and inducing apoptosis. Cancer Cell Int. 2015, 15, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, Y.Q.; Chen, H.; Lin, Y.W.; Hu, Z.H.; Wu, J.; Xu, X.; Xu, X.L.; Qin, J.; Xie, L.P. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of t24 human bladder cancer cells. Cancer Cell Int. 2013, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.R.; Yao, X.Q.; Wen, G.; Fan, Q.; Li, Y.J.; Fu, X.Q.; Li, C.K.; Sun, X.G. Apigenin suppresses the growth of colorectal cancer xenografts via phosphorylation and up-regulated fadd expression. Oncol. Lett. 2011, 2, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, X.; Gao, Y.; Zheng, J.; Xu, Q.; Sun, Y.; Guan, H.; Yu, H.; Sun, Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma bcpap cells. Food Funct. 2015, 6, 3464–3472. [Google Scholar] [CrossRef]
- Liu, R.; Ji, P.; Liu, B.; Qiao, H.; Wang, X.; Zhou, L.; Deng, T.; Ba, Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol. Lett. 2017, 13, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Marzocchella, L.; Quaranta, A.; Palumbo, C.; Pompa, G.; Izzi, V.; Canini, A.; Modesti, A.; Galvano, F.; Bei, R. Apigenin induces apoptosis and impairs head and neck carcinomas egfr/erbb2 signaling. Front. Biosci. 2011, 16, 1060–1068. [Google Scholar] [CrossRef]
- Masuelli, L.; Benvenuto, M.; Mattera, R.; Di Stefano, E.; Zago, E.; Taffera, G.; Tresoldi, I.; Giganti, M.G.; Frajese, G.V.; Berardi, G.; et al. In vitro and in vivo anti-tumoral effects of the flavonoid apigenin in malignant mesothelioma. Front. Pharmacol. 2017, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin-induced cell cycle arrest is mediated by modulation of mapk, pi3k-akt, and loss of cyclin d1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle 2007, 6, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.H.; Nafees, S.; Khan, A.; Rizvi, M.A. Chrysin: A promising anticancer agent its current trends and future imedpub journals chrysin: A promising anticancer agent its current trends and future perspectives. Eur. J. Exp. Biol. 2018, 8, 16. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; Athira, K.V.; Gogoi, R.; Barua, C.C. Chemopreventive and Therapeutic Potential of Chrysin in Cancer: Mechanistic Perspectives; Elsevier Ireland Ltd.: Amsterdam, The Netherlands, 2015; Volume 233, pp. 214–225. [Google Scholar]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.N.; Huang, J.M.; Xiong, X.K.; Chen, M.F.; Ong, C.N.; Shen, H.M.; Yang, X.F. Chrysin promotes tumor necrosis factor (tnf)-related apoptosis-inducing ligand (trail) induced apoptosis in human cancer cell lines. Toxicol. In Vitro 2011, 25, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ros and er stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Y.; Hu, D.N.; Iacob, C.; Lu, C.; Huang, Z. Chrysin induces cell apoptosis in human uveal melanoma cells via intrinsic apoptosis. Oncol. Lett. 2016, 4813–4820. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.M.; Wang, J.N.; Xiong, X.K.; Yang, X.F.; Zou, F. Combination of chrysin and cisplatin promotes the apoptosis of hep g2 cells by up-regulating p53. Chem.-Biol. Interact. 2015, 232, 12–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, S.; Liu, B.; Liu, J.; Zhu, R.; Li, M. Chrysin induces cell apoptosis via activation of the p53/bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp. Ther. Med. 2016, 12, 469–474. [Google Scholar] [CrossRef]
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of flos chrysanthemi extract in rats. Fitoterapia 2012, 83, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Molecular targets of luteolin in cancer. Eur. J. Cancer Prev. 2016, 25, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Park, O.J.; Lee, Y.K.; Sung, M.J.; Hur, H.J.; Kim, M.S.; Ha, J.H.; Kwon, D.Y. Anti-tumor effect of luteolin is accompanied by amp-activated protein kinase and nuclear factor-κb modulation in hepg2 hepatocarcinoma cells. Int. J. Mol. Med. 2011, 28, 25–31. [Google Scholar] [PubMed]
- Lee, H.J.; Wang, C.J.; Kuo, H.C.; Chou, F.P.; Jean, L.F.; Tseng, T.H. Induction apoptosis of luteolin in human hepatoma hepg2 cells involving mitochondria translocation of bax/bak and activation of jnk. Toxicol. Appl. Pharmacol. 2005, 203, 124–131. [Google Scholar] [CrossRef]
- Cai, X.; Ye, T.; Liu, C.; Lu, W.; Lu, M.; Zhang, J.; Wang, M.; Cao, P. Luteolin induced g2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol. In Vitro 2011, 25, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Q.; Li, M.-H.; Qin, Y.-M.; Jiang, H.-Y.; Zhang, X.; Wu, M.-H. Luteolin inhibits tumorigenesis and induces apoptosis of non-small cell lung cancer cells via regulation of microrna-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Yang, W.E.; Chang, H.R.; Chu, S.C.; Hsieh, Y.S. Luteolin induces apoptosis in oral squamous cancer cells. J. Dent. Res. 2008, 87, 401–406. [Google Scholar] [CrossRef]
