Proton Partial Breast Irradiation: Detailed Description of Acute Clinico-Radiologic Effects
Abstract
:1. Introduction
2. Patients and Methods
2.1. Assessment of Skin Changes
2.2. Assessment of Radiologic Changes
3. Results
3.1. Skin Toxicity
3.2. Radiologic Correlate
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Czechura, T.; Winchester, D.J.; Pesce, C.; Huo, D.; Winchester, D.P.; Yao, K. Accelerated partial-breast irradiation versus whole-breast irradiation for early-stage breast cancer patients undergoing breast conservation, 2003–2010: A report from the national cancer data base. Ann. Surg. Oncol. 2013, 20, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Taghian, A.G.; Kozak, K.R.; Katz, A.; Adams, J.; Lu, H.M.; Powell, S.N.; DeLaney, T.F. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Pignol, J.P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Shaitelman, S.F.; Kim, L.H. Accelerated partial-breast irradiation: The current state of our knowledge. Oncology 2013, 27, 329–342. [Google Scholar] [PubMed]
- Galland-Girodet, S.; Pashtan, I.; MacDonald, S.M.; Ancukiewicz, M.; Hirsch, A.E.; Kachnic, L.A.; Specht, M.; Gadd, M.; Smith, B.L.; Powell, S.N.; et al. Long-term cosmetic outcomes and toxicities of proton beam therapy compared with photon-based 3-dimensional conformal accelerated partial-breast irradiation: A phase 1 trial. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.A.; Do, S.; Lum, S.; Garberoglio, C.; Mirshahidi, H.; Patyal, B.; Grove, R.; Slater, J.D. Partial breast radiation therapy with proton beam: 5-year results with cosmetic outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Lee, N.K.; Kim, J.Y.; Kim, Y.J.; Moon, S.H.; Kim, T.H.; Kim, J.Y.; Kim, D.Y.; Cho, K.H.; Shin, K.H. Phase II trial of proton beam accelerated partial breast irradiation in breast cancer. Radiother Oncol. 2013, 108, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Li, X.; Amos, R.A.; Shaitelman, S.F.; Hoffman, K.; Howell, R.; Salehpour, M.; Zhang, S.X.; Sun, T.L.; et al. Accelerated partial-breast irradiation using intensity-modulated proton radiotherapy: Do uncertainties outweigh potential benefits? Br. J. Radiol. 2013, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Depauw, N.; Batin, E.; Daartz, J.; Rosenfeld, A.; Adams, J.; Kooy, H.; MacDonald, S.; Lu, H.M. A novel approach to postmastectomy radiation therapy using scanned proton beams. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Strom, E.A.; Ovalle, V. Initial clinical experience using protons for accelerated partial-breast irradiation: Longer-term results. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Kozak, K.R.; Smith, B.L.; Adams, J.; Kornmehl, E.; Katz, A.; Gadd, M.; Specht, M.; Hughes, K.; Gioioso, V.; Lu, H.M. Accelerated partial-breast irradiation using proton beams: Initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.A.; Slater, J.D.; Garberoglio, C.; Do, S.; Lum, S.; Slater, J.M. Partial breast irradiation delivered with proton beam: Results of a phase ii trial. Clin. Breast Cancer 2011, 11, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Strom, E.A.; Amos, R.A.; Shaitelman, S.F.; Kerr, M.D.; Hoffman, K.E.; Smith, B.D.; Tereffe, W.; Stauder, M.C.; Perkins, G.H.; Amin, M.D.; et al. Proton partial breast irradiation in the supine position: Treatment description and reproducibility of a multibeam technique. Pract. Radiat. Oncol. 2015, 5, e283–e290. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 4 April 2018).
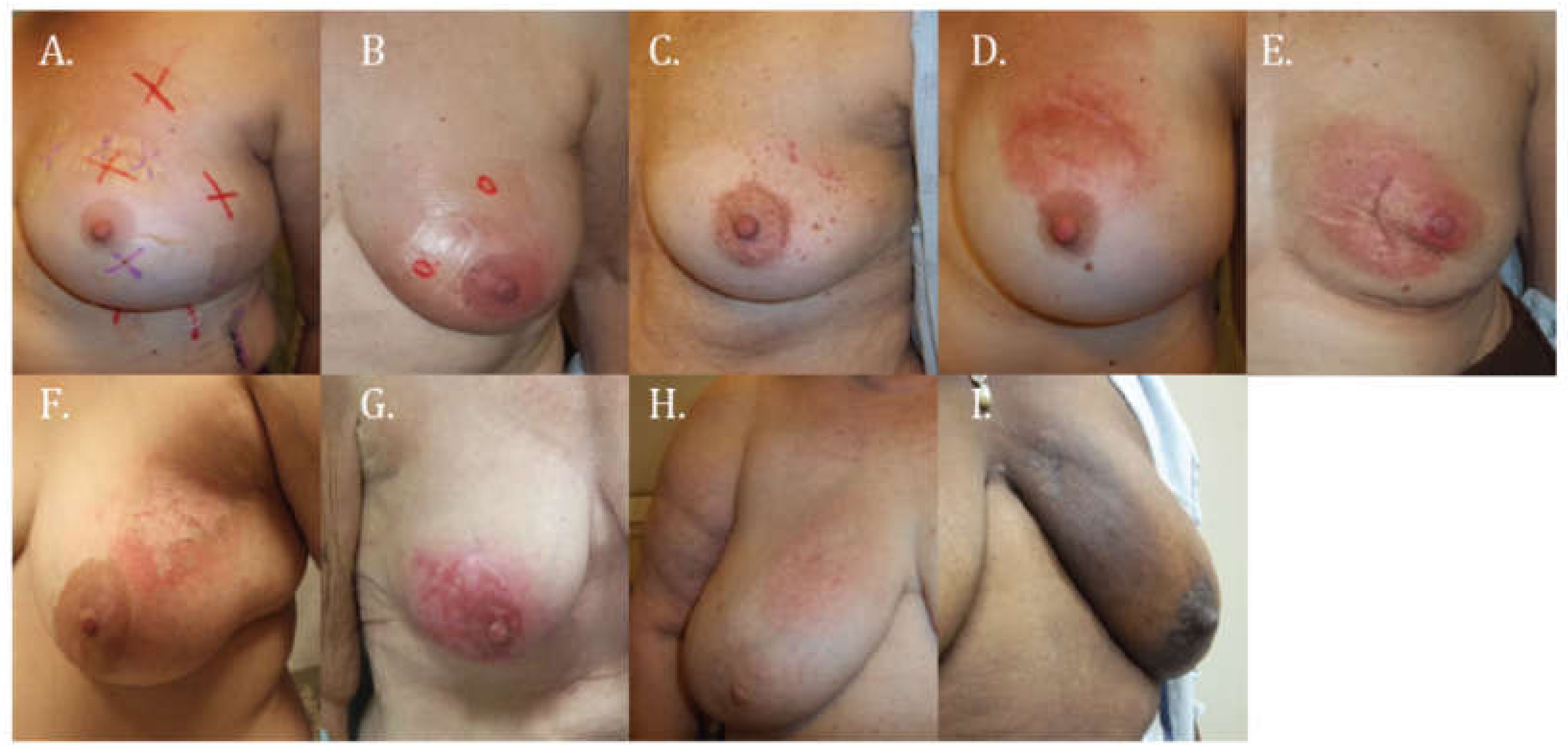

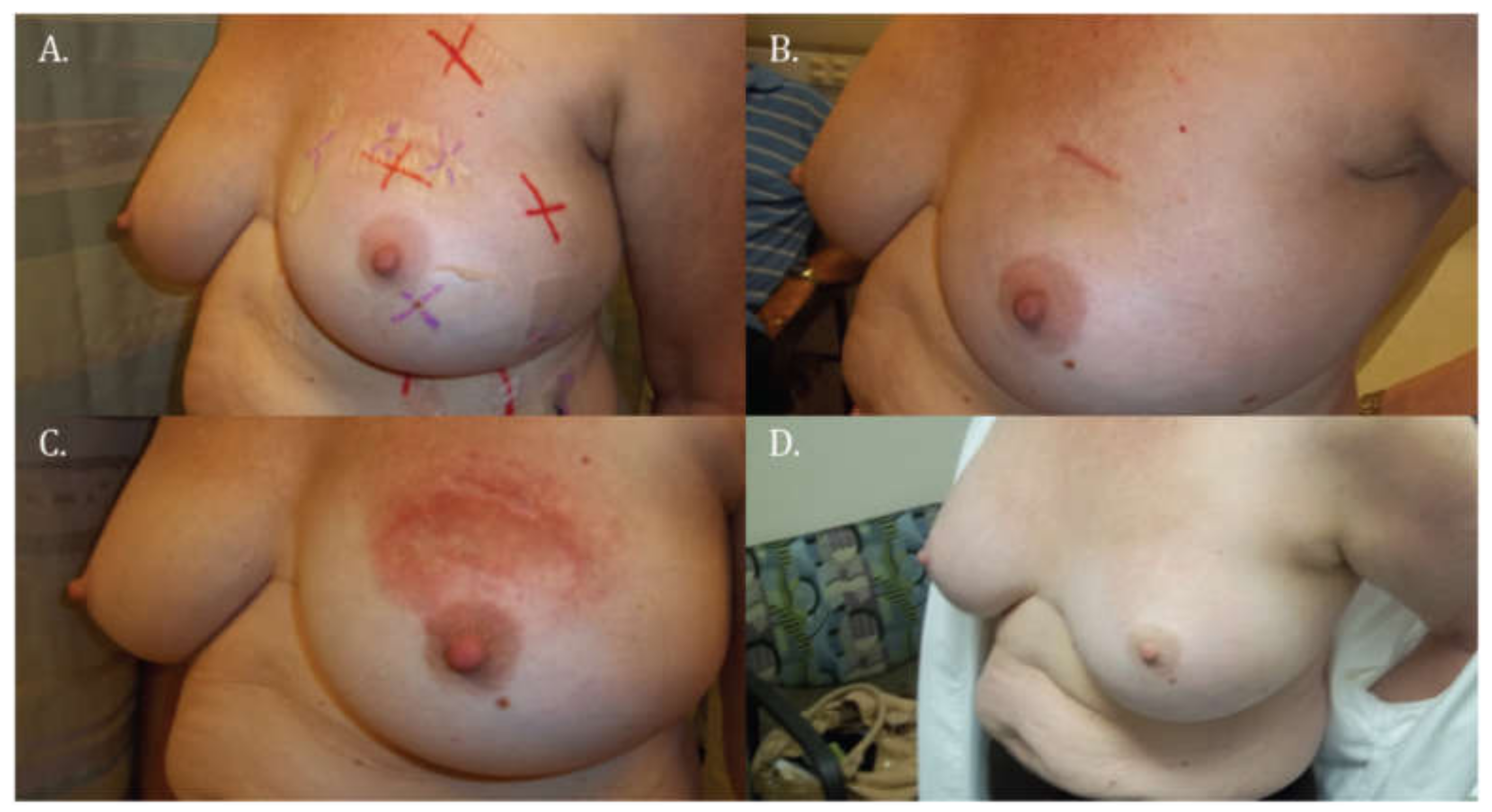



| Main Skin Reaction | CTCAE 4.0 Grade (Subcategorized) | Description |
|---|---|---|
| Erythema | 0 | No visual Changes |
| 1(a) | Faint erythema | |
| 1(b) | Patchy erythema in ≤50% of the treated skin area | |
| 1(c) | Patchy erythema in >50% of the treated skin area | |
| 2(a) | Confluent erythema over entire treated area | |
| Desquamation | 1(d) | Dry desquamation limited to treated area |
| 3 | Moist desquamation limited to treated area not in skin folds | |
| Hyperpigmentation | 1(a) | Mild hyperpigmentation limited to treated area |
| 1(b) | Moderate/severe hyperpigmentation limited to treated area |
| Mammographic Finding | CTCAE 4.0 Grade |
|---|---|
| Skin thickening | NA |
| Seroma/hematoma | 1 |
| Fat necrosis | 1 |
| Retraction/Asymmetry | Mild (1) |
| Moderate (2) | |
| Severe (3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovalle, V.; Strom, E.A.; Shaitelman, S.; Hoffman, K.; Amos, R.; Perkins, G.; Tereffe, W.; Smith, B.D.; Stauder, M.; Woodward, W. Proton Partial Breast Irradiation: Detailed Description of Acute Clinico-Radiologic Effects. Cancers 2018, 10, 111. https://doi.org/10.3390/cancers10040111
Ovalle V, Strom EA, Shaitelman S, Hoffman K, Amos R, Perkins G, Tereffe W, Smith BD, Stauder M, Woodward W. Proton Partial Breast Irradiation: Detailed Description of Acute Clinico-Radiologic Effects. Cancers. 2018; 10(4):111. https://doi.org/10.3390/cancers10040111
Chicago/Turabian StyleOvalle, Valentina, Eric A. Strom, Simona Shaitelman, Karen Hoffman, Richard Amos, George Perkins, Welela Tereffe, Benjamin D. Smith, Michael Stauder, and Wendy Woodward. 2018. "Proton Partial Breast Irradiation: Detailed Description of Acute Clinico-Radiologic Effects" Cancers 10, no. 4: 111. https://doi.org/10.3390/cancers10040111
APA StyleOvalle, V., Strom, E. A., Shaitelman, S., Hoffman, K., Amos, R., Perkins, G., Tereffe, W., Smith, B. D., Stauder, M., & Woodward, W. (2018). Proton Partial Breast Irradiation: Detailed Description of Acute Clinico-Radiologic Effects. Cancers, 10(4), 111. https://doi.org/10.3390/cancers10040111