- Park, S.H.; Park, H.S.; Lee, J.H.; Chi, G.Y.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Hyun, J.W.; Kim, W.J.; Choi, Y.H. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in nci-h460 lung carcinoma cells. Food Chem. Toxicol. 2013, 56, 100–109. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Jia, Y.; Pan, H.; Ding, H. Luteolin induces apoptosis by ros/er stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother. Pharmacol. 2017, 79, 1031–1041. [Google Scholar] [CrossRef]
- Johnson, J.L.; Gonzalez de Mejia, E. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem. Toxicol. 2013, 60, 83–91. [Google Scholar] [CrossRef]
- Yang, M.Y.; Wang, C.J.; Chen, N.F.; Ho, W.H.; Lu, F.J.; Tseng, T.H. Luteolin enhances paclitaxel-induced apoptosis in human breast cancer mda-mb-231 cells by blocking stat3. Chem.-Biol. Interact. 2014, 213, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Hyder, S.M. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer Targets Ther. 2016, 9, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Kim, K.H.; Kwon, T.H.; Bak, Y.; Lee, D.H.; Song, Y.S.; Park, S.H.; Park, Y.S.; Kim, M.S.; Kang, J.W.; et al. Luteolin induces intrinsic apoptosis via inhibition of e6/e7 oncogenes and activation of extrinsic and intrinsic signaling pathways in hpv-18-associated cells. Oncol. Rep. 2014, 31, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Zhao, X.H. Apigenin induces both intrinsic and extrinsic pathways of apoptosis in human colon carcinoma hct-116 cells. Oncol. Rep. 2017, 37, 1132–1140. [Google Scholar]
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014, 354, 5–11. [Google Scholar] [CrossRef]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef]
- Takahashi, H.; Chen, M.C.; Pham, H.; Angst, E.; King, J.C.; Park, J.; Brovman, E.Y.; Ishiguro, H.; Harris, D.M.; Reber, H.A.; et al. Baicalein, a component of scutellaria baicalensis, induces apoptosis by mcl-1 down-regulation in human pancreatic cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1465–1474. [Google Scholar] [CrossRef]
- Zhou, R.-T.; He, M.; Yu, Z.; Liang, Y.; Nie, Y.; Tai, S.; Teng, C.-B. Baicalein inhibits pancreatic cancer cell proliferation and invasion via suppression of nedd9 expression and its downstream akt and erk signaling pathways. Oncotarget 2017, 8, 56351–56363. [Google Scholar] [CrossRef]
- Chai, Y.; Xu, J.; Yan, B. The anti-metastatic effect of baicalein on colorectal cancer. Oncol. Rep. 2017, 37, 2317–2323. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.J.; Kim, H.R.; Lee, S.H.; Cho, S.D.; Choi, C.S.; Nam, J.S.; Jung, J.Y. Antitumor actions of baicalein and wogonin in ht-29 human colorectal cancer cells. Mol. Med. Rep. 2012, 6, 1443–1449. [Google Scholar] [CrossRef]
- Mu, J.; Liu, T.; Jiang, L.; Wu, X.; Cao, Y.; Li, M.; Dong, Q.; Liu, Y.; Xu, H. The traditional chinese medicine baicalein potently inhibits gastric cancer cells. J. Cancer 2016, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The fascinating effects of baicalein on cancer: A review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Guo, C.; Yang, Y.; Li, F.; Zhang, Y.; Jiang, B.; Li, Q. Baicalein induces apoptosis of human cervical cancer hela cells in vitro. Mol. Med. Rep. 2015, 11, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, C.; Chen, W.; Zhang, G.; Luo, D.; Cao, Y.; Wu, J.; Ding, Y.; Liu, B. Baicalein induces apoptosis and autophagy via endoplasmic reticulum stress in hepatocellular carcinoma cells. Biomed. Res. Int. 2014, 2014, 732516. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Maugeri, A.; Calapai, G.; Gangemi, S.; Navarra, M. Chemopreventive agents and inhibitors of cancer hallmarks: May citrus offer new perspectives? Nutrients 2016, 8, 698. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, K.; Baskaran, K.; Ilakkia, A.; Vanitha, K.; Selvaraj, S.; Sakthisekaran, D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7,12-dimethylbenz(a) anthracene-induced proliferative breast cancer in sprague-dawley rats. Cancer Chemother. Pharmacol. 2015, 75, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, L.; Sorimuthu Pillai, S. Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7,12-dimethylbenz(α)anthracene-induced rat mammary carcinoma. J. Nutr. Biochem. 2014, 25, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer ags cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, D.W.; Yu, X.D.; Zhou, Y.L. Tangeretin induces cell cycle arrest and apoptosis through upregulation of pten expression in glioma cells. Biomed. Pharmacother. 2016, 81, 491–496. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Liu, Y.; Zhang, T. Tangeretin sensitises human lung cancer cells to trail-induced apoptosis via ros-jnk/erk-chop pathway-mediated up-regulation of death receptor 5. Trop. J. Pharm. Res. 2017, 16, 17–29. [Google Scholar] [CrossRef]
- Kim, C.D.; Cha, J.D.; Li, S.; Cha, I.H. The mechanism of acacetin-induced apoptosis on oral squamous cell carcinoma. Arch. Oral Biol. 2015, 60, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Park, C.G.; Jung, J.Y. Acacetin (5,7-dihydroxy-4′-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of nf-kb/akt signaling in prostate cancer cells. Int. J. Mol. Med. 2014, 33, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yomogida, S. Acacetin induces apoptosis in human t cell leukemia jurkat cells via activation of a caspase cascade. Oncol. Rep. 2011, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Roudkenar, M.H.; Sadeghi, L.; Mohseni, A.; Seydi, E.; Pirahmadi, N.; Pourahmad, J. Selective anticancer activity of acacetin against chronic lymphocytic leukemia using both in vivo and in vitro methods: Key role of oxidative stress and cancerous mitochondria. Nutr. Cancer 2016, 68, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-H.; Hung, S.-H.; Yin, L.-T.; Huang, C.-S.; Chao, C.-H.; Liu, C.-L.; Shih, Y.-W. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer du145 cells via inactivation of the p38 mapk signaling pathway. Mol. Cell. Biochem. 2010, 333, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.-Y.; Park, J.-H.; Paik, H.-D.; Nah, S.-Y.; Kim, D.S.H.L.; Han, Y.S. Molecules and acacetin-induced apoptosis of human breast cancer mcf-7 cells involves caspase cascade, mitochondria-mediated death signaling and sapk/jnk1/2-c-jun activation. Mol. Cells 2007, 24, 95–104. [Google Scholar] [PubMed]
- Ni, W.; Ji, J.; Dai, Z.; Papp, A.; Johnson, A.J.; Ahn, S.; Farley, K.L.; Lin, T.S.; Dalton, J.T.; Li, X.; et al. Flavopiridol pharmacogenetics: Clinical and functional evidence for the role of slco1b1/oatp1b1 in flavopiridol disposition. PLoS ONE 2010, 5, e13792. [Google Scholar] [CrossRef]
- Li, L.; Pongtornpipat, P.; Tiutan, T.; Kendrick, S.L.; Park, S.; Persky, D.O.; Rimsza, L.M.; Puvvada, S.D.; Schatz, J.H. Synergistic induction of apoptosis in high-risk dlbcl by bcl2 inhibition with abt-199 combined with pharmacologic loss of mcl1. Leukemia 2015, 29, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, E.; Lucas, D.M.; Gupta, S.V.; Wagner, A.J.; Herman, S.E.M.; Smith, L.L.; Yeh, Y.-Y.; Andritsos, L.; Jones, J.A.; Flynn, J.M.; et al. Er stress and autophagy: New players in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Blood 2012, 120, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Wiernik, P.H. Alvocidib (flavopiridol) for the treatment of chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 2016, 25, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Li, G.Q.; Zhang, Y.; Guo, W.Z.; Zhang, J.K.; Li, J.; Lv, J.F.; Zhang, S.J. Upregulation of mcl-1 inhibits jq1-triggered anticancer activity in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2018, 495, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Yu, S.J.; Yoon, J.H.; Lee, S.H.; Lee, S.M.; Lee, J.H.; Kim, Y.J.; Lee, H.S.; Kim, C.Y. Synergistic anti-tumor efficacy of doxorubicin and flavopiridol in an in vivo hepatocellular carcinoma model. J. Cancer Res. Clin. Oncol. 2015, 141, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Gokce, O.; Dogan Turacli, I.; Ilke Onen, H.; Erdem, O.; Erguven Kayaa, E.; Ekmekci, A. Flavopiridol induces apoptosis via mitochondrial pathway in b16f10 murine melanoma cells and a subcutaneous melanoma tumor model. Acta Dermatovenerol. Croat. 2016, 24, 2–12. [Google Scholar] [PubMed]
- Zocchi, L.; Wu, S.C.; Wu, J.; Hayama, K.L.; Benavente, C.A. The cyclin-dependent kinase inhibitor flavopiridol (alvocidib) inhibits metastasis of human osteosarcoma cells. Oncotarget 2018, 9, 23505–23518. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.L.; Sharma, N.; Kumar Singh, A.; Singh Sodhi, S.; Zhang, J.J.; Mongre, R.K.; Ghosh, M.; Kim, N.; Ho Park, Y.; Kee Jeong, D. Anti-tumor activity of wogonin, an extract from scutellaria baicalensis, through regulating different signaling pathways. Chin. J. Nat. Med. 2017, 15, 15–40. [Google Scholar] [CrossRef]
- Ge, W.; Yin, Q.; Xian, H. Wogonin induced mitochondrial dysfunction and endoplasmic reticulum stress in human malignant neuroblastoma cells via ire1α-dependent pathway. J. Mol. Neurosci. 2015, 56, 652–662. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, Q.; Zheng, X.L.; Yan, J.Q.; Yang, L.; Sun, H.; Hu, L.N.; Lin, Y.; Wang, X. Wogonin potentiates cisplatin-induced cancer cell apoptosis through accumulation of intracellular reactive oxygen species. Oncol. Rep. 2012, 28, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Ruibin, J.; Bo, J.; Danying, W.; Chihong, Z.; Jianguo, F.; Linhui, G. Therapy effects of wogonin on ovarian cancer cells. BioMed Res. Int. 2017, 2017, 9381513. [Google Scholar] [CrossRef]
- Xu, M.; Lu, N.; Zhang, H.; Dai, Q.; Wei, L.; Li, Z.; You, Q.; Guo, Q. Wogonin induced cytotoxicity in human hepatocellular carcinoma cells by activation of unfolded protein response and inactivation of akt. Hepatol. Res. 2013, 43, 890–905. [Google Scholar] [CrossRef]
- Li, S.-J.; Sun, S.-J.; Gao, J.; Sun, F.-B. Wogonin induces beclin-1/pi3k and reactive oxygen species-mediated autophagy in human pancreatic cancer cells. Oncol. Lett. 2016, 12, 5059–5067. [Google Scholar] [CrossRef]
- Androutsopoulos, V.; Arroo, R.R.J.; Hall, J.F.; Surichan, S.; Potter, G.A. Antiproliferative and cytostatic effects of the natural product eupatorin on mda-mb-468 human breast cancer cells due to cyp1-mediated metabolism. Breast Cancer Res. 2008, 10, 1–12. [Google Scholar] [CrossRef]
- Lee, K.; Hyun Lee, D.; Jung, Y.J.; Shin, S.Y.; Lee, Y.H. The natural flavone eupatorin induces cell cycle arrest at the g2/m phase and apoptosis in hela cells. Appl. Biol. Chem. 2016, 59, 193–199. [Google Scholar] [CrossRef]
- Sarvestani, N.N.; Sepehri, H.; Farimani, M.M. Anticancer effect of eupatorin via bax/bcl-2 and mitochondrial membrane potential changes through ros mediated pathway in human colon cancer. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1039–1046. [Google Scholar]
- Sarvestani, N.N.; Sepehri, H.; Delphi, L.; Farimani, M.M. Eupatorin and salvigenin potentiate doxorubicin-induced apoptosis and cell cycle arrest in ht-29 and sw948 human colon cancer cells. Asian Pac. J. Cancer Prev. 2018, 19, 131–139. [Google Scholar]
- Estévez, S.; Marrero, M.T.; Quintana, J.; Estévez, F. Eupatorin-induced cell death in human leukemia cells is dependent on caspases and activates the mitogen-activated protein kinase pathway. PLoS ONE 2014, 9, e112536. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lu, H.; Ma, H.; Feng, F.; Hu, X.; Zhang, Q.; Wang, J.; Xu, Y.; Zhao, Q. Bioactive compounds of eriocaulon sieboldianum blocking proliferation and inducing apoptosis of hepg2 cells might be involved in aurora kinase inhibition. Food Funct. 2015, 6, 3746–3759. [Google Scholar] [CrossRef] [PubMed]
- López De Las Hazas, M.C.; Mosele, J.I.; Macià, A.; Ludwig, I.A.; Motilva, M.J. Exploring the colonic metabolism of grape and strawberry anthocyanins and their in vitro apoptotic effects in ht-29 colon cancer cells. J. Agric. Food Chem. 2017, 65, 6477–6487. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-o-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Sorrenti, V.; Vanella, L.; Acquaviva, R.; Cardile, V.; Giofrè, S.; Di Giacomo, C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015, 47, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.M.; Karimi, A.; Behroozaghdam, M.; Javidi, M.A.; Ghiasvand, S.; Bereimipour, A.; Aryan, H.; Nassiri, F.; Jangholi, E. Cytotoxic and apoptogenic effects of cyanidin-3-glucoside on the glioblastoma cell line. World Neurosurg. 2017, 108, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Oroudjev, E.; Wilson, L.; Ayoub, G. Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in mcf7 human breast cancer cells. Integr. Cancer Sci. Ther. 2015, 2, 82–86. [Google Scholar]
- Liu, X.; Zhang, D.; Hao, Y.; Liu, Q.; Wu, Y.; Liu, X.; Luo, J.; Zhou, T.; Sun, B.; Luo, X.; et al. Cyanidin curtails renal cell carcinoma tumorigenesis. Cell. Physiol. Biochem. 2018, 46, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.W.; Chung, H.S. Cyanidin and malvidin from oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytic leukemia cells by arrest of g(2)/m phase and induction of apoptosis. J. Agric. Food Chem. 2004, 52, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-tumor properties of anthocyanins from lonicera caerulea ‘beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Karthi, N.; Kalaiyarasu, T.; Kandakumar, S.; Mariyappan, P.; Manju, V. Pelargonidin induces apoptosis and cell cycle arrest: Via a mitochondria mediated intrinsic apoptotic pathway in ht29 cells. RSC Adv. 2016, 6, 45064–45076. [Google Scholar] [CrossRef]
- Wang, L.S.; Sun, X.D.; Cao, Y.; Wang, L.; Li, F.J.; Wang, Y.F. Antioxidant and pro-oxidant properties of acylated pelargonidin derivatives extracted from red radish (raphanus sativus var. Niger, brassicaceae). Food Chem. Toxicol. 2010, 48, 2712–2718. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Geng, B.; Yi, Z. Pelargonidin induces antitumor effects in human osteosar—Coma cells via autophagy induction, loss of mitochondrial membrane potential, g2/m cell cycle arrest and downregula—tion of pi3k/akt signalling pathway. J. BUON 2018, 23, 735–740. [Google Scholar]
- Ko, H.; Jeong, M.-H.; Jeon, H.; Sung, G.-J.; So, Y.; Kim, I.; Son, J.; Lee, S.-W.; Yoon, H.-G.; Choi, K.-C. Delphinidin sensitizes prostate cancer cells to trail-induced apoptosis, by inducing dr5 and causing caspase-mediated hdac3 cleavage. Oncotarget 2015, 6, 9970–9984. [Google Scholar] [CrossRef]
- Lim, W.; Song, G. Inhibitory effects of delphinidin on the proliferation of ovarian cancer cells via pi3k/akt and erk 1/2 mapk signal transduction. Oncol. Lett. 2017, 14, 810–818. [Google Scholar] [CrossRef]
- Pal, H.C.; Sharma, S.; Strickland, L.R.; Agarwal, J.; Athar, M.; Elmets, C.A.; Afaq, F. Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting egfr/vegfr2 signaling pathways. PLoS ONE 2013, 8, e77270. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M.; León-González, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.M.; Mauvieux, L.; Herbrecht, R.; et al. Bilberry extract (antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the bcl-2/bad pathway. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, B.; Asim, M.; Siddiqui, I.A.; Adhami, V.M.; Murtaza, I.; Mukhtar, H. Delphinidin, a dietary anthocyanidin in pigmented fruits and vegetables: A new weapon to blunt prostate cancer growth. Cell Cycle 2008, 7, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer pc3 cells in vitro and in vivo: Involvement of nuclear factor-kappab signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef]
- Li, H.Q.; Luo, Y.; Qiao, C.H. The mechanisms of anticancer agents by genistein and synthetic derivatives of isoflavone. Mini-Rev. Med. Chem. 2012, 12, 350–362. [Google Scholar] [PubMed]
- Spagnuolu, C.; Russo, G.L.; Orhan, l.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. Int. Rev. J. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Choi, E.J.; Jung, J.Y.; Kim, G.-H. Genistein inhibits the proliferation and differentiation of mcf-7 and 3t3-l1 cells via the regulation of erα expression and induction of apoptosis. Exp. Ther. Med. 2014, 8, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhou, W.; He, W.; Liu, X.; Ding, Q.; Ling, L.; Zha, X.; Wang, S. Genistein inhibits mda-mb-231 triple-negative breast cancer cell growth by inhibiting nf-κb activity via the notch-1 pathway. Int. J. Mol. Med. 2012, 30, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Solomon, L.A.; Ali, S.; Banerjee, S.; Munkarah, A.R.; Morris, R.T.; Sarkar, F.H. Sensitization of ovarian cancer cells to cisplatin by genistein: the role of NF-kappaB. J. Ovarian Res. 2008, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Krol, W. Soy isoflavones augment the effect of trail-mediated apoptotic death in prostate cancer cells. Oncol. Rep. 2011, 26, 533–541. [Google Scholar] [PubMed]
- Dong, X.; Xu, W.; Sikes, R.A.; Wu, C. Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone. Food Chem. 2013, 141, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Kumi-Diaka, J.; Merchant, K.; Haces, A.; Hormann, V.; Johnson, M. Genistein-selenium combination induces growth arrest in prostate cancer cells. J. Med. Food 2010, 13, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Weber, C.R.; Wasland, K.; Savkovic, S.D. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor foxo3 activity. BMC Cancer 2011, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Teng, J.; Zhu, Z.; Chen, J.; Huang, W.J. Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of akt in colorectal cancer cells. Pharm. Biol. 2016, 54, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Saidijam, M.; Tavilani, H.; Ghasemkhani, N.; Khodadadi, I. Genistein induces apoptosis and inhibits proliferation of ht29 colon cancer cells. Int. J. Mol. Cell. Med. 2016, 5, 178–191. [Google Scholar]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein induces mcf-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef]
- Liu, X.; Suzuki, N.; Laxmi, Y.R.S.; Okamoto, Y.; Shibutani, S. Anti-breast cancer potential of daidzein in rodents. Life Sci. 2012, 91, 415–419. [Google Scholar] [CrossRef]
- Park, H.J.; Jeon, Y.K.; You, D.H.; Nam, M.J. Daidzein causes cytochrome c-mediated apoptosis via the bcl-2 family in human hepatic cancer cells. Food Chem. Toxicol. 2013, 60, 542–549. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol egcg blunts androgen receptor function in prostate cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (egcg) inhibits pc-3 prostate cancer cell proliferation via mek-independent erk1/2 activation. Chem.-Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sadava, D.; Whitlock, E.; Kane, S.E. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem. Biophys. Res. Commun. 2007, 360, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Rieger-Christ, K.M.; Hanley, R.; Lodowsky, C.; Bernier, T.; Vemulapalli, P.; Roth, M.; Kim, J.; Yee, A.S.; Le, S.M.; Marie, P.J.; et al. The green tea compound, (−)-epigallocatechin-3-gallate downregulates n-cadherin and suppresses migration of bladder carcinoma cells. J. Cell. Biochem. 2007, 102, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Righeschi, C.; Eichhorn, T.; Karioti, A.; Bilia, A.R.; Efferth, T. Microarray-based mrna expression profiling of leukemia cells treated with the flavonoid, casticin. Cancer Genom. Proteomics 2012, 9, 143–151. [Google Scholar]
- Liu, F.; Cao, X.; Liu, Z.; Guo, H.; Ren, K.; Quan, M.; Zhou, Y.; Xiang, H.; Cao, J. Casticin suppresses self-renewal and invasion of lung cancer stem-like cells from a549 cells through down-regulation of pakt. Acta Biochim. Biophys. Sin. 2014, 46, 15–21. [Google Scholar] [CrossRef]
- Shen, J.-K.; Du, H.-P.; Yang, M.; Wang, Y.-G.; Jin, J. Casticin induces leukemic cell death through apoptosis and mitotic catastrophe. Ann.Hematol. 2009, 88, 743–752. [Google Scholar] [CrossRef]
- Roh, J.S.; Han, J.Y.; Kim, J.H.; Hwang, J.K. Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biol. Pharm. Bull. 2004, 27, 1976–1978. [Google Scholar] [CrossRef]
- Wirger, A.; Perabo, F.G.E.; Burgemeister, S.; Haase, L.; Schmidt, D.H.; Doehn, C.; Mueller, S.C.; Jocham, D. Flavopiridol, an inhibitor of cyclin-dependent kinases, induces growth inhibition and apoptosis in bladder cancer cells in vitro and in vivo. Anticancer Res. 2005, 25, 4341–4347. [Google Scholar]
- Mayer, F.; Mueller, S.; Malenke, E.; Kuczyk, M.; Hartmann, J.T.; Bokemeyer, C. Induction of apoptosis by flavopiridol unrelated to cell cycle arrest in germ cell tumour derived cell lines. Investig. New Drugs 2005, 23, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Schrump, D.S.; Matthews, W.; Chen, G.A.; Mixon, A.; Altorki, N.K. Flavopiridol mediates cell cycle arrest and apoptosis in esophageal cancer cells. Clin Cancer Res. 1998, 4, 2885–2890. [Google Scholar] [PubMed]
- Patel, V.; Senderowicz, A.M.; Pinto, D., Jr.; Igishi, T.; Raffeld, M.; Quintanilla-Martinez, L.; Ensley, J.F.; Sausville, E.A.; Gutkind, J.S. Flavopiridol, a novel cyclin-dependent kinase inhibitor, suppresses the growth of head and neck squamous cell carcinomas by inducing apoptosis. J. Clin. Investig. 1998, 102, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.W.; Kaur, G.; Nieves-Neira, W.; Taimi, M.; Kohlhagen, G.; Shimizu, T.; Losiewicz, M.D.; Pommier, Y.; Sausville, E.A.; Senderowicz, A.M. Early induction of apoptosis in hematopoietic cell lines after exposure to flavopiridol. Blood 1998, 91, 458–465. [Google Scholar] [PubMed]
- Dolečková, I.; Rárová, L.; Grúz, J.; Vondrusová, M.; Strnad, M.; Kryštof, V. Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of orthosiphon stamineus leaves. Fitoterapia 2012, 83, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Yuan, B.; Okusumi, S.; Aoyama, R.; Murota, R.; Kikuchi, H.; Takagi, N.; Toyoda, H. Enhanced cytotoxic effects of arsenite in combination with anthocyanidin compound, delphinidin, against a human leukemia cell line, hl-60. Chem.-Biol. Interact. 2018, 294, 9–17. [Google Scholar] [CrossRef]
- Kamenickova, A.; Anzenbacherova, E.; Pavek, P.; Soshilov, A.A.; Denison, M.S.; Anzenbacher, P.; Dvorak, Z. Pelargonidin activates the ahr and induces cyp1a1 in primary human hepatocytes and human cancer cell lines hepg2 and ls174t. Toxico. Lett. 2013, 218, 253–259. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F.; Valiani, A.; Ghobadifar, M.A. Effect of genistein on apoptosis and proliferation of hepatocellular carcinoma hepa1-6 cell line. Int. J. Prev. Med. 2018, 9, 12. [Google Scholar]
- Bi, Y.L.; Min, M.; Shen, W.; Liu, Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, g0/g1cell cycle arrest and regulation of stat3 signalling pathway. Phytomed. Int. J. Phytother. Phytopharmacol. 2018, 39, 10–16. [Google Scholar]
- Gundogdu, G.; Dodurga, Y.; Cetin, M.; Secme, M.; Cicek, B. The cytotoxic and genotoxic effects of daidzein on mia paca-2 human pancreatic carcinoma cells and ht-29 human colon cancer cells. Drug chem. Toxicol. 2018. [Google Scholar] [CrossRef]
- Hua, F.; Li, C.H.; Chen, X.G.; Liu, X.P. Daidzein exerts anticancer activity towards skov3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the raf/mek/erk cascade. Int. J. Mol. Med. 2018, 41, 3485–3492. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-J.; Li, W.; Jiang, G.-B.; Lai, S.-H.; Zhang, C.; Zeng, C.-C.; Liu, Y.-J. Effects of daidzein in regards to cytotoxicity in vitro, apoptosis, reactive oxygen species level, cell cycle arrest and the expression of caspase and bcl-2 family proteins. Oncol. Rep. 2015, 34, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Viskupičová, J.; Ondrejovič, M.; Šturdík, E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 2008, 47, 151–162. [Google Scholar]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of polyphenols and flavonoids in the era of precision medicine. Mol. Pharm. 2017, 14, 2861–2863. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Barnes, S. Soy isoflavones--phytoestrogens and what else? J. Nutr. 2004, 134, 1225S–1228S. [Google Scholar] [CrossRef]
- Messina, M.J.; Loprinzi, C.L. Soy for breast cancer survivors: A critical review of the literature. J. Nutr. 2001, 131, 3095S–3108S. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Chen, M.; Wang, Y. Delivering flavonoids into solid tumors using nanotechnologies. Expert Opin. Drug Deliv. 2013, 10, 1411–1428. [Google Scholar] [CrossRef] [PubMed]
- Goniotaki, M.; Hatziantoniou, S.; Dimas, K.; Wagner, M.; Demetzos, C. Encapsulation of naturally occurring flavonoids into liposomes: Physicochemical properties and biological activity against human cancer cell lines. J. Pharm. Pharmacol. 2004, 56, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wu, C.; Li, J.; Guo, A.; Li, Q.; Jiang, H.; Chen, B.; Wang, X. Synergistic effect of functionalized nickel nanoparticles and quercetin on inhibition of the smmc-7721 cells proliferation. Nanoscale Res. Lett. 2009, 4, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Chiu, G.N. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model. Nanomedicine 2011, 7, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Yang, R.; Li, J.; Liang, W.; Zhang, Y.; Dong, M.; Besenbacher, F.; Wang, C. Enhancement of biological activities of nanostructured hydrophobic drug species. Nanoscale 2012, 4, 2078–2082. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.P.; Chen, L.J.; Wei, Y.Q.; Fan, L.Y.; Tang, M.H.; Yang, G.L. Nanoliposomal quercetin inhibits formation of malignant ascites of hepatocellular carcinoma. Ai Zheng 2006, 25, 941–945. [Google Scholar] [PubMed]
- Yuan, Z.P.; Chen, L.J.; Fan, L.Y.; Tang, M.H.; Yang, G.L.; Yang, H.S.; Du, X.B.; Wang, G.Q.; Yao, W.X.; Zhao, Q.M.; et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006, 12, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, P.; Asensi, M.; Segarra, R.; Ortega, A.; Benlloch, M.; Obrador, E.; Varea, M.T.; Asensio, G.; Jorda, L.; Estrela, J.M. Association between pterostilbene and quercetin inhibits metastatic activity of b16 melanoma. Neoplasia 2005, 7, 37–47. [Google Scholar] [CrossRef]
- Harper, C.E.; Cook, L.M.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Arabshahi, A.; Shirai, T.; Lamartiniere, C.A. Genistein and resveratrol, alone and in combination, suppress prostate cancer in sv-40 tag rats. Prostate 2009, 69, 1668–1682. [Google Scholar] [CrossRef]
- Sakamoto, K. Synergistic effects of thearubigin and genistein on human prostate tumor cell (pc-3) growth via cell cycle arrest. Cancer Lett. 2000, 151, 103–109. [Google Scholar] [CrossRef]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Vadgama, J.V.; Said, J.W.; Magyar, C.E.; Doan, N.; Heber, D.; Henning, S.M. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J. Nutr. Biochem. 2014, 25, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Wang, D.; Zhang, H.; Peng, S.; Shin, H.J.; Brandes, J.C.; Tighiouart, M.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Enhanced anti-tumor activity by the combination of the natural compounds (-)-epigallocatechin-3-gallate and luteolin: Potential role of p53. J. Biol. Chem. 2010, 285, 34557–34565. [Google Scholar] [CrossRef] [PubMed]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The combination of epigallocatechin gallate and curcumin suppresses er alpha-breast cancer cell growth in vitro and in vivo. Int. J. Cancer 2008, 122, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Bomser, J.A.; Romero, C.; Talcott, S.T.; Percival, S.S. Ellagic acid potentiates the effect of quercetin on p21waf1/cip1, p53, and map-kinases without affecting intracellular generation of reactive oxygen species in vitro. J. Nutr. 2005, 135, 609–614. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Talcott, S.T.; Percival, S.S. Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in molt-4 human leukemia cells. J. Nutr. 2003, 133, 2669–2674. [Google Scholar] [CrossRef]
- Suganuma, M.; Okabe, S.; Kai, Y.; Sueoka, N.; Sueoka, E.; Fujiki, H. Synergistic effects of (—)-epigallocatechin gallate with (—)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line pc-9. Cancer Res. 1999, 59, 44–47. [Google Scholar]
- Suganuma, M.; Kurusu, M.; Suzuki, K.; Tasaki, E.; Fujiki, H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of gadd153 gene. Int. J. Cancer 2006, 119, 33–40. [Google Scholar] [CrossRef]
- Adhami, V.M.; Malik, A.; Zaman, N.; Sarfaraz, S.; Siddiqui, I.A.; Syed, D.N.; Afaq, F.; Pasha, F.S.; Saleem, M.; Mukhtar, H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin. Cancer Res. 2007, 13, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.E.; Wang, M. Synergistic effects of the green tea extract epigallocatechin-3-gallate and taxane in eradication of malignant human prostate tumors. Transl. Oncol. 2011, 4, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.E.; Amatangelo, M.D.; Varma, D.; Sell, C.; Goodyear, S.M. Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: Inhibition of metastatic tumor growth in severe combined immunodeficiency mice. Am. J. Pathol. 2010, 177, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Tang, A.; Lin, X.; Li, L.; Zhang, S.; Huang, Z.; Tang, H.; Li, Q.Q. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int. J. Oncol. 2010, 37, 111–123. [Google Scholar] [PubMed]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (−)-epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010, 12, R8. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.; Soprano, K.J.; Weinstein, K.; Fong, D. Epigallocatechin-3-gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J. Cell Physiol. 2006, 207, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Fu, J.; Shankar, S.; Srivastava, R.K. Egcg enhances the therapeutic potential of gemcitabine and cp690550 by inhibiting stat3 signaling pathway in human pancreatic cancer. PLoS ONE 2012, 7, e31067. [Google Scholar] [CrossRef] [PubMed]
- Staedler, D.; Idrizi, E.; Kenzaoui, B.H.; Juillerat-Jeanneret, L. Drug combinations with quercetin: Doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Sen, S.; Singh, N. Molecular pathways in the chemosensitization of cisplatin by quercetin in human head and neck cancer. Cancer Biol. Ther. 2005, 4, 949–955. [Google Scholar] [CrossRef]
- Banerjee, S.; Zhang, Y.; Ali, S.; Bhuiyan, M.; Wang, Z.; Chiao, P.J.; Philip, P.A.; Abbruzzese, J.; Sarkar, F.H. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005, 65, 9064–9072. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Banerjee, S.; Li, Y.; Aboukameel, A.; Kucuk, O.; Sarkar, F.H. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in bxpc-3 pancreatic tumor xenografts. Cancer 2006, 106, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zhang, Y.; Wang, Z.; Che, M.; Chiao, P.J.; Abbruzzese, J.L.; Sarkar, F.H. In vitro and in vivo molecular evidence of genistein action in augmenting the efficacy of cisplatin in pancreatic cancer. Int. J. Cancer 2007, 120, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Sarkar, F.H.; Hillman, G.G. Radiosensitization of prostate cancer by soy isoflavones. Curr. Cancer Drug Targets 2007, 7, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Singh-Gupta, V.; Knoll, Z.E.; Fite, A.; Zhang, H.; Abrams, J.; Sarkar, F.H.; Hillman, G.G. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007, 67, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Che, M.; Knoll, Z.E.; Doerge, D.R.; Abrams, J.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int. J. Cancer 2007, 120, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Saldanha, S.N.; Tollefsbol, T.O. The role of nutraceuticals in chemoprevention and chemotherapy and their clinical outcomes. J. Oncol. 2012, 2012, 192464. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A phase i study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, K.; Liu, Q.; Yang, C.; et al. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef]
| Compound | Cancer Type/Cell Line | Time Point (h) | IC50 | Reference |
|---|---|---|---|---|
| Hesperetin | Myeloid | 24 | 500 ± 100 µM | [222] |
| Naringenin | Lymphoid | 48 | 164.9 ± 21.83 µM | [222] |
| EGCG | Breast T47D | 72 | 14.17 µM | [49] |
| Prostrate LNCaP | 48 | 0.4 µM | [223] | |
| Prostrate PC3 | 48 | 38.95 µM | [224] | |
| Lung | 24 | 70 µM | [225] | |
| Bladder | 70–80 µM | [226] | ||
| Acute T-lymphoblastic leukemia (CCRF-CEM) | 16.04 ± 1.56 µM | [227] | ||
| Quercetin | Bladder | 48 | 876.9 ± 13.1 µM | [222] |
| Kaempferol | Bone | 24 | 148.4 µM | [222] |
| Fisetin | Erythroid | 48 | 15 ± 2 µM | [222] |
| Myricetin | Colon | 72 | 68.0 ± 20.4 µM | [222] |
| Galangin | Liver | 24 | 100.4 ± 17.0 µM | [222] |
| Casticin | Lung A549 | 48 | 0.4 µMol/L | [228] |
| Actute T-lymphoblastic leukemia (CCRF-CEM) | 24 & 72 | 0.28 ± 0.02 µM | [227] | |
| Leukemia K562 | 48 | 5.95 µM | [229] | |
| Leukemia Kasumi-1 | 48 | 15.56 µM | [229] | |
| Leukemia HL-60 | 48 | 4.82 µM | [229] | |
| Apigenin | ER- breast | 24 | 60.4 ± 15.8 µM | [222] |
| Chrysin | ER+ breast | 48 | 82.5 µM | [222] |
| Luteolin | Lung | 72 | 35.9 ± 9.3 µM | [222] |
| Baicalein | Stomach | 72 | 64.3 µM | [222] |
| Tangertin | Melanoma | 48 | 0.3 µM | [222] |
| Acacetin | Oral squamous HSC-3 | 24 | 25 µg/mL | [162] |
| MCF7 | 24 | 26.4 ± 0.7 µM | [167] | |
| Melanoma | 0.79 mM | [230] | ||
| Flavopiridol | Bladder RT4/RTI12 | 24–72 | 150–350 nM | [231] |
| Bladder T24/SUP | 24–72 | 1000 nM | [231] | |
| GCT | 72 | 60–70 nM | [232] | |
| SKOV, MCF-7, HeLa | 72 | 280–350 nM | [232] | |
| Esophageal | 72 | 100–150 mM | [233] | |
| HNSCC tumor xenografts | 10 weeks | 5 mg/kg/day | [234] | |
| Hematopoietic SUDHL4 | 12 | 120 mMol/L | [235] | |
| Prostrate PC3 | 12 | 203 mMol/L | [235] | |
| Wogonin | Ovarian | 72 | 19.9 ± 1.2 µM | [222] |
| Eupatorin | HeLa | 11.72 ± 2.86 µM | [236] | |
| K562 | 4.29 ± 1.35 µM | [236] | ||
| MCF7 | 16.61 ± 5.56 µM | [236] | ||
| RPMI8226 | 4.77 ± 0.51 µM | [236] | ||
| HL-60 | 14.09 ± 0.55 µM | [236] | ||
| MOLT | 4.74 ± 0.43 µM | [236] | ||
| MDA-MB-468 | 96 | 0.5 µM | [182] | |
| MCF-7 | 96 | 50 µM | [182] | |
| Cyanidin | HL-60 | 48 | 31.6 µM | [237] |
| Pelargonidin | HL-60 | 48 | 85.2 µM | [237] |
| Osteosarcoma U2OS | 15 µM | [200] | ||
| HepG2 | 24 | 33 µM | [238] | |
| Delphinidin | HepG2 | 24 | 77 µM | [238] |
| HL-60 | 48 | 10.9 µM | [237] | |
| Genistein | Hepatocellular Hepa1-6 | 24 | 20 µM | [239] |
| Pancreatic Mia-PaCa2 | 24 | 20 µM | [240] | |
| Pancreatic PANC-1 | 24 | 25 µM | [240] | |
| Pancreatic H6C7 | 24 | 120 µM | [240] | |
| Colorectal HCT 116 | 24 | 690 µM | [217] | |
| Colorectal HCT 116 | 48 | 135 µM | [217] | |
| Colorectal HCT 116 | 72 | 61 µM | [217] | |
| Daidzein | Colorectal HT-29 | 48 | 200 µM | [241] |
| MIA PaCa-2 | 48 | 200 µM | [241] | |
| Ovarian SKOV3 [224] | 24 | 20 µM | [242] | |
| BEL-7402 | 48 | 59.7 ± 8.1 µM | [243] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. https://doi.org/10.3390/cancers11010028
Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Büsselberg D. Flavonoids in Cancer and Apoptosis. Cancers. 2019; 11(1):28. https://doi.org/10.3390/cancers11010028
Chicago/Turabian StyleAbotaleb, Mariam, Samson Mathews Samuel, Elizabeth Varghese, Sharon Varghese, Peter Kubatka, Alena Liskova, and Dietrich Büsselberg. 2019. "Flavonoids in Cancer and Apoptosis" Cancers 11, no. 1: 28. https://doi.org/10.3390/cancers11010028
APA StyleAbotaleb, M., Samuel, S. M., Varghese, E., Varghese, S., Kubatka, P., Liskova, A., & Büsselberg, D. (2019). Flavonoids in Cancer and Apoptosis. Cancers, 11(1), 28. https://doi.org/10.3390/cancers11010028







